Abstract
The reflex fibular muscle sympathetic nerve (MSNA) response to dynamic handgrip exercise is elicited at a lower threshold in heart failure with reduced ejection fraction (HFrEF). The present aim was to test the hypothesis that the contralateral MSNA response to mild to moderate dynamic one-legged exercise is augmented in HFrEF relative to age- and sex-matched controls. Heart rate (HR), blood pressure and MSNA were recorded in 16 patients with HFrEF (left ventricular ejection fraction = 31 ± 2%; age 62 ± 3 years, mean ± SE) and 13 healthy control subjects (56 ± 2 years) before and during 2 min of upright one-legged unloaded cycling followed by 2 min at 50% of peak oxygen uptake (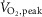 ). Resting HR and blood pressure were similar between groups whereas MSNA burst frequency was higher (50.0 ± 2.0 vs. 42.3 ± 2.7 bursts min−1, P = 0.03) and
). Resting HR and blood pressure were similar between groups whereas MSNA burst frequency was higher (50.0 ± 2.0 vs. 42.3 ± 2.7 bursts min−1, P = 0.03) and 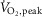 lower (18.0 ± 2.0 vs. 32.6 ± 2.8 ml kg−1 min−1, P < 0.001) in HFrEF. Exercise increased HR (P < 0.001) with no group difference (P = 0.1). MSNA burst frequency decreased during mild to moderate dynamic exercise in the healthy controls but increased in HFrEF (−5.5 ± 2.0 vs. 6.9 ± 1.8 bursts min−1, P < 0.001). Exercise capacity correlated inversely with MSNA burst frequency at 50%
lower (18.0 ± 2.0 vs. 32.6 ± 2.8 ml kg−1 min−1, P < 0.001) in HFrEF. Exercise increased HR (P < 0.001) with no group difference (P = 0.1). MSNA burst frequency decreased during mild to moderate dynamic exercise in the healthy controls but increased in HFrEF (−5.5 ± 2.0 vs. 6.9 ± 1.8 bursts min−1, P < 0.001). Exercise capacity correlated inversely with MSNA burst frequency at 50% 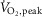 (n = 29; r = −0.64; P < 0.001). At the same relative workload, one-legged dynamic exercise elicited a fall in MSNA burst frequency in healthy subjects but sympathoexcitation in HFrEF, a divergence probably reflecting between-group differences in reflexes engaged by cycling. This finding, coupled with an inverse relationship between MSNA burst frequency during loaded cycling and subjects’
(n = 29; r = −0.64; P < 0.001). At the same relative workload, one-legged dynamic exercise elicited a fall in MSNA burst frequency in healthy subjects but sympathoexcitation in HFrEF, a divergence probably reflecting between-group differences in reflexes engaged by cycling. This finding, coupled with an inverse relationship between MSNA burst frequency during loaded cycling and subjects’ 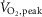 , is consistent with a neurogenic determinant of exercise capacity in HFrEF.
, is consistent with a neurogenic determinant of exercise capacity in HFrEF.
Key points
People with diminished ventricular contraction who develop heart failure have higher sympathetic nerve firing rates at rest compared with healthy individuals of a similar age and this is associated with less exercise capacity.
During handgrip exercise, sympathetic nerve activity to muscle is higher in patients with heart failure but the response to leg exercise is unknown because its recording requires stillness.
We measured sympathetic activity from one leg while the other leg cycled at a moderate level and observed a decrease in nerve firing rate in healthy subjects but an increase in subjects with heart failure.
Because these nerves release noradrenaline, which can restrict muscle blood flow, this observation helps explain the limited exercise capacity of patients with heart failure.
Lower nerve traffic during exercise was associated with greater peak oxygen uptake, suggesting that if exercise training attenuated sympathetic outflow functional capacity in heart failure would improve.
Introduction
Two important markers of heart failure severity with adverse implications for prognosis are increased resting muscle sympathetic nerve activity (MSNA) and decreased peak oxygen uptake (Stelken et al. 1996; Barretto et al. 2009; Floras, 2009). In previous investigations involving patients with heart failure with reduced ejection fraction (HFrEF), we detected a significant inverse relationship between peak oxygen uptake during exercise and resting efferent MSNA (but not cardiac noradrenaline spillover) (Notarius et al. 1999, 2002), signifying the potential for peripheral neurogenic vasoconstriction to limit such patients’ exercise capacity.
For practical reasons, handgrip has been the intervention applied conventionally to quantify, using fibular nerve recordings, the effects of exercise on MSNA (Sterns et al. 1991; Kingwell et al. 1995; Notarius et al. 2001a; Middlekauff et al. 2004; Soares-Miranda et al. 2011). When subjects perform static and dynamic handgrip at similar relative workloads, the latter elicits a reflex increase in MSNA burst frequency at a lower intensity in middle-aged patients with HFrEF than in age-matched controls, indicating a lesser threshold for this neurogenic response (Notarius et al. 2001a). However, handgrip is rarely a symptom-generating activity in HFrEF and the MSNA response to static handgrip is unrelated to peak 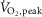 (Kuniyoshi et al. 2014). If fibular MSNA could be recorded during dynamic leg exercise, such data could yield more compelling evidence in favour of the hypothesis that peripheral sympathetic vasoconstriction can limit exercise capacity.
