Abstract
Endurance exercise training can increase the ability to perform prolonged strenuous exercise. The major adaptation responsible for this increase in endurance is an increase in muscle mitochondria. This adaptation occurs too slowly to provide a survival advantage when there is a sudden change in environment that necessitates prolonged exercise. In the present study, we discovered another, more rapid adaptation, a downregulation of expression of the glycogenolytic and glycolytic enzymes in muscle that mediates a slowing of muscle glycogen depletion and lactic acid accumulation. This adaptation, which appears to be mediated by PGC-1α, occurs in response to a single exercise bout and is further enhanced by two additional daily exercise bouts. It is biologically significant, because glycogen depletion and lactic acid accumulation are two of the major causes of muscle fatigue and exhaustion.
Key points
Long-term endurance exercise training results in a reduction in the rates of muscle glycogen depletion and lactic acid accumulation during submaximal exercise; this adaptation is mediated by an increase in muscle mitochondria.
There is evidence suggesting that short-term training induces adaptations that downregulate glycogenolysis before there is an increase in functional mitochondria.
We discovered that a single long bout of exercise induces decreases in expression of glycogenolytic and glycolytic enzymes in rat skeletal muscle; this adaptation results in slower rates of glycogenolysis and lactic acid accumulation in muscle during contractile activity.
Two additional days of training amplified the adaptive response, which appears to be mediated by PGC-1α; this adaptation is biologically significant, because glycogen depletion and lactic acid accumulation are major causes of muscle fatigue.
Introduction
Endurance exercise training induces adaptations that increase the ability to perform prolonged vigorous exercise. This increase in endurance is, in part, mediated by a slower depletion of glycogen and reduced production of lactic acid in the working muscles during exercise of the same intensity after, as compared to before, training. The glycogen sparing effect of training is important because glycogen depletion is a major cause of exhaustion that forces cessation of prolonged, strenuous exercise (Ahlborg et al. 1967; Hermansen et al. 1967; Baldwin et al. 1973a), while lactic acid accumulation in muscle can cause fatigue during brief, very intense exercise (Hermansen, 1981; Knuth et al. 2006; Fitts, 2008). The glycogen sparing effect of training appears to be mediated by an exercise-induced increase in muscle mitochondria (Constable et al. 1987).
The increase in mitochondrial biogenesis induced by exercise is initiated by rapid increases in peroxisome proliferator activated receptor γ coactivator-1α (PGC-1α) activity and expression (Baar et al. 2002; Terada et al. 2002; Wright et al. 2007). PGC-1α coactivates the transcription factors that control expression of genes encoding mitochondrial proteins and, thus, stimulates mitochondrial biogenesis (Wu et al. 1999; Kelly & Scarpulla, 2004; Handschin & Spiegelman, 2006). Many of the mitochondrial enzyme proteins involved in substrate oxidation have short half-lives, and their expression peaks within 18–24 h after a bout of exercise (Wright et al. 2007). Others, including cytochrome c and a number of citrate cycle enzymes have long half-lives of ∼7 days and increase more slowly (Booth & Holloszy, 1977). The newly synthesized proteins, along with various lipids and lipoproteins, have to be integrated into existing mitochondria or used to form new mitochondria. As a result, it takes more than 3 days before an exercise-induced increase in functional mitochondria, as evidenced by an increase in the capacity for substrate oxidation, begins to occur in skeletal muscle.
Adaptive responses were selected for because they enhance the ability to adjust to and survive changes in the environment. An adaptation to exercise that take longer than 3 days before it begins to enhance performance can provide a survival advantage in situations when there is time to prepare for a challenge requiring enhanced physical performance. However, such a relatively slow adaptation is of no benefit in the case of a sudden emergency that requires a prolonged increase in physical activity for survival such as escaping from invading predators or an advancing flood. This consideration, together with reports that as few as three to five daily endurance training sessions result in a slowing of glycogenolysis and lactate accumulation (Green et al. 1992; Phillips et al. 1996), led us to examine the hypothesis that the increase in functional mitochondria induced by exercise is preceded by an adaptive decrease in muscle glycogenolytic and glycolytic capacity.
