Abstract
Importance
Epilepsy is a debilitating condition, often with neither a known etiology nor an effective treatment. Autoimmune mechanisms have been increasingly identified.
Objective
To conduct a population-level study investigating the relationship between epilepsy and several common autoimmune diseases.
Design, Setting, and Participant
A retrospective population-based study using claims from a nation-wide employer-provided health insurance plan in the United States. Subjects were beneficiaries enrolled between 1999 and 2006 (n= 2,518,034).
Main Outcomes and Measures
We examined the relationship between epilepsy and 12 autoimmune diseases: type 1 diabetes, psoriasis, rheumatoid arthritis, Graves’ disease, Hashimoto’s thyroiditis, Crohn’s disease, ulcerative colitis, systemic lupus erythematosus, antiphospholipid syndrome, Sjögren’s syndrome, myasthenia gravis and celiac disease.
Results
The risk of epilepsy is significantly heightened among patients with autoimmune diseases (OR 3.8, 95% CI 3.6–4.0, P<0.001), and is especially pronounced in children (OR 5.2, 95% CI 4.1–6.5; P<0.001). Elevated risk is consistently observed across all 12 autoimmune diseases.
Conclusions and Relevance
Epilepsy and AD frequently co-occur and patients with either condition should undergo surveillance for the other. The potential role of autoimmunity must be given due consideration in epilepsy, so we are not overlooking a treatable etiology.
Introduction
Epilepsy is a debilitating condition affecting 0.5% to 1% of the world’s population. Therapies address manifestations rather than the underlying etiology, which remains unelucidated in most patients, one third of which are refractory to anticonvulsant therapy.1 Surgical interventions are ineffective in many epilepsy patients, with seizures recurring in 50% of patients within 5 years of surgery,2 and the number of patients who remain seizure free decreases further over the years to 38%.3 A deeper understanding of the underlying etiologies is necessary to develop new therapeutic approaches.
Specific autoimmune causes, typically associated with autoantibodies, have been increasingly identified in a subset of previously idiopathic seizure disorders.4–5.6–10 In some of these situations, seizures are associated with other neurological manifestations, while in others they are the only sign of neurologic autoimmunity. Small case studies and disease specific investigations also report a high incidence of seizures in AD such as systemic lupus erythematosus (SLE)11–12 and Hashimoto’s thyroiditis.13–14 Further, published reports document success in treating a substantial proportion of patients with presumed autoimmune mechanisms for their seizures with immunotherapy.15
Establishing an autoimmune basis in patients with idiopathic epilepsy is important, as it highlights opportunities for developing new strategies for the treatment of medically refractory epilepsy. To date, evidence on the role of autoimmune process in epileptogenesis is mainly based on animal studies and small sample, disease-specific clinical observations. Here, we conduct the largest population study to investigate the relationship between epilepsy and several common autoimmune diseases. Since clinical presentation of seizures, their etiology, and the presence of comorbidities in the elderly differ considerably from younger patients, our study focuses on epilepsy in children (<18 years of age) and non-elderly adults (<=65 years of age).
Methods
Study population and design
We conducted a population-based retrospective cohort study, using claims from a major, nation-wide employer-provided health insurance plan in the United States. Data included dates of enrollment in the insurance program, outpatient and inpatient visits, and prescription drugs dispensed. Demographic data included gender and age. All encounters were coded with up to four International Classification of Diseases, ninth version (ICD-9) codes. Prescription drugs were reported using the National Drug Code.
Subjects were beneficiaries between January 1, 1999 and December 31, 2006, excluding elderly adults greater than 65 years of age. To ensure adequate follow-up, we included only individuals continuously enrolled for at least four years, and we considered only individuals diagnosed with epilepsy two or more years after entry into our study, and with at least two years follow-up after the first recorded epilepsy diagnosis.
The Boston Children’s Hospital IRB approved the study and granted a waiver of consent.
Outcome measures
We assessed the relationship between epilepsy and 12 AD selected a priori: type 1 diabetes, psoriasis, rheumatoid arthritis, Graves’ disease, Hashimoto’s thyroiditis, Crohn’s disease, ulcerative colitis, SLE, antiphospholipid syndrome, Sjögren’s syndrome, myasthenia gravis and celiac disease. Outcomes were stratified based on age groups: (1) children (<18 years of age); (2) non-elderly adults (<=65 years of age).