(Kuniyoshi et al. 2014). If fibular MSNA could be recorded during dynamic leg exercise, such data could yield more compelling evidence in favour of the hypothesis that peripheral sympathetic vasoconstriction can limit exercise capacity.
Ray et al. (1993) recorded MSNA in young healthy men during contralateral dynamic knee extension and reported a reduction in MSNA burst frequency at mild to moderate workloads. A similar decrease was observed when MSNA was recorded from the median nerve (i.e. in the arm) during mild intensity two-legged cycling at intensities up to 40% of peak oxygen uptake. At higher intensities there was a progressive rise in MSNA (Ichinose et al. 2008). Breathing a hypoxic gas mixture reduced the threshold for MSNA activation (Katayama et al. 2011), analogous to the lesser dynamic handgrip exercise workload required to elicit sympathetic activation in middle-aged patients with HFrEF as compared with healthy controls (Notarius et al. 2001a).
To test the hypothesis that, in contrast to reductions in controls, MSNA burst frequency would increase in patients with HFrEF during mild (unloaded) dynamic leg exercise cycling and diverge further from MSNA responses of control subjects during a moderate workload, we developed the capacity to acquire stable neurograms from the stationary contralateral leg during one-legged cycling. In our proof-of-concept analysis 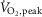 related inversely to MSNA recorded during moderate-intensity dynamic leg exercise (Notarius et al. 2014). The aim of the present study was to determine and compare, in middle-aged patients with HFrEF and age-matched control subjects, MSNA responses to mild and moderate intensity dynamic one-legged cycling exercise.
related inversely to MSNA recorded during moderate-intensity dynamic leg exercise (Notarius et al. 2014). The aim of the present study was to determine and compare, in middle-aged patients with HFrEF and age-matched control subjects, MSNA responses to mild and moderate intensity dynamic one-legged cycling exercise.
Methods
Subjects
We recruited 16 stable patients with HFrEF [four women; mean age 62 ± 3 SE (range 42–79) years], all in sinus rhythm, who were referred to the Toronto Rehabilitation Institute Cardiovascular Prevention and Rehabilitation Program. Their aetiology was either ischaemic (n = 12) or non-ischaemic dilated (n = 4) cardiomyopathy. Only one had an implanted cardiac device, a defibrillator, but his rhythm was never paced. Their mean left ventricular ejection fraction was 31 ± 2% (range 19–40). None had participated previously in an exercise-training programme. Diabetic patients were excluded because of their potential for autonomic neuropathy. To assure the clinical generalizability of any findings, and to avoid any adverse rebound effect of drug withdrawal, participants were maintained on their prescribed heart failure therapy: angiotensin-converting-enzyme inhibitors (n = 10; 62%); beta-adrenoceptor antagonists (n = 16; 100%); diuretics (n = 12; 75%); aspirin (n = 14; 88%); and anticoagulants (n = 7; 44%). Of those prescribed diuretics, four were receiving a loop diuretic, four a mineralocorticoid receptor antagonist and four the combination of both.
Thirteen healthy, age-matched, medication-free volunteers (three woman) were recruited through local advertisement and screened by medical history to serve as control subjects. Their mean age was 56 ± 2 (range 48–69) years. All participants abstained from caffeine for 12 h before the study. An exploratory multiple regression analysis comprising individual data from a subgroup of this cohort has been the subject of recent correspondence (Notarius et al. 2014).
Procedures and protocol
This study represents one element of a larger protocol that was approved by our Institution’s Research Ethics Board. Informed written consent was obtained from all subjects. Subjects were studied in a quiet temperature-controlled laboratory 2 h following the last food intake, on two separate days at least 1 week apart.