The present results show that a single, long bout of exercise results in a downregulation of expression of phosphorylase, phosphorylase kinase, phosphofructokinase, and other glycolytic enzymes, with a reduction in the rates of glycogen breakdown and lactic acid accumulation in muscles during contractile activity. Our findings provide evidence that this adaptive downregulation of glycogenolytic and glygolytic enzymes is mediated by PGC-1α.
Methods
Animals and exercise programme
This research was approved by the Animal Studies Committee of Washington University School of Medicine. Sixty Wistar male rats weighing ∼100 g were obtained from Charles River (Wilmington, MA, USA) and maintained on a diet of Purina chow and water. Rats were accustomed to swimming for 10 min day–1 for 3 days. Rats were exercised by swimming in water maintained at 30°C using a modification of the protocol described by Ploug et al. (1990); exercise was performed in two 3 h sessions separated by a 45 min rest. One group exercised on one day, another group exercised on three consecutive days.
Muscle stimulation protocol
Sixteen to 20 h after the last exercise bout, rats were anaesthetized with pentobarbital sodium (5 mg (100 g body weight)–1) and given oxygen via a face mask. The right forelimb was prepared for stimulation of the triceps muscle via the radial nerve (Favier et al. 1986). After a 5 min recovery, the muscles were stimulated with 100 ms long trains of 50 Hz at a rate of 60 trains min–1 (Favier et al. 1986). After 3 min of stimulation, the contracting triceps muscle was clamp-frozen. Anaesthetized rats were killed by exsanguination.
Analytical methods
Frozen muscle was used for measurement of glycogen (Keppler & Decker, 1974), lactic acid (Gutman & Wahlefeld, 1974), ATP (Lamprecht & Trautschold, 1974), creatine phosphate (Lamprecht et al. 1974) and inorganic phosphate (Guynn et al. 1972). Protein was measured by the method of Lowry et al. (1951).
Muscle electroporation
Transfection of PGC-1α DNA into rat triceps muscle was performed using an electric pulse-mediated gene transfer technique (Higashida et al. 2013). Rats weighing ∼60 g were anaesthetized with isoflurane gas. A triceps muscle was injected with 100 μg of plasmid DNA containing either empty vector, or a pEGFP-PGC-1 vector (Puigserver et al. 1998; Addgene, Plasmid 4; Cambridge, MA, USA) in 100 μl saline, using a 27 gauge needle, at a rate of 40 μl min−1. After injection an electric field was applied to the triceps muscle (Higashida et al. 2013). Muscles were harvested 8 days after electroporation.
Western blot analysis
Muscle extracts were prepared and Western blotting was performed as described previously (Hancock et al. 2011). Blots were probed with the following antibodies: PGC-1α (Calbiochem, 516557; San Diego, CA, USA); glycogen phosphorylase (Santa Cruz, SC-46347; Dallas, TX, USA); phosphorylase kinase (Gene Tex, GTX109401; Irvine, CA, USA); phosphofructokinase (Santa Cruz, SC1667222); glyceraldehydes phosphate dehydrogenase (Sigma, G8795; St. Louis, MO, USA); lactate dehydrogenase (Abcam, ab7638; Cambridge, UK); β-actin (Sigma, A5441). The blots were then incubated with the appropriate horseradish-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, USA). Antibody bound protein was detected by enhanced chemiluminescence (ECL).
Pyruvate oxidation
Triceps muscles were homogenized and the capacity of whole muscle homogenates to oxidize pyruvate was measured as described previously (Winder et al. 1975) except that an Oxygraph-2 K (Oroboros, Innsbruck, Austria) was used to measure oxygen uptake.