Further, we examined the potential effects of common therapies used for treating AD, including aminosalicylates, disease-modifying antirheumatic drugs (DMARD), systemic glucocorticoids, non-steroidal anti-inflammatory drugs (NSAID), anti-TNF agents, and other biologics (eTable 1). Only exposures that occurred prior to the first epileptic seizure were considered. Gender and age were included as covariates. Exposure to medications was expressed as a dichotomous variable.
Case identification
Epilepsy was defined as two or more seizures occurring at least 24 hours apart within a period of two years. Individuals with diagnoses of epilepsy and AD were identified using ICD-9 diagnostic codes (eTable 2), according to previously validated criteria:16 (1) at least one acute inpatient encounter with the relevant ICD-9 as the primary diagnosis; or (2) at least two healthcare encounters with the relevant ICD-9 within a period of two years. These criteria have been demonstrated to achieve high accuracy in identifying patients from administrative data (sensitivity=92.9%; specificity=91.2%).16 To further strengthen the specificity of epilepsy case identification, we considered only individuals prescribed at least one course of an anticonvulsant.
Statistical analysis
The risk of epilepsy in AD patients was compared against the risk of epilepsy in individuals without AD using logistic regression, expressed as odds ratios (OR) with 95% confidence interval (CI). All analyses were performed using SPSS software version 21. All statistical tests were 2-sided.
Results
Prevalence of epilepsy
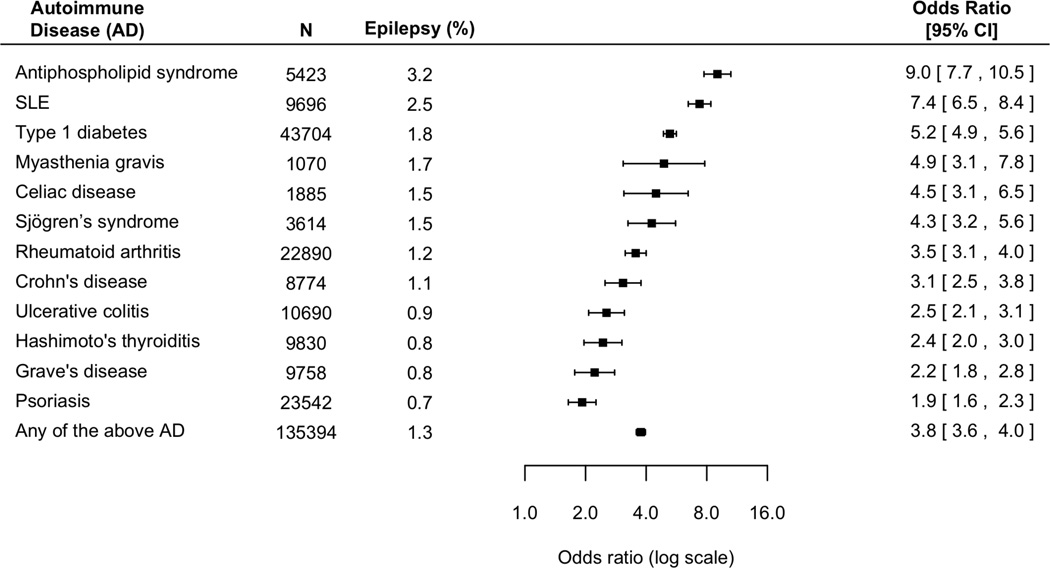
A total of 2,518,034 subjects were included in our study. 0.4% of the study population developed epilepsy (Table 1). The risk of epilepsy is significantly heightened among patients with AD (OR 3.8, 95% CI 3.6–4.0, P<0.001) (Figure 1). Collectively, individuals with AD account for 17.5% of epilepsy patients in the study population.
Table 1.
Demographic characteristics.