On the first study day, 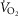 was assessed on a cycle ergometer during a 15 W min–1 ramped protocol to peak effort and expressed as a percentage of that predicted based on age, sex and weight (Jones et al. 1985). On the second study day, subjects were studied seated in a comfortable chair with the left leg supported on a stool while the right leg was secured to the pedal of a cycle ergometer placed on the floor (Monark Rehab Ergometer Trainer 881, Monark Exercise AB, Vansbro, Sweden). Blood pressure was monitored from the right arm manually every minute by sphygmomanometer (Dinamap Pro 100, Critikon, Tampa, FL, USA). Heart rate was derived from lead II of an electrocardiogram and a respiratory belt encircled the abdomen. After 10 min of quiet rest, baseline signals were acquired during 7 min of spontaneous breathing. We recorded MSNA by microneurography (left fibular nerve) at rest and during one-legged cycling (right leg) for 4 min (2 min at zero load and 2 min at 50%
was assessed on a cycle ergometer during a 15 W min–1 ramped protocol to peak effort and expressed as a percentage of that predicted based on age, sex and weight (Jones et al. 1985). On the second study day, subjects were studied seated in a comfortable chair with the left leg supported on a stool while the right leg was secured to the pedal of a cycle ergometer placed on the floor (Monark Rehab Ergometer Trainer 881, Monark Exercise AB, Vansbro, Sweden). Blood pressure was monitored from the right arm manually every minute by sphygmomanometer (Dinamap Pro 100, Critikon, Tampa, FL, USA). Heart rate was derived from lead II of an electrocardiogram and a respiratory belt encircled the abdomen. After 10 min of quiet rest, baseline signals were acquired during 7 min of spontaneous breathing. We recorded MSNA by microneurography (left fibular nerve) at rest and during one-legged cycling (right leg) for 4 min (2 min at zero load and 2 min at 50% 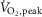 ). Heart rate, blood pressure and rating of perceived exertion (RPE; modified Borg scale 0–10) (Noble et al. 1983) also were assessed.
). Heart rate, blood pressure and rating of perceived exertion (RPE; modified Borg scale 0–10) (Noble et al. 1983) also were assessed.
Microneurography
Multiunit recordings of post-ganglionic MSNA were obtained with a unipolar tungsten electrode inserted selectively into a muscle–nerve fascicle of the left peroneal (fibular) nerve, posterior to the fibular head as previously described (Notarius et al. 1999). MSNA was expressed as burst frequency (bursts min–1) and burst incidence (bursts 100 heart beats–1), to allow for differences in heart rate between groups, and was computed by a customized analytic program using a LabVIEW® software platform (National Instruments, Austin, TX, USA). Because both the neural noradrenaline release rate and the resulting vasoconstriction are a direct function of burst frequency (not burst incidence, which is temporally independent, and therefore applied when evaluating efferent central sympathetic responses to reflexes that concurrently alter heart rate), the principal representation of MSNA with respect to its influence on exercise capacity in the present study was as bursts min–1 (Wallin et al. 1992).
Statistical analysis
Data are presented as mean ± SE. Unpaired t tests or Mann–Whitney rank sum tests (if the data did not follow a normal Gaussian distribution) were performed to assess differences between group means for dependent variables. A comparison of the absolute change from baseline in dependent variables during the second minute of unloaded and loaded dynamic one-legged cycling between the heart failure and healthy subject groups was determined by a two-factor repeated measures analysis of variance (ANOVA) (SigmaStat™ for Windows, Ver. 3.5; Systat Software Inc., Chicago, IL, USA) with group (HFrEF and control subjects) and exercise intensity (zero load, 50% of 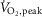 cycling) as the two factors. A post-hoc Student–Newman–Keuls test was applied to assess individual differences between means. Linear regression was used to assess the relationship between
cycling) as the two factors. A post-hoc Student–Newman–Keuls test was applied to assess individual differences between means. Linear regression was used to assess the relationship between 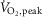 and MSNA during exercise in the entire study cohort.
and MSNA during exercise in the entire study cohort.
Results
Physical characteristics and baseline measures
Data are summarized in Table1. Heart failure and healthy control groups were similar with respect to age, height, weight, resting heart rate, and both systolic and diastolic blood pressure. MSNA burst frequency and burst incidence at rest were significantly higher in the HFrEF group (P = 0.03 and 0.005 respectively). 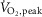 , whether expressed in absolute, relative to body mass or normalized as the percentage of predicted
, whether expressed in absolute, relative to body mass or normalized as the percentage of predicted 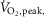 was lower in patients with HFrEF (all P < 0.001).
was lower in patients with HFrEF (all P < 0.001).
Table 1.
Physical characteristics and baseline data
| Healthy subjects | Heart failure | ||
|---|---|---|---|
| Variable | n = 13 | n = 16 | P value |
| Age (years) | 55.6 ± 1.9 | 62.2 ± 2.6 | 0.06 |
| Height (cm) | 174.3 ± 3.0 | 167.6 ± 2.0 | 0.08 |
| Body weight (kg) | 77.2 ± 3.4 | 75.6 ± 2.8 | 0.71 |
| Heart rate (beats min−1) | 64.6 ± 2.8 | 62.0 ± 2.0 | 0.44 |
| Systolic blood pressure (mmHg) | 110.0 ± 4.7 | 112.5 ± 3.9 | 0.68 |
| Diastolic blood pressure | 66.8 ± 2.5 | 66.7 ± 1.8 | 0.95 |
| MSNA (bursts min−1) | 42.3 ± 2.7 | 50.0 ± 2.0 | 0.03 |
| MSNA (bursts/100 heart beat) | 66.2 ± 4.1 | 82.1 ± 3.4 | 0.005 |
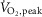 (l min−1) (l min−1) |
2.6 ± 0.2 | 1.40 ± 0.1 | <0.001 |
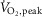 (ml kg−1 min−1) (ml kg−1 min−1) |
32.6 ± 2.8 | 18.0 ± 2.0 | <0.001 |
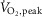 (% predicted) (% predicted) |
116.8 ± 7.6 | 69.0 ± 6.4 | <0.001 |
Mean ± SE. MSNA, muscle sympathetic nerve activity; 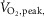 peak oxygen uptake.
peak oxygen uptake.