Cell culture and PGC-1α shRNA transfection
C2C12 myoblasts were grown in DMEM (4.5 g glucose l–1, Invitrogen) containing 10% fetal bovine serum, 100 mU ml−1 penicillin, and 100 mU ml−1 streptomycin. When the myoblasts were 70% confluent, they were transfected with either a PGC-1α shRNA-plasmid or scrambled shRNA-plasmid (GeneCopia, Rockville, MD, USA) using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 24 h of transfection cells were differentiated by switching to medium containing 2% heat inactivated horse serum. After 48 h of differentiation, myotubes were harvested.
Statistics
Values are expressed as means ± SEM. Statistically significant differences were determined using unpaired Student's t tests, or ANOVA for multiple comparisons.
Results
One and three daily bouts of exercise training reduce expression of glycogenolytic and glycolytic enzymes
Phosphorylase and phosphorylase kinase protein expression levels in triceps muscle decreased ∼40% over an ∼18 h period after one bout of swimming (Fig.1). Similar decreases occurred in the expression of phosphofructokinase, glyceraldehyde phosphate dehydrogenase and total lactate dehydrogenase proteins (Fig.1). Two additional daily bouts of exercise resulted in further decreases in the expression of these enzymes, so that after 3 days of exercise the muscle protein levels were ∼60% to 80% lower than the control values (Fig. 1).
Figure 1. Effect of exercise on rat triceps muscle enzyme activity.
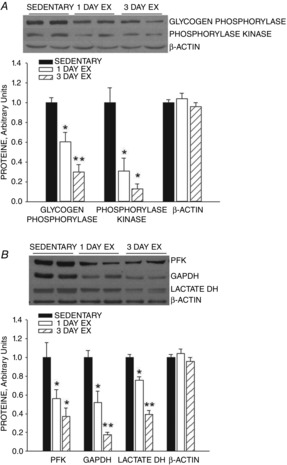
One bout of exercise results in downregulation of expression of glycogen phosphorylase, phosphorylase kinase, phosphofructokinase (PFK), glyceraldehyde phosphate dehydrogenase (GAPDH) and total lactate dehydrogenase in rat triceps muscle. Two additional daily exercise bouts result in a further decrease in expression of these enzymes. Values are means ± SEM for 6 animals per group. *P < 0.01, exercise vs. sedentary; **P < 0.001, 3 days exercise vs. 1 day exercise and sedentary.
One and three daily bouts of exercise training decrease the rates of glycogenolysis and lactic acid accumulation in muscle during contractile activity
The decrease in glycogen in muscles stimulated to contract in situ averaged 18 μmol (gm muscle)–1 in the no-exercise group (Fig.2A). Glycogen utilization in muscles stimulated to contract using the same protocol was 30% lower in rats studied ∼18 h after one exercise bout, and 55% lower in rats studied ∼18 h after the third exercise bout than in muscles of rats that had not been exercised (Fig. 2A and B). The increase in lactic acid in muscles stimulated to contract in situ in non-exercised rats averaged 12.6 μmol (gm muscle)–1 (Fig. 2B). Lactic acid accumulation in muscles stimulated to contract was 23% lower in rats studied ∼18 h after one exercise bout, and 47% lower in rats studied ∼18 h after a third exercise bout (Fig. 2C and D).
Figure 2. Effect of exercise on rat triceps muscle glycogen depletion and lactate accumulation.