| Patients, n | 2,518,034 |
| Gender, n (%) | |
| Female | 1,302,709 (51.7) |
| Male | 1,215,325 (48.3) |
| Age, n (%) | |
| Children (<18 years old) | 476,805 (18.9) |
| Adults (<=65 years old) | 2,041,229 (81.1) |
| Length of follow-up (days)(mean ± SD) | 2571 ± 490 |
| Epilepsy prevalence, n (%) | |
| All ages | 10,041 (0.4) |
| Children (<18 years old) | 1,796 (0.4) |
| Adults (<=65 years old) | 8,245 (0.4) |
| Autoimmune disease prevalence, n (%) | |
| Type 1 diabetes | 43,704 (1.7) |
| Psoriasis | 23,542 (0.9) |
| Rheumatoid arthritis | 22,890 (0.9) |
| Ulcerative colitis | 10,690 (0.4) |
| Hashimoto’s thyroiditis | 9,830 (0.4) |
| Grave’s disease | 9,758 (0.4) |
| Systemic lupus erythematosus | 9,696 (0.4) |
| Crohn’s disease | 8,774 (0.3) |
| Antiphospholipid syndrome | 5,423 (0.2) |
| Sjögren’s Syndrome | 3,614 (0.1) |
| Celiac disorder | 1,885 (0.1) |
| Myasthenia gravis | 1,070 (0.04) |
| Any of the above AD | 137,398 (5.5) |
| Medications*, n (%) | |
| Aminosalicylates | 24,303 (1.0) |
| Disease-modifying antirheumatic drugs | 33,557 (1.3) |
| Systemic glucocorticoids | 790,045 (31.4) |
| Anti-TNF agents | 7,114 (0.3) |
| Other biologics | 2,915 (0.1) |
| Non-steroidal anti-inflammatory agents | 945,892 (37.6) |
(*excluding medications taken after the first epileptic seizure)
Figure 1. The risk of epilepsy in children (<18 years of age) and non-elderly adults (<=65 years of age) with autoimmune diseases, compared with individuals without autoimmune diseases.
Epilepsy susceptibility is consistently heightened in patients with autoimmune disorders (P<0.001). Collectively, patients with any of the autoimmune diseases under study constituted 17.5% of the total epilepsy population.
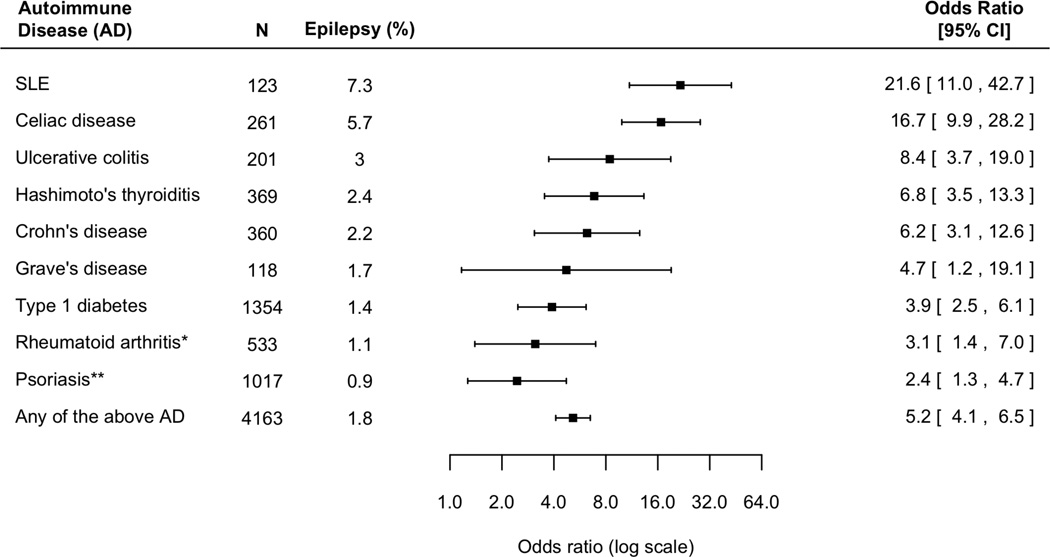
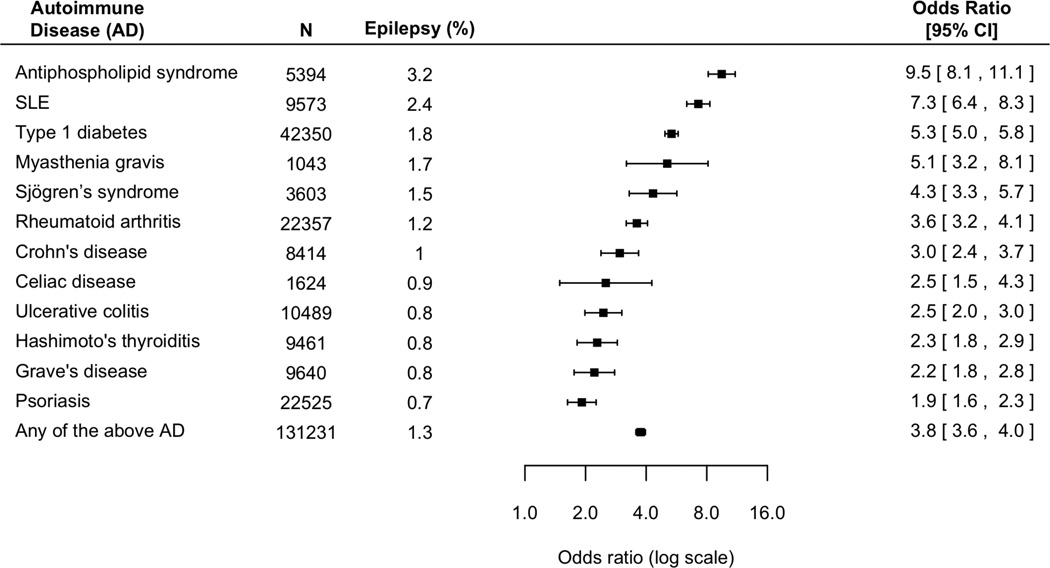
Elevated risk is consistent across different AD. Patients with antiphospholipid syndrome and SLE had the highest risk, with an nine-fold and seven-fold increased risk of epilepsy respectively; followed by patients with type 1 diabetes and myasthenia gravis, eliciting a five-fold increased risk of epilepsy. The risk of epilepsy is especially pronounced in children. Overall, children with AD have a five-fold increased risk of epilepsy (Figure 2). In comparison, non-elderly adults with AD have a four-fold increased risk of epilepsy (Figure 3).
Figure 2. The risk of epilepsy in children (<18 years of age) with autoimmune diseases, compared with children without autoimmune diseases.
Overall, children with an autoimmune condition have a five-fold increased risk of epilepsy (P<0.001 in all cases, except otherwise indicated; *P=0.006; **P=0.008).
Figure 3. The risk of epilepsy in non-elderly adults (<=65 years of age) with autoimmune diseases, compared with non-elderly adults without autoimmune diseases.
Overall, adults with an autoimmune condition have a four-fold increased risk of epilepsy (P<0.001 in all cases).