One-legged cycling
Representative multiunit microneurographic MSNA recordings from a healthy control subject at rest, and a patient with HFrEF acquired at rest, during mild (zero load) and during moderate intensity (50% of 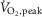 ) one-legged cycling are shown in Fig. 1. There was no significant difference between groups in the mean RPE as assessed by the modified Borg scale (0–10) at either work rate (HFrEF vs. controls at zero load cycling: 1.8 ± 0.2 vs. 1.8 ± 0.3; at 50%
) one-legged cycling are shown in Fig. 1. There was no significant difference between groups in the mean RPE as assessed by the modified Borg scale (0–10) at either work rate (HFrEF vs. controls at zero load cycling: 1.8 ± 0.2 vs. 1.8 ± 0.3; at 50% 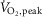 cycling: 4.0 ± 0.4 vs. 3.8 ± 0.5). Mean heart rate increased during both the mild and moderate cycling work rates (P < 0.001) with no difference in absolute change from baseline between groups (P = 0.10) (Fig. 2A).
cycling: 4.0 ± 0.4 vs. 3.8 ± 0.5). Mean heart rate increased during both the mild and moderate cycling work rates (P < 0.001) with no difference in absolute change from baseline between groups (P = 0.10) (Fig. 2A).
Figure 1. Example of individual multifibre muscle sympathetic nerve activity recordings with burst frequency count in a representative patient with heart failure and age-matched healthy control subject at rest, during the second minute of mild (zero load) cycling and moderate cyclingy (50% 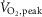 ).
).
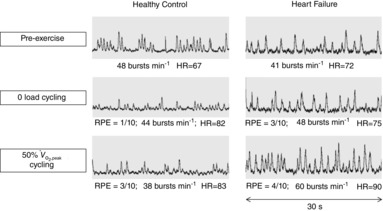
HR, heart rate; RPE, rating of perceived exertion (0–10 Borg scale).
Figure 2. Divergent MSNA burst frequency and incidence responses to dynamic exercise in patients with HFrEF and control subjects.
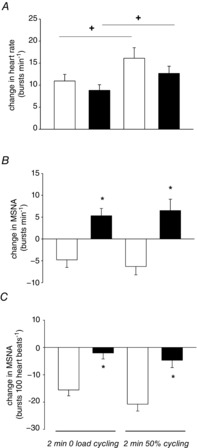
A, mean change in heart rate from baseline during mild and moderate dynamic leg exercise. Heart rate increased significantly in response to increasing exercise intensity during the second minute of one-legged cycling in both healthy controls (white bars) and subjects with heart failure (black bars) (P < 0.003 for 50% vs. zero load cycling) with no between-group difference (P = 0.10). B, mean change in MSNA burst frequency (bursts min–1) from baseline during mild and moderate dynamic leg exercise. MSNA decreased significantly during both exercise intensities in healthy control subjects (white bars) but increased significantly during both exercise intensities in patients with heart failure (black bars) (*P < 0.001 HFrEF vs. control). There was no significant difference in MSNA between exercise intensities within groups (P = 0.78). C, mean change in MSNA burst incidence (bursts 100 heart beats–1) from baseline during mild and moderate dynamic leg exercise. MSNA burst incidence decreased significantly during both exercise intensities in healthy controls (white bars) but not in patients with heart failure (black bars) (*P < 0.001 HFrEF vs. control at each intensity). This divergence became more pronounced as exercise intensity increased (P = 0.01) with no between-group interaction. HFrEF, heart failure with reduced ejection fraction; MSNA, muscle sympathetic nerve activity.
Mean MSNA burst frequency (as proxy for neural noradrenaline release rate), increased from baseline during exercise in the HFrEF group. In contrast, MSNA decreased in the healthy controls (main effect of group, P < 0.001), and was independent of exercise intensity (no main effect, P = 0.78) (Fig. 2B). The reflex response with respect to MSNA burst incidence was a significant drop during exercise only in control subjects (main effect of group, P < 0.001). This divergence was more pronounced at moderate exercise intensity (significant main effect, P = 0.01) (Fig. 2C).