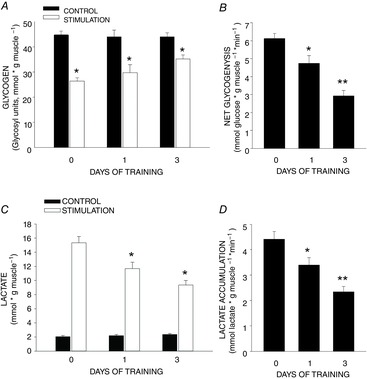
One bout of exercise results in a reduction in glycogen depletion and lactate accumulation in rat triceps muscle stimulated to contract in situ ∼18 h after exercise. Two additional daily exercise bouts enhance this adaptation. A, decreases in muscle glycogen. *P < 0.01, control muscle versus muscles stimulated to contract. B, rates of glycogenolysis. *P < 0.01, 1 day of training versus no training; **P < 0.001, 3 days training versus no training and 1 day of training. C, increases in muscle lactate concentration. *P < 0.01, 1 and 3 days training versus no training. D, rates of lactate accumulation. *P < 0.01, 1 day training versus no training; **P < 0.001, 3 days training versus no training and 1 day of training. Values are means ± SEM for 14 to 20 muscles per group.
ATP, phosphocreatine (PC) and inorganic phosphate (Pi)
The decreases in PC and ATP and the increase in Pi concentrations induced by contractile activity were the same in the muscles of the rats that were trained for 1 or 3 days and the unexercised controls (Table1). This is in contrast to muscles that have adapted to longer periods of training, in which there is a smaller decrease in high energy phosphates and a smaller increase in Pi in response to the same contractile activity (Constable et al. 1987) or exercise stimulus (Phillips et al. 1996).
Table 1.
Muscle phosphocreatine (PC), ATP, and inorganic phosphate concentrations
| PC | ATP | Pi | ||||
|---|---|---|---|---|---|---|
| Rest | Stimulation | Rest | Stimulation | Rest | Stimulation | |
| Sedentary | 22.8 ± 0.9 | 14.8 ± 1.2 | 5.8 ± 0.16 | 5.43 ± 0.26 | 2.09 ± 0.12 | 19.2 ± 1.3 |
| Exercise, 1 day | 22.6 ± 0.7 | 14.0 ± 0.8 | 6.19 ± 0.19 | 5.67 ± 0.20 | 2.24 ± 0.13 | 20.2 ± 1.3 |
| Exercise, 3 days | 24.1 ± 0.8 | 15.8 ± 1.2 | 6.11 ± 0.19 | 5.67 ± 0.14 | 2.52 ± 0.17 | 19.7 ± 1.5 |
Lack of effect of one or three bouts of exercise training on the decreases in phosphocreatine (PC), ATP and inorganic phosphate (Pi) in muscles stimulated to contract for 3 min. Values are expressed as μmol g (muscle wet weight)−1 and are the means ± SEM for 12 to 14 muscles per group.
Three days of exercise training does not result in an increase in functional mitochondria
Our finding that 3 days of exercise training did not result in smaller decreases in CP and ATP or a smaller increase in Pi provides evidence that 3 days of exercise is not sufficient to bring about an increase in functional mitochondria. The absence of an increase in functional mitochondria is confirmed by the finding that there was no increase in the capacity of whole muscle homogenates to oxidize pyruvate. (Fig. 3A).
Figure 3. Effect of exercise on skeletal muscle mitochondria.
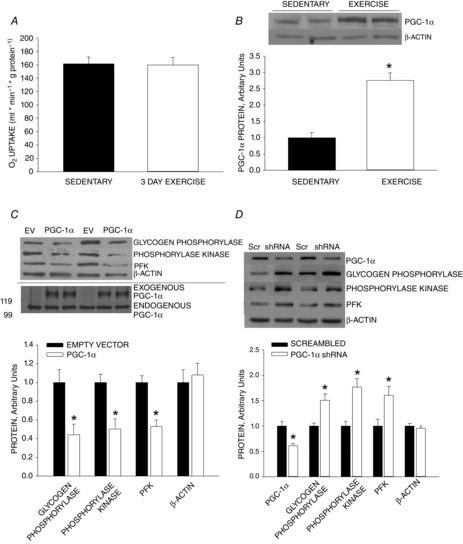
A, three daily bouts of exercise do not result in an increase in functional mitochondria in skeletal muscle as evidenced by no increase in the capacity for pyruvate oxidation. B, one bout of exercise results in an increase in PGC-1α expression measured ∼18 h after exercise. *P < 0.01, exercise versus sedentary. C, overexpression of PGC-1α in rat triceps muscle by means of electroporation resulted in downregulation of expression of phosphorylase, phosphorylase kinase and phosphofructokinase (PFK). D, knockdown of PGC-1α in C2C12 myotubes by transfection with PGC-1α shRNA results in increased expression of phosphorylase, phosphorylase kinase and phosphofructokinase. *P < 0.01, control versus empty vector (EV) or scrambled RNA (Scr). Values are means ± SEM for 6 rats per group.