Timing of epilepsy
Patients experienced their first epileptic seizure both before and after the onset of AD. The onset of epilepsy preceded the diagnosis of AD in 30% of cases. And in 30% of cases, the first epileptic seizure occurred within the first year of disease diagnosis.
Effects of medications
More than 70% patients with AD and epilepsy were not exposed to anticonvulsants for at least two years prior to the diagnosis of AD. The risk of epilepsy in AD patients persists after adjusting for medication use, including aminosalicylates, DMARD, systemic glucocorticoids, NSAID, anti-TNF agents and other biologics (OR 4.1, 95 CI% 3.9–4.3, P<0.001)(Table 2). There is no evidence that any of the medications lead to increased risk of epilepsy. Patients exposed to aminosalicylates, NSAID, anti-TNF agents and other biologics appear to have a reduced risk of epilepsy.
Table 2. The effect of autoimmune therapies on epilepsy risk.
We performed a multivariate logistic regression analysis to evaluate epilepsy risk in patients with autoimmune disease, accounting for common autoimmune therapies, as well as gender and age. Even after adjustment, the presence of autoimmune disease remained a strong predictor of epilepsy occurrence.
| Variable | Epilepsy Risk OR (95% CI) |
P-value |
|---|---|---|
| Any autoimmune disease | 4.1 (3.9 – 4.3) | <0.001 |
| Gender (female) | 1.2 (1.1 – 1.2) | <0.001 |
| Children (< 18 years of age) | 1.0 (0.97 – 1.1) | 0.370 |
| Aminosalicylates | 0.6 (0.5 – 0.8) | <0.001 |
| Disease modifying antirheumatic drugs | 1.0 (0.9 – 1.1) | 0.977 |
| Systemic glucocorticoids | 1.1 (1.0 – 1.1) | 0.001 |
| Anti-TNF agents | 0.4 (0.3 – 0.6) | <0.001 |
| Other biologics | 0.3 (0.1 – 0.8) | 0.012 |
| Non-steroidal anti-inflammatory drugs | 0.9 (0.8 – 0.9) | <0.001 |
Discussion
Clinicians caring for patients with either AD or epilepsy should be aware of the strong association between them. Indeed, nearly one in five epilepsy patients has a coexisting AD. Elevated epilepsy prevalence has been previously reported in AD where pathology directly involves the brain. SLE is associated with a range of inflammatory mechanisms in the brain, and cerebral ischemia is a common manifestation of antiphospholipid syndrome. Rates of epilepsy in SLE vary between 4 and 51%,11–12,17 and in antiphospholipid syndrome from 3% to 8%.18–19 Our analysis is consistent with these published data. In addition, we establish the association across a wide range of AD including those where the primary pathology is not known to directly affect the brain. Of note, patients with myasthenia gravis had a 5-fold increased risk of epilepsy. While our data do not elucidate the underlying causes of this relationship, they strongly support further effort to explore the potential role of autoimmunity in epileptogenesis. A focus on autoimmune mechanisms can focus and guide translational approaches to new therapeutic options.