Considering the cohort as a whole, approximately 41% of the variance in subjects’ 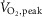 (expressed as percentage of predicted) could be predicted by MSNA burst frequency during the second minute of cycling at 50% of
(expressed as percentage of predicted) could be predicted by MSNA burst frequency during the second minute of cycling at 50% of 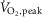 (r = −0.64, P < 0.001) (Fig. 3). Such relationships also were significant when MSNA was expressed as its change from baseline (r = −0.55, P = 0.002) or as absolute burst incidence (r = −0.50, P = 0.006).
(r = −0.64, P < 0.001) (Fig. 3). Such relationships also were significant when MSNA was expressed as its change from baseline (r = −0.55, P = 0.002) or as absolute burst incidence (r = −0.50, P = 0.006).
Figure 3. Inverse relationship (n = 29; r = −0.64; P < 0.001) between MSNA burst frequency and peak oxygen uptake (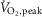 ) during the second minute of one-legged cycling performed at 50%
) during the second minute of one-legged cycling performed at 50% 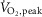 .
.
Controls (n = 13) are presented as open circles and subjects with heart failure (n = 16) as filled circles. MSNA, muscle sympathetic nerve activity.
Discussion
This study represents the first report of MSNA responses in the leg during mild and moderate dynamic one-legged cycling exercise in either heart failure or in middle-aged healthy subjects. The principal novel finding was the increase in MSNA burst frequency elicited by exercise in patients with HFrEF, in contrast to the reduction documented in the age-matched healthy control subjects. A neurogenic limit to exercise tolerance in patients with HFrEF may be the most important functional consequence of such sympathoexcitation (Notarius et al. 1999). Indeed, approximately 41% of the variance in these middle-aged subjects’ peak exercise capacity could be attributed to their MSNA response to moderate one-legged cycling.
Studies involving electrically induced static hindlimb muscle contraction have demonstrated, in rat models of dilated cardiomyopathy, increased discharge relative to control groups from mechanically sensitive type III afferents (Wang et al. 2010) and an augmented blood pressure and heart rate response (Smith et al. 2005).
In humans, previous studies examining directly efferent sympathetic traffic to muscle during dynamic leg exercise have recruited exclusively young healthy men in whom MSNA was recorded either from the median or radial nerve of the arm during leg cycling (Saito et al. 1993; Callister et al. 1994; Ichinose et al. 2008; Katayama et al. 2011) or from the tibial or fibular nerve of the contralateral leg during one-legged knee extension (Saito ´ Mano, 1991; Ray et al. 1993). In contrast to one-legged static leg exercise, tibial nerve MSNA decreased during dynamic one-legged leg cycling at mild to moderate intensities (Saito ´ Mano, 1991), as did median nerve MSNA during two-legged cycling at work rates less than 40%  . In one study, radial nerve MSNA was inhibited both immediately before and upon the initiation of cycling (Callister et al. 1994). At workloads greater than 60%
. In one study, radial nerve MSNA was inhibited both immediately before and upon the initiation of cycling (Callister et al. 1994). At workloads greater than 60%  , both median and radial nerve MSNA increased above baseline and continued to increase progressively up to peak exercise (Saito et al. 1993; Callister et al. 1994; Ichinose et al. 2008). Similarly, a drop in fibular MSNA in the contralateral leg during upright dynamic leg extension occurred during mild and moderate intensities and was associated with increased central venous pressure (Ray et al. 1993). The present findings confirm and extend these observations to middle-aged healthy men and women, in whom mean sympathetic outflow to skeletal muscle also fell during dynamic leg exercise.
, both median and radial nerve MSNA increased above baseline and continued to increase progressively up to peak exercise (Saito et al. 1993; Callister et al. 1994; Ichinose et al. 2008). Similarly, a drop in fibular MSNA in the contralateral leg during upright dynamic leg extension occurred during mild and moderate intensities and was associated with increased central venous pressure (Ray et al. 1993). The present findings confirm and extend these observations to middle-aged healthy men and women, in whom mean sympathetic outflow to skeletal muscle also fell during dynamic leg exercise.
Initial evaluations of sympathetic responses to dynamic leg exercise activity in HFrEF utilized the more indirect and global indices of plasma noradrenaline and noradrenaline spillover. A small (six patients) study involving supine cycling reported similar increases in noradrenaline release into plasma for the same relative work rate, but considerable variance in subject responses (Hasking et al. 1988). Other investigators have proposed a causal relationship in HFrEF between augmented plasma noradrenaline responses to exercise and concurrent reductions in leg blood flow, as these could be reversed by the central sympatholytic agent, clonidine (Lang et al. 1997). When noradrenaline spillover at peak exercise was measured during both bilateral dynamic leg cycling and one-legged dynamic leg extension, this was higher in patients with HFrEF than in age-matched controls (Esposito et al. 2010). The arterial noradrenaline concentration in HFrEF was highest during peak one-legged exercise, which utilizes a relatively lower muscle mass and consequently peak oxygen uptake, compared with two-legged exercise. These several findings with respect to noradrenaline kinetics are concordant with our previous microneurographic observations established at rest (Notarius et al. 1999) and during moderate one-legged cycling exercise (Notarius et al. 2014).