Role of PGC-1α
As in previous studies (Baar et al. 2002; Wright et al. 2007), the swimming protocol resulted in an increase in PGC-1α protein expression measured in triceps muscle ∼18 h following exercise (Fig. 3B). Overexpression of PGC-1α in triceps muscles by electroporation resulted in ∼50% decreases in expression of glycogen phosphorylase, phosphorylase kinase, and phosphofructokinase proteins in rat triceps muscle (Fig.3C), showing that short-term overexpression of PGC-1α downregulates glycogenolytic and glycolytic enzymes. Knockdown of PGC-1α had the opposite effect, resulting in increased expression of phosphorylase, phosphorylase kinase and phosphfructokinase (PFK) (Fig. 3D). These findings provide evidence that the downregulation of expression of glycogenolytic and glycolytic enzymes in muscle in response to exercise is mediated by PGC-1α.
Discussion
The results of this study show that a single long bout of exercise induces adaptive decreases in the expression of glycogen phosphorylase, phosphorylase kinase and glycolytic enzymes, including phosphofructokinase, glyceraldehyde phosphate dehydrogenase, and lactate dehydrogenase, in skeletal muscle. Two more exercise bouts over the next 2 days resulted in a further decrease in the expression of these enzymes. Associated with the decreases in these glycogenolytic and glycolytic enzymes were significant reductions in the rates of glycogen depletion and lactic acid accumulation in muscles stimulated to contract in situ. These findings have biological significance, because muscle glycogen depletion is frequently the major cause of fatigue/exhaustion that forces cessation of prolonged vigorous exercise (Ahlborg et al. 1967; Hermansen et al. 1967; Baldwin et al. 1973a), while lactic acid accumulation in muscle is a major cause of fatigue during brief intense exercise (Hermansen, 1981; Knuth et al. 2006; Fitts, 2008).
Long-term endurance exercise training results in a reduction in the rates of muscle glycogen depletion and lactic acid accumulation during exercise at a given work rate (Hermansen et al. 1967; Karlsson et al. 1972, 1974; Baldwin et al. 1973a; Fitts et al. 1975). The glycogen sparing effect of endurance exercise training is mediated by an increase in muscle mitochondria that results in smaller decreases in ATP and phosphocreatine and smaller increases in inorganic phosphate (Pi), ADP and AMP in the working muscles in the trained than in the untrained state (Favier et al. 1986; Constable et al. 1987; Phillips et al. 1996; Holloszy, 2011). Pi is rate-limiting for conversion of glycogen to glucose-1-P and plays a key role in regulating the rate of glycogenolysis (Chasiotis, 1983; Ren et al. 1992; Holloszy, 2011). The lower concentration of Pi attained in endurance trained than in untrained muscle at the same work rate (Favier et al. 1986; Constable et al. 1987; Phillips et al. 1996) appears to mediate the slowing of glycogenolysis and lactate production (Holloszy, 2011).