Seizures tend to occur within the first 1 to 2 years after the AD diagnosis. The risk of epilepsy is consistently higher in children with AD, compared with adults with the same AD. Both clinical and biological features of AD may be influenced by the age at disease onset,20–21 with childhood onset AD often more severe than adult onset AD.22–23 Consistent with a previous study11, our data showed that the female gender is associated with a higher risk of epilepsy.
Effects of medications
Prior studies on the effects of anticonvulsants have produced inconsistent results. Anticonvulsants have been reported to exert anti-inflammatory effects,24 but also, there are reported cases of SLE-like symptoms caused by carbamazepine;25 however, evidence for causation remains largely anecdotal and inconsistent, with other studies failing to show a relationship between anticonvulsant use and SLE.26 Our main findings are clearly not explained purely by immunological side effects of anticonvulsants, as 70% patients with AD and epilepsy were not exposed to anticonvulsants for at least two years prior to the diagnosis of AD.
We also explore whether specific treatments for AD may cause or prevent seizures. In some prior studies, corticosteroids, certain immunologic agents and NSAID have been found to induce seizures,27–29 and to reduce the risk of seizures in others.30–32 We find the risk of epilepsy in patients with autoimmune diseases persists, even after adjustment for these medications in regression models. Exposures to aminosalicylates, NSAID and biologics appear to reduce epilepsy risk. However, since we did not study the duration and dose of exposures, whether or not these medications confer a protective effect against developing epilepsy is unknown, and beyond the scope of this study.
What is the nature of the relationship?
Among the growing list of neuronal autoantibodies identified in some patients with epilepsy, some appear to play a pathogenic role while others may merely be markers of disease. For example, compelling clinical and laboratory evidence supports the pathogenicity of antibodies against the NR1 subunit of the N-methyl D-aspartate receptor (NMDAR). Antibodies from patients with anti-NMDAR encephalitis cause a decrease of the density of NMDAR through antibody-mediated cross-linking and internalization, resulting in the impairment of NMDAR-mediated synaptic function.33–34 Clinical outcome of these patients has been found to correlate with antibody tires in CSF, and in most cases, symptoms are reversible by immunotherapy.35–36 On the other hand, the pathogenic role of other autoantibodies, particularly those directed against intracellular antigens such as glutamic acid decarboxylase, is less clear.
The immune response in AD involves the adaptive immune system for which the presence of autoimmune antibodies is just one manifestation, as well as the innate arm, as characterized by the increased synthesis and release of pro-inflammatory cytokines and chemokines. Thus, the occurrence of epilepsy in patients with AD might be attributable to the inflammatory component of AD, as evidenced by the expression of sustained inflammatory response in the resected brain tissue from patients undergoing surgery for refractory epilepsy, including activation of microglia and astrocytes and production of pro-inflammatory molecules.37–39 Additionally, there is evidence of the anticonvulsant effects of selected anti-inflammatory drugs,40–41 and the anti-inflammatory properties of some anticonvulsant medications, such as valproate42 and carbamazepine.43
While autoimmunity and neuroinflammation are likely to play a role in a subset of epilepsy in autoimmune patients, seizures may also be a result of the cerebrovascular complications that are commonly associated with many autoimmune conditions, including SLE, RA, Sjögren’s syndrome, type 1 diabetes and inflammatory bowel disease. Thus the risk of seizures in these patients is heightened independent of immunologic causes. However, given that susceptibility to cerebrovascular conditions correlates strongly with advancing age, our findings showing children with AD at a much higher risk of epilepsy compared with adults with the same autoimmune condition suggest that the relationship cannot be entirely attributable to the cerebrovascular complications secondary to AD. Further, the association between epilepsy and AD that do not directly affect the brain, including myasthenia gravis and psoriasis, strongly implicates that other mechanisms are at play.