Owing to technical challenges, comparisons to date of MSNA responses to dynamic exercise between subjects with HFrEF and age-matched controls have been confined to the handgrip model (Silber et al. 1998; Notarius et al. 2001a; Kuniyoshi et al. 2014). We previously reported that, compared to age-matched control subjects, the elevated resting MSNA burst frequency of supine patients with HFrEF was stimulated by a lower dynamic handgrip workload, was greater in magnitude, and was sustained during post-handgrip ischaemia (Notarius et al. 2001a), observations indicating augmentation, in heart failure, of reflexes arising from skeletal muscle. Importantly, the MSNA response was greatest in patients whose peak oxygen uptake was less than 56% of predicted by their age, sex and weight (Notarius et al. 2001a).
In the present study, MSNA burst frequency increased in HFrEF during mild to moderate dynamic cycling exercise but decreased in age-matched controls. This divergence was observed even though all subjects were exercising at the same relative exercise intensities as evidenced by the nearly identical mean RPE values and by comparable graded heart rate responses during exercise.
Net MSNA responses elicited by exercise represent the interaction and integration of several autonomic reflexes, with some eliciting qualitatively similar efferent effects, while others have directionally opposite responses (Floras, 2009). For example, in healthy young subjects, activation of the sympathoinhibitory cardiopulmonary baroreflex (Saito et al. 1993; Ichinose et al. 2008), triggered by acute elevations in central venous pressure during exercise induced by the muscle pump effect on venous return, has been proposed to explain the drop in MSNA at lower work rates, a finding that was reversed during supine posture (Ray et al. 1993). Opposing this are sympathoexcitatory reflexes, which include: the muscle metaboreflex triggered by the accumulation of metabolites released by contracting muscle, which stimulate type III and IV muscle afferents (Mitchell et al. 1983); the muscle mechanoreflex, activated by the immediate mechanical effects of muscle contraction; and the arterial chemoreceptor reflex, which when evoked by hypoxia lowers the threshold for MSNA activation (Katayama et al. 2011). Of note, in many patients with HFrEF augmentation of these particular sympathoexcitatory reflexes elevates MSNA even at rest (Floras, 2009).
Previous work by others and ourselves leads us to suggest that the probable mechanism for divergent net MSNA responses to one-legged cycling observed in the present series is the development, in HFrEF, of quantitative or qualitative differences with respect to each reflex elicited (Floras, 2009). The sympathoexcitatory muscle metaboreflex response to the dynamic handgrip exercise has been demonstrated by us (Notarius et al. 2001a) and others (Piepoli et al. 1996; Silber et al. 1998) to be augmented in HFrEF [and abolished by pretreatment with caffeine, a non-specific blocker of adenosine receptors (Notarius et al. 2001b)]. Its functional consequence is increased peripheral vasoconstriction (Crisafulli et al. 2007). Others have argued that the muscle metaboreflex is diminished in HFrEF (Kon et al. 2004) and the muscle mechanoreflex, augmented (Middlekauff et al. 2004), perhaps due to between-laboratory differences with respect to the autonomic integrity of HFrEF and control populations studied, the posture at which handgrip was performed and the exercise protocols themselves (Notarius ´ Floras, 2007). However, augmentation of the metaboreflex in HFrEF is not the only possible explanation for the divergent MSNA burst frequency and burst incidence responses to one-legged cycling observed in the present experiments. With respect to cardiopulmonary reflexes, our group has established evidence, using both cardiac noradrenaline spillover and single fibre MSNA recordings, for the existence in humans with HFrEF of an additional, sympathoexcitatory, cardiopulmonary reflex, which is by contrast relatively quiescent in healthy subjects (Azevedo et al. 2000; Millar et al. 2013). Indeed, the relative proportion of efferent single MSNA units discharging in response to an acute increase in pre-load induced by non-hypertensive lower body positive pressure is increased significantly in HFrEF (Millar et al. 2014). It would be reasonable to anticipate that this sympathoexcitatory response would be evoked similarly by exercise-induced increases in atrial pressures. In healthy subjects, the arterial chemoreflex accounts for approximately 30% of the MSNA response during dynamic handgrip (Stickland ´ Miller, 2008). Exaggerated arterial chemoreceptor sensitivity, known to be present in a substantial proportion of patients with HFrEF (Depas et al. 2012), also would be anticipated to augment their MSNA response to dynamic leg exercise. The present study marks an important first step in characterizing a neurogenic limit to exercise tolerance in HFrEF. Elucidation of the specific contribution of each of these sympathoexcitatory reflexes to the net multi-unit MSNA response to exercise and its functional consequence with respect to leg blood flow represents an opportunity for future investigation.