It was thought that the endurance exercise-induced increase in muscle mitochondria develops gradually in response to weeks of vigorous training (Holloszy, 1967; Booth & Holloszy, 1977). After the discovery of PGC-1α and its regulatory role in mitochondrial biogenesis (Wu et al. 1999; Kelly & Scarpulla, 2004; Handschin & Spiegelman, 2006), it was found that a single bout of exercise results in an almost immediate activation of PGC-1α, followed within a few hours by increases in the expression of PGC-1α and many of the mitochondrial enzymes involved in substrate oxidation and in oxidative phosphorylation (Baar et al. 2002; Wright et al. 2007). However, a number of key enzymes, including citrate synthase, α-ketoglutarate dehydrogenase, and cytochrome c have long half-lives of ∼7 days, so their protein expression increases more slowly (Booth & Holloszy, 1977). Furthermore, after they are synthesized, the mitochondrial proteins, along with various lipids, have to be assembled to form new mitochondria or integrated into existing mitochondria. As a consequence it takes more than 3 days before there is an increase in functional muscle mitochondria as evidenced by an increase in the capacity for substrate oxidation. Thereafter, substrate oxidative capacity increases for 3–4 weeks if exercise intensity and duration are held constant (Booth & Holloszy, 1977; Hickson et al. 1981), or longer if training intensity and duration are progressively increased.
Adaptations that occur over a period of weeks, such as the increases in muscle mitochondria and physiological cardiac hypertrophy that develop in response to endurance exercise, provide major survival advantages by preparing individuals for future challenges. However, an adaptation that takes more than 3 days before it starts to improve functional capacity does not have survival value when there is a sudden change in environment that necessitates prolonged increases in physical activity. Reports that 3 to 5 days of daily endurance exercise training result in a slowing of glycogenolysis and muscle lactic acid accumulation (Green et al. 1992; Phillips et al. 1996) suggested the possibility that, in addition to the increase in mitochondria, endurance exercise might elicit another, more rapid, adaptation that enhances the ability to perform prolonged exercise. Our findings show that this additional adaptation is a downregulation of the expression of glycogenolytic and glycolytic enzymes, and provide evidence that this adaptation is also mediated by PGC-1α.
That the decrease in expression of glycogenolytic and glycolytic enzymes in response to exercise is mediated by the increase in PGC-1α induced by a bout of exercise is supported by our findings that the overexpression of PGC-1α in muscle by electroporation mimics the exercise-induced decrease in expression of glycogenolytic and glycolytic enzymes, while knockdown of PGC-1α results in increased expression of glycogenolytic and glycolytic enzymes. Surprisingly, the decrease in expression of glycogenolytic and glycolytic enzymes is, to a large extent, reversed in rats that have adapted to long-term endurance exercise and are highly trained (Baldwin et al. 1973b).
PGC-1α is a transcription coactivator, so it seems probable that it mediates downregulation of glycogenolytic and glycolytic enzymes by an indirect mechanism such as activation of a transcription factor that upregulates expression of transcriptional repressors of the genes encoding these enzymes. Although the mechanism remains unclear, it is well documented that an increase in PGC-1α also results in repression of expression of a number of other proteins including myosin heavy chain IIb (Mortensen et al. 2006), various inflammatory cytokines (Eisele et al. 2013), atrogin-1, MuRF-1 and cathepsin L (Sandri et al. 2006).
In conclusion, this study provides the new information that endurance exercise induces a rapid decrease in expression of the enzymes of the glycogenolytic–glycolytic pathway and that this adaptation results in slowing of glycogen breakdown and lactic acid accumulation during muscle contractile activity. This adaptation, which appears to be mediated by PGC-1α, is already biologically significant the day following a single exercise bout.
Acknowledgments
We thank Mrs Victoria Reckamp for assistance in preparation of the manuscript and Mrs Robin Fitzgerald for technical assistant.
Glossary
Abbreviations
- ECL
enhanced chemiluminescence
- EV
empty vector
- GAPDH
glyceraldehyde phosphate dehydrogenase
- PC
phosphocreatine
- PFK
phosphofructokinase
- Pi
iinorganic phosphate
Additional information
Competing interests
There are no competing interests.