In reality, epilepsy is not a single disease entity, but a variety of disorders reflecting the underlying brain dysfunction that may result from many different causes. Similarly, it is likely that multiple factors contribute to the risk of epilepsy in patients with autoimmune conditions. First, some patients with AD may have coincidental epilepsy that is unrelated to autoimmune mechanisms; we have accounted for this via the comparison to the non-AD population. Second, some patients with AD may have non-inflammatory brain pathology that gives rise to epilepsy; as above, while this is likely to be true, we do not think it fully explains the heightened risk as described above. Third, some patients with AD and epilepsy may have known autoantibodies such as NMDAR antibodies. Lastly, we speculate that the largest subgroup consists of patients where as yet undefined autoimmune and inflammatory mechanisms lead to their epilepsy. Further studies are needed to elucidate the pathogenesis of epilepsy in autoimmune patients, especially in the latter subgroup. Identifying these mechanisms may also yield insights and novel treatment approaches for patients with epilepsy but without AD, particularly those without a known etiology and/or refractory epilepsy.
Study limitations
There are several limitations to our study. First, claims data have limited resolution and do not permit fine-grained classification of epilepsy type. However, the validity of using ICD codes in administrative data for identifying epilepsy cases has been demonstrated in several studies.16,44–45 To maximize the specificity of our case identification, we have chosen a stringent case definition for diagnosis of epilepsy that has been previously validated.16 In addition, we selected only epilepsy patients who were prescribed antiepileptic medications, thus minimizing the likelihood of misclassification of non-epileptic seizures, such as those induced by hypoglycemia, alcohol or drugs. The consistency between our results and published data on the relationships between epilepsy and several AD, including SLE and antiphospholipid syndrome, further validates our approach. Second, because our study participants are privately insured individuals, findings from these data may not generalize to other populations. Third, since our data spanned seven years and the set of AD included in our study is non-exhaustive, incidence of epilepsy in AD patients may be underestimated.
While there are limitations to claims data, the availability of large number of patients makes it possible to study the relationships between rare diseases, which may not have been observable in traditional studies involving chart reviews or surveys. Further, claims data are systematically collected, and provide longitudinal information that cross facilities, geographical locations and population demographics, thereby enhancing the generalizability of the research and limiting selection biases. While our study does not prove that epilepsy and AD indeed share a common pathophysiology, which remains to be elucidated, our data provide important epidemiology evidence that lends support to the hypothesis, and highlights the need for in-depth investigation.
Conclusions
Epilepsy and AD frequently co-occur and patients with either condition should undergo surveillance for the other. Because AD affect 8% of the population, and for reasons unknown their prevalence is rising, the link between autoimmunity and epilepsy will underpin a rise in the global burden of neurological disease. The potential role of autoimmunity must be given due consideration in refractory epilepsy, so we are not overlooking a treatable etiology.
Supplementary Material
Acknowledgments
We thank Marc Natter MD (Boston Children’s Hospital) for his intellectual input. M.S.O. is supported by a fellowship from the National Health and Medical Research Council, Australia. I.K. was funded in part by the i2b2 National Center for Biomedical Computing (NIH/NLM U54 LM008748) and the Conte Center for Computational System Genomics of Neuropsychiatric Phenotypes (NIH P50MH94267). The funding bodies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
M.S.O. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author contributions: K.M. and M.S.O. conceived the study. M.S.O. conducted data analysis and drafted the manuscript. I.K., T.C., M.G., K.M. participated in study design, statistical analysis, data interpretation, and writing of manuscript. All authors read and approved the final manuscript.
Competing interests: The authors have no potential conflicts of interest to be disclosed.