In conclusion, we demonstrate for the first time that patients with heart failure due to reduced ejection fraction augment their already elevated resting sympathetic nerve traffic to muscle during mild and moderate dynamic leg cycling, in contrast to the decrease observed in age-matched healthy controls at similar relative work rates. In addition, peak exercise capacity related inversely to MSNA burst frequency elicited by mild to moderate exercise. Taken together, these findings are consistent with the concept of a neurogenic limit to exercise tolerance in medically treated patients with HFrEF. Importantly, such sympathetic activation is potentially modifiable by non-pharmacologic interventions such as exercise training.
Glossary
Abbreviations
- HFrEF
heart failure due to reduced ejection fraction
- HR
heart rate
- MSNA
muscle sympathetic nerve activity
- RPE
rating of perceived exertion
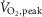
peak oxygen uptake
Additional information
Competing interests
None to disclose.
Author contributions
C.N. and J.S.F. were responsible for the conception and design of the experiment. C.N., S.M. and P.O. recruited subjects with HFrEF and S.M. and P.O. characterized each patient’s peak exercise capacity by performing graded exercise testing before the laboratory experiment day. C.N., P.J.M., H.M. and B.L.M. conducted the experiments with C.N. and P.J.M. performing the study analysis. C.N., P.J.M., H.M., B.L.M., S.M., P.O. and J.S.F. interpreted data. C.N. and J.S.F. drafted the manuscript. C.N., P.J.M., H.M., S.M., P.O. and J.S.F. edited and revised manuscript; C.N., P.J.M., H.M., B.L.M., S.M., P.O. and J.S.F. approved final version of the manuscript.
Funding
This study was supported by Grants-in-Aid from the Heart and Stroke Foundation of Ontario (T4938, NA6298). P.J.M. was supported by a Canadian Institutes of Health Research Postdoctoral Fellowship. H.M. was supported by a Bluma Appel International Fellowship of the Mount Sinai Hospital Department of Medicine, Toronto, and by the Heart and Stroke Foundation of Ontario (SPEC 6580). P.O. holds the GoodLife Fitness Chair in Cardiovascular Rehabilitation and Prevention. J.S.F. holds the Canada Research Chair in Integrative Cardiovascular Biology.
References
- Azevedo ER, Newton GE, Floras JS. Parker JD. Reducing cardiac filling pressure lowers norepinephrine spillover in patients with chronic heart failure. Circulation. 2000;101:2053–2059. doi: 10.1161/01.cir.101.17.2053. [DOI] [PubMed] [Google Scholar]
- Barretto ACP, Santos AC, Munhoz R, Rondon MUPB, Franco FG, Trombetta IC, Roveda F, De Matos LDNJ, Braga AMW, Middlekauf HR. Negrao CE. Increased muscle sympathetic nerve activity predicts morality in heart failure patients. Int J Cardiol. 2009;135:302–307. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- Callister R, Ng AV, Seals DR. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise in humans. J Appl Physiol. 1994;77:1402–1410. doi: 10.1152/jappl.1994.77.3.1403. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliara P. Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol. 2007;292:H2988–H2996. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- Depas F, Lambert E, Vaccaro A, Labrunee M, Franchitto N, Lebrun M, Galinier M, Senard JM, Lambert G, Esler M. Pathak A. Peripheral chemoreflex activation contributes to sympathetic baroreflex impairment in chronic heart failure. J Hypertens. 2012;30:753–760. doi: 10.1097/HJH.0b013e328350136c. [DOI] [PubMed] [Google Scholar]
- Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD. Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: Partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–1955. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–385. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Hasking GJ, Esler MD, Jennings GL, Dewar E. Lambert G. Norepinephrine spillover to plasma during steady-state supine bicycle exercise: comparison of patients with congestive heart failure and normal subjects. Circulation. 1988;78:516–521. doi: 10.1161/01.cir.78.3.516. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N. Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol. 2008;586:2753–2766. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NL, Makrides L, Hitchcock C, Chychar T. McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131:700–708. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- Katayama K, Ishida K, Iwamoto E, Iemitsu M, Koike T. Saito M. Hypoxia augments muscle sympathetic neural response to leg cycling. Am J Physiol Regul Integr Comp Physiol. 2011;301:R456–R464. doi: 10.1152/ajpregu.00119.2011. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Thompson JM, McPherson GA, Kaye DM, Jennings GL. Esler MD. Comparison of heart rate spectral analysis with cardiac noradrenaline spillover and muscle sympathetic nerve activity in human subjects. In: Zanchetti A, editor; Di Rienzo M, Mancia G, Parati G, Pedotti A, et al., editors. Computer Analysis of Cardiovascular Signals. Amsterdam: IOS Press; 1995. pp. 167–176. [Google Scholar]