Author contributions
The experiments were performed at Washington University School of Medicine. D.H.H. and J.O.H. designed the study. S.H.K., J.H.K. and K.H. contributed to the design of experiments and performed experiments. S.R.J. performed experiments. D.H.H., S.H.K. and J.H.K. analysed and interpreted the data. J.O.H. interpreted data and wrote the paper. All authors approved the final version of the manuscript, and all those who qualify for authorship are listed.
Funding
The research was supported by National Institute of Aging grant AG000425 and NIDDK grant P30DK056341.
References
- Ahlborg B, Bergström J, Ekelund LG. Hultman E. Muscle glycogen and muscle electrolytes during prolonged physical exercise. Acta Physiol Scand. 1967;70:129–142. [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP. Holloszy JO. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Reitman JS, Terjung RL, Winder WW. Holloszy JO. Substrate depletion in different types of muscle and in liver during prolonged running. Am J Physiol. 1973a;225:1045–1050. doi: 10.1152/ajplegacy.1973.225.5.1045. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Winder WW, Terjung RL. Holloszy JO. Glycolytic enzyme activities in red, white and intermediate skeletal muscle: Adaptive response to exercise. Am J Physiol. 1973b;225:962–966. doi: 10.1152/ajplegacy.1973.225.4.962. [DOI] [PubMed] [Google Scholar]
- Booth FW. Holloszy JO. Cytochrome c turnover in rat skeletal muscles. J Biol Chem. 1977;252:416–419. [PubMed] [Google Scholar]
- Chasiotis D. The regulation of glycogen phosphorylase and glycogen breakdown in human skeletal muscle. Acta Physiol Scand Suppl. 1983;518:1–68. [PubMed] [Google Scholar]
- Constable SH, Favier RJ, McLane JA, Fell RD, Chen M. Holloszy JO. Energy metabolism in contracting rat skeletal muscle: Adaptation to exercise-training. Am J Physiol Cell Physiol. 1987;253:C316–C322. doi: 10.1152/ajpcell.1987.253.2.C316. [DOI] [PubMed] [Google Scholar]
- Eisele PS, Salatino S, Sobek J, Hottiger MO. Handschin C. The peroxisome proliferator-activated receptor γ coactivator 1α/β (PGC-1) coactivators repress the transcriptional activity of NF-KB in skeletal muscle cells. J Biol Chem. 2013;288:2246–2260. doi: 10.1074/jbc.M112.375253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier RJ, Constable SH, Chen M. Holloszy JO. Endurance exercise-training reduces lactate production. J Appl Physiol. 1986;61:885–889. doi: 10.1152/jappl.1986.61.3.885. [DOI] [PubMed] [Google Scholar]
- Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Booth FW, Winder WW. Holloszy JO. Skeletal muscle respiratory capacity, endurance and glycogen utilization. Am J Physiol. 1975;228:1029–1033. doi: 10.1152/ajplegacy.1975.228.4.1029. [DOI] [PubMed] [Google Scholar]
- Green HJ, Helyar R, Ball-Burnett M, Kowalchuk N, Symon S. Farrance B. Metabolic adaptations to training precede changes in muscle mitochondrial capacity. J Appl Physiol. 1992;72:484–491. doi: 10.1152/jappl.1992.72.2.484. [DOI] [PubMed] [Google Scholar]
- Gutman I. Wahlefeld AW. L-(+)-lactate determination with lactate dehydrogenase and NAD. In: Bergmeyer HU, editor; Methods of Enzymatic Analysis. New York: Academic; 1974. pp. 1464–1468. [Google Scholar]
- Guynn RW, Veloso D. Veech RL. Enzymatic determination of inorganic phosphate in the presence of creatine phosphate. Anal Biochem. 1972;45:277–285. doi: 10.1016/0003-2697(72)90028-0. [DOI] [PubMed] [Google Scholar]
- Hancock CR, Han D-H, Higashida K, Kim SH. Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. 2011;25:785–791. doi: 10.1096/fj.10-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C. Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Hermansen L. Effect of metabolic changes on force generation in skeletal muscle during maximal exercise. Ciba Found Symp. 1981;82:75–88. doi: 10.1002/9780470715420.ch5. [DOI] [PubMed] [Google Scholar]