References
- 1.Kwan P, Brodie MJ. Early Identification of Refractory Epilepsy. N Eng J Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2.de Tisi J, Bell GS, Peacock JL, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378(9800):1388–1395. doi: 10.1016/S0140-6736(11)60890-8. [DOI] [PubMed] [Google Scholar]
- 3.Wieser HG, Ortega M, Friedman A, Yonekawa Y. Long-term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98(4):751–763. doi: 10.3171/jns.2003.98.4.0751. [DOI] [PubMed] [Google Scholar]
- 4.Bergey GK. Autoantibodies in the patient with drug-resistant epilepsy: are we missing a treatable etiology? Arch Neurol. 2012;69(5):565–566. doi: 10.1001/archneurol.2012.354. [DOI] [PubMed] [Google Scholar]
- 5.Palace J, Lang B. Epilepsy: an autoimmune disease? J Neurol Neurosurg Psychiatry. 2000;69(6):711–714. doi: 10.1136/jnnp.69.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKnight K, Hart Y, Cavey A, et al. Serum antibodies in epilepsy and seizure-associated disorders. Neurology. 2005;65(11):1730–1736. doi: 10.1212/01.wnl.0000187129.66353.13. [DOI] [PubMed] [Google Scholar]
- 7.Barajas RF, Collins DE, Cha S, Geschwind MD. Adult-onset drug-refractory seizure disorder associated with anti-voltage-gated potassium-channel antibody. Epilepsia. 2010;51(3):473–477. doi: 10.1111/j.1528-1167.2009.02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liimatainen S, Peltola M, Sabater L, et al. Clinical significance of glutamic acid decarboxylase antibodies in patients with epilepsy. Epilepsia. 2010;51(5):760–767. doi: 10.1111/j.1528-1167.2009.02325.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin JJ, Lin KL, Hsia SH, et al. Antiglutamic acid decarboxylase antibodies in children with encephalitis and status epilepticus. Pediatr Neurol. 2012;47(4):252–258. doi: 10.1016/j.pediatrneurol.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanly JG, Urowitz MB, Su L, et al. Seizure disorders in systemic lupus erythematosus results from an international, prospective, inception cohort study. Ann Rheum Dis. 2012;71(9):1502–1509. doi: 10.1136/annrheumdis-2011-201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appenzeller S, Cendes F, Costallat LT. Epileptic seizures in systemic lupus erythematosus. Neurology. 2004;63(10):1808–1812. doi: 10.1212/01.wnl.0000144178.32208.4f. [DOI] [PubMed] [Google Scholar]
- 13.Berger I, Castiel Y, Dor T. Paediatric Hashimoto encephalopathy, refractory epilepsy and immunoglobulin treatment – unusual case report and review of the literature. Acta Paediatr. 2010;99(12):1903–1905. doi: 10.1111/j.1651-2227.2010.01967.x. [DOI] [PubMed] [Google Scholar]
- 14.Arain A, Abou-Khalil B, Moses H. Hashimoto’s encephalopathy: documentation of mesial temporal seizure origin by ictal EEG. Seizure. 2010;10(6):438–441. doi: 10.1053/seiz.2001.0531. [DOI] [PubMed] [Google Scholar]
- 15.Quek AM, Britton JW, McKeon A, et al. Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol. 2012;69(5):582–593. doi: 10.1001/archneurol.2011.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid AY, St Germaine-Smith C, Liu M, et al. Development and validation of a case definition for epilepsy for use with administrative health data. Epilepsy Res. 2012;102(3):173–179. doi: 10.1016/j.eplepsyres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Andrade RM, Alarcón GS, González LA, et al. Seizures in patients with systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA LIV) Ann Rheum Dis. 2008;67(6):829–834. doi: 10.1136/ard.2007.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoenfeld Y, Lev S, Blatt I, et al. Features associated with epilepsy in the antiphospholipid syndrome. J Rheumatol. 2004;31(7):1344–1348. [PubMed] [Google Scholar]
- 19.Cervera R, Piette JC, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46(4):1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 20.Carreño L, López-Longo FJ, Monteagudo I, et al. Immunological and clinical differences between juvenile and adult onset of systemic lupus erythematosus. Lupus. 1999;8(4):287–292. doi: 10.1191/096120399678847786. [DOI] [PubMed] [Google Scholar]
- 21.Barron KS, Silverman ED, Gonzales J, Reveille JD. Clinical, serologic, and immunogenetic studies in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1993;36(3):348–354. doi: 10.1002/art.1780360310. [DOI] [PubMed] [Google Scholar]
- 22.Descloux E, Durieu I, Cochat P, et al. Influence of age at disease onset in the outcome of paediatric systemic lupus erythematosus. Rheumatology. 2009;48(7):779–784. doi: 10.1093/rheumatology/kep067. [DOI] [PubMed] [Google Scholar]