- Kon H, Nakamura M, Arakawa N. Hiramori K. Muscle metaboreflex is blunted with reduced vascular resistance response of nonexercised limb in patients with chronic heart failure. J Cardiac Fail. 2004;10:503–510. doi: 10.1016/j.cardfail.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kuniyoshi RR, Matinelli M, Negrao CE, Siqueira SF, Rondon MU, Trombetta IC, Kuniyoshi FH, Laterza MC, Nishioka SA, Costa R, Tamaki WT, Crevelari ES, Peizoto GD, Ramires JA. Kalil R. Effects of cardiac resynchronization therapy on muscle sympathetic nerve activity. Pacing Clin Electrophysiol. 2014;37:11–18. doi: 10.1111/pace.12254. [DOI] [PubMed] [Google Scholar]
- Lang CC, Rayos GH, Chomsky DB, Wood AJJ. Wilson JR. Effect of sympathoinhibition on exercise performance in patients with heart failure. Circulation. 1997;96:238–245. doi: 10.1161/01.cir.96.1.238. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, MacLellan WR, Hage A, Moriguchi J. Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–H1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- Millar PJ, Murai H, Floras JS. Paradoxical muscle sympathetic reflex activation in human heart failure. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.010765. DOI: 10.1161/CIRCULATIONAHA.114.010765. [DOI] [PubMed] [Google Scholar]
- Millar PJ, Murai H, Morris BL. Floras JS. Microneurographic evidence in healthy middle-aged humans for a sympathoexcitatory reflex activated by atrial pressure. Am J Physiol Heart Circ Physiol. 2013;305:H931–H938. doi: 10.1152/ajpheart.00375.2013. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP. Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferents mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Noble BJ, Borg GAV, Jacobs I, Ruggaro C. Kaiser P. A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc. 1983;15:523–528. [PubMed] [Google Scholar]
- Notarius CF. Floras JS. Point:counterpoint: Increased mechanoreceptor/metaboreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol. 2007;102:824. doi: 10.1152/japplphysiol.01330.2006. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Ando S, Rongen GA. Floras JS. Resting muscle sympathetic nerve activity and peak oxygen uptake in heart failure and normal subjects. Eur Heart J. 1999;20:880–887. doi: 10.1053/euhj.1998.1447. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Atchison DJ. Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001a;280:H969–H976. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Atchison DJ, Rongen GA. Floras JS. Effect of adenosine receptor blockade with caffeine on sympathetic response to handgrip exercise in heart failure. Am J Physiol Heart Circ Physiol. 2001b;281:H1312–H1318. doi: 10.1152/ajpheart.2001.281.3.H1312. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Azevedo ER, Parker JD. Floras JS. Peak oxygen uptake is not determined by cardiac noradrenaline spillover in heart failure. Eur Heart J. 2002;23:800–805. doi: 10.1053/euhj.2001.2942. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Millar PJ, Murai H, Morris BL. Floras JS. Inverse relationship between muscle sympathetic nerve activity during exercise and peak oxygen uptake in subjects with and without heart failure. J Am Coll Cardiol. 2014;63:605–606. doi: 10.1016/j.jacc.2013.08.693. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P. Coats AJS. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- Ray CA, Rea RF, Clary MP. Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol Heart Circ Physiol. 1993;264:H1–H7. doi: 10.1152/ajpheart.1993.264.1.H1. [DOI] [PubMed] [Google Scholar]
- Saito M. Mano T. Exercise mode affects muscle sympathetic nerve responsiveness. Jpn J Physiol. 1991;41:143–151. doi: 10.2170/jjphysiol.41.143. [DOI] [PubMed] [Google Scholar]
- Saito M, Tsukanaka A, Yanagihara D. Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI. Leuenberger U. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol. 1998;84:1551–1559. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- Soares-Miranda L, Franco FG, Roveda F, Martinez DG, Rondon MUPB, Mota J, Brum PC, Antunes-Correa LM, Nobre TS, Barretto ACP, Middlekauff HR. Negrao CE. Effects of exercise training on neurovascular responses during handgrip exercise in heart failure patients. Int J Cardiol. 2011;146:122–125. doi: 10.1016/j.ijcard.2010.09.091. [DOI] [PubMed] [Google Scholar]
- Stelken AM, Younis LT, Jennison SH, Miller DD, Miller LW, Shaw LJ, Kargl D. Chaitman BR. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J Am Coll Cardiol. 1996;27:345–352. doi: 10.1016/0735-1097(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Sterns DA, Ettinger SE, Gray KS, Whisler SK, Mosher TJ, Smith MB. Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84:2034–2039. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- Stickland MK. Miller JD. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol. 2008;586:1743–1754. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol. 2010;588:5033–5047. doi: 10.1113/jphysiol.2010.199562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac norepinephrine spillover and sympathetic outflow to skeletal muscle. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]