- Hermansen L, Hultman E. Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967;71:129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- Hickson RC, Hagberg JM, Ehsani AA. Holloszy JO. Time-course of the adaptive responses of aerobic power and heart rate to training. Med Sci Sports Exerc. 1981;13:17–20. [PubMed] [Google Scholar]
- Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO. Han D-H. Effects of resveratrol and SIRT1 on PGC-11537; activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial O2 uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Holloszy JO. Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol. 2011;1:921–940. doi: 10.1002/cphy.c100052. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Nordsejo L-O, Jorfeldt L. Saltin B. Muscle lactate, ATP, and CP levels during exercise after physical training in man. J Appl Physiol. 1972;33:199–203. doi: 10.1152/jappl.1972.33.2.199. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Nordesjo L-O. Saltin B. Muscle glycogen utilization during exercise after physical training. Acta Physiol Scand. 1974;90:210–217. doi: 10.1111/j.1748-1716.1974.tb05579.x. [DOI] [PubMed] [Google Scholar]
- Kelly DP. Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- Keppler D. Decker K. Glycogen. Determination with amyloglucosidase. In: Bergmeyer HU, editor; Methods of Enzymatic Analysis. New York: Academic; 1974. pp. 1127–1131. [Google Scholar]
- Knuth ST, Dave H, Peters JR. Fitts RH. Low cell pH depresses peak power in rat skeletal muscle fibres at both 30°C and 15°C: implications for muscle fatigue. J Physiol. 2006;575:887–899. doi: 10.1113/jphysiol.2006.106732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht W, Stein P, Heinz F. Weisser H. Creatine phosphate. In: Bergmeyer HU, editor; Methods of Enzymatic Analysis. New York: Academic; 1974. pp. 1777–1781. [Google Scholar]
- Lamprecht W. Trautschold I. ATP-determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor; Methods of Enzymatic Analysis. New York: Academic; 1974. pp. 2101–2110. [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL. Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mortensen OH, Frandsen L, Schjerling P, Nishimura E. Grunnet N. PGC-1α and PGC-11538; have both similar and distinct effects on myofiber switching toward an oxidative phenotype. Am J Physiol Endocrinol Metab. 2006;291:E807–E816. doi: 10.1152/ajpendo.00591.2005. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJF. Grant SM. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol:Endocrin Metab. 1996;270:E265–E272. doi: 10.1152/ajpendo.1996.270.2.E265. [DOI] [PubMed] [Google Scholar]
- Ploug T, Stallknecht BM, Pedersen O, Kahn BB, Ohkuwa T, Vinten J. Galbo H. Effect of endurance-training on glucose transport capacity and glucose transporter expression in rat skeletal muscle. Am J Physiol. 1990;259:E778–E786. doi: 10.1152/ajpendo.1990.259.6.E778. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M. Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Ren JM, Gulve EA, Cartee GD. Holloszy JO. Hypoxia causes glycogenolysis without an increase in percent phosphorylase a in rat skeletal muscle. Am J Physiol Endocrinol Metab. 1992;263:E1086–E1091. doi: 10.1152/ajpendo.2006.263.6.E1086. [DOI] [PubMed] [Google Scholar]
- Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL. Spiegelman BM. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T. Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002;296:350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- Winder WW, Baldwin KM, Terjung RL. Holloszy JO. Effects of thyroid hormone administration on skeletal muscle mitochondria. Am J Physiol. 1975;228:1341–1345. doi: 10.1152/ajplegacy.1975.228.5.1341. [DOI] [PubMed] [Google Scholar]
- Wright DC, Han D-H, Garcia-Roves PM, Geiger PC, Jones TE. Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC. Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]