- 23.Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis. 2010;16(6):953–961. doi: 10.1002/ibd.21152. [DOI] [PubMed] [Google Scholar]
- 24.Bianchi M, Rossoni G, Sacerdote P, Panerai AE, Berti F. Carbamazepine exerts anti-inflammatory effects in the rat. Eur J Pharmacol. 1995;294(1):71–74. doi: 10.1016/0014-2999(95)00516-1. [DOI] [PubMed] [Google Scholar]
- 25.Pelizza L, De Luca P, La Pesa M, Minervino A. Drug-induced systemic lupus erythematosus after 7 years of treatment with carbamazepine. Acta Biomed. 2006;77(1):17–19. [PubMed] [Google Scholar]
- 26.Jain KK. Systemic lupus erythematosus (SLE)-like syndromes associated with carbamazepine therapy. Drug Saf. 1991;6(5):350–360. doi: 10.2165/00002018-199106050-00005. [DOI] [PubMed] [Google Scholar]
- 27.Sternthal MB, Murphy SJ, George J, Kornbluth A, Lichtiger S, Present DH. Adverse events associated with the use of cyclosporine in patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103(4):937–943. doi: 10.1111/j.1572-0241.2007.01718.x. [DOI] [PubMed] [Google Scholar]
- 28.Roberts AJ, Keith LD. Corticosteroids enhance convulsion susceptibility via central mineralocorticoid receptors. Psychoneuroendocrinology. 1995;20(8):891–902. doi: 10.1016/0306-4530(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Hernandez MC, Delgado J, Navarro AM, Orta JC, Hernandez M, Conde J. Seizures induced by NSAID. Allergy. 1999;54(1):90–91. doi: 10.1034/j.1398-9995.1999.00931.x. [DOI] [PubMed] [Google Scholar]
- 30.Buzatu M, Bulteau C, Altuzarra C, Dulac O, Van Bogaert P. Corticosteroids as treatment of epileptic syndromes with continuous spike-waves during slow-wave sleep. Epilepsia. 2009;50(Suppl 7):68–72. doi: 10.1111/j.1528-1167.2009.02224.x. [DOI] [PubMed] [Google Scholar]
- 31.Jung S, Yang H, Kim BS, Chu K, Lee SK, Jeon D. The immunosuppressant cyclosporin A inhibits recurrent seizures in an experimental model of temporal lobe epilepsy. Neurosci Lett. 2012;529(2):133–138. doi: 10.1016/j.neulet.2012.08.087. [DOI] [PubMed] [Google Scholar]
- 32.Wallenstein MC. Attenuation of epileptogenesis by nonsteroidal anti-inflammatory drugs in the rat. Neuropharmacology. 1991;30(6):657–663. doi: 10.1016/0028-3908(91)90087-r. [DOI] [PubMed] [Google Scholar]
- 33.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. Lancet Neurol. 2011;10(1):63–74. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manto M, Dalmau J, Didelot A, Rogemond V, Honnorat J. In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: further evidence of synaptic glutamatergic dysfunction. Orphanet J Rare Dis. 2010;5:31. doi: 10.1186/1750-1172-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655e67. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29(1):142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. doi: 10.1111/j.1528-1167.2011.03033.x. [DOI] [PubMed] [Google Scholar]
- 39.Crespel A, Coubes P, Rousset MC, et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952(2):159–169. doi: 10.1016/s0006-8993(02)03050-0. [DOI] [PubMed] [Google Scholar]
- 40.Verhelst H, Boon P, Buyse G, et al. Steroids in intractable childhood epilepsy: clinical experience and review of the literature. Seizure. 2005;14(6):412–421. doi: 10.1016/j.seizure.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Marchi N, Granata T, Freri E, et al. Infantile spasms: therapy and outcome. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One. 2011;6(3):e18200. doi: 10.1371/journal.pone.0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichiyama T, Okada K, Lipton JM, Matsubara T, Hayashi T, Furukawa S. Sodium valproate inhibits production of TNF-alpha and IL-6 and activation of NF-kappaB. Brain Res. 2000;857(1–2):246–251. doi: 10.1016/s0006-8993(99)02439-7. [DOI] [PubMed] [Google Scholar]
- 43.Matoth I, Pinto F, Sicsic C, Brenner T. Inhibitory effect of carbamazepine on inflammatory mediators produced by stimulated glial cells. Neurosci Res. 2000;38(2):209–212. doi: 10.1016/s0168-0102(00)00127-9. [DOI] [PubMed] [Google Scholar]
- 44.Jetté N, Reid AY, Quan H, Hill MD, Wiebe S. How accurate is ICD coding for epilepsy? Epilepsia. 2010;51(1):62–69. doi: 10.1111/j.1528-1167.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 45.St Germaine-Smith C, Metcalfe A, Pringsheim T, et al. Recommendations for optimal ICD codes to study neurologic conditions: a systematic review. Neurology. 2012;79(10):1049–1055. doi: 10.1212/WNL.0b013e3182684707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.