SUMMARY
Tissue-resident memory T (Trm) cells provide enhanced protection against infection at mucosal sites. Here we found that CD4+ T cells are important for the formation of functional lung-resident CD8+ T cells after influenza virus infection. In the absence of CD4+ T cells, CD8+ T cells displayed reduced expression of CD103 (Itgae), were mislocalized away from airway epithelia, and demonstrated an impaired ability to recruit CD8+ T cells to the lung air-ways upon heterosubtypic challenge. CD4+ T cell-derived interferon-γ was necessary for generating lung-resident CD103+ CD8+ Trm CD8 T cells. Furthermore, expression of the transcription factor T-bet was increased in “unhelped” lung Trm cells, and a reduction in T-bet rescued CD103 expression in the absence of CD4+ T cell help. Thus, CD4+ T cell-dependent signals are important to limit expression of T-bet and allow for the development of CD103+ CD8+ Trm cells in the lung airways following respiratory infection.
INTRODUCTION
Influenza virus remains a significant public health threat and is responsible for 200,000 hospitalizations and 3,000–49,000 deaths each year in the United States (Centers for Disease Control and Prevention, 2010; Thompson et al., 2003). While annual influenza vaccines are capable of protecting against seasonal virus strains, they offer little resistance in the case of the emergence of a novel influenza strain such as H5N1 or H7N9 (Epstein and Price, 2010). CD8+ T cells can recognize internal, conserved influenza epitopes and offer heterosubtypic influenza virus protection (Kreijtz et al., 2007; Ulmer et al., 1998). Lung-resident CD8+ T cells, in particular, are indispensable for the generation of heterosubtypic protection (Wu et al., 2014). Therefore, a vaccine capable of inducing lung-resident memory CD8+ T cells offers the potential to generate potent heterosubtypic influenza virus protection, and for these reasons it is critical to understand the signals that dictate their formation and residence in the lungs.
During an immune response, effector T cells are licensed to migrate to nonlymphoid tissue (NLT) in a process dependent on chemokine receptor expression and the production of chemokines at the site of infection (von Andrian and Mackay, 2000). Whereas many of these effector cells lose their ability to reside in NLT upon the cessation of inflammatory signals, a subset of T cells lodge in the tissue and survive long term without returning to circulation (Masopust et al., 2001). These tissue-resident memory T (Trm) cells are characterized by their expression of CD69, an inhibitor of sphingosine-1-phosphate receptor-1 (S1PR1) function, and in some cases, the integrin αEβ7 (CD103) (Casey et al., 2012; Gebhardt et al., 2009; Hofmann and Pircher, 2011; Shiow et al., 2006). Trm cells have been identified in a variety of tissues and act as sentinels to provide immediate protection upon local secondary infection through direct effector function and the recruitment of circulating memory T cells (Sathaliyawala et al., 2013; Schenkel et al., 2013; Shin and Iwasaki, 2012; Wu et al., 2014). Recent work has identified interleukin-15 (IL-15) and transforming growth factor β (TGF-β) (Mackay et al., 2013; Zhang and Bevan, 2013), as well as downregulation of Krüppel-like Factor 2 (KLF2) expression (Skon et al., 2013) as being important in their formation. Despite this progress, much remains unclear about the exact mechanism by which T cells are exposed to the signals necessary for their differentiation into protective Trm cells.
CD4+ T cells are important for the formation of functional memory CD8+ T cells during infection and vaccination (Sun and Bevan, 2003). The former are critical for CD8+ T cell priming through licensing of dendritic cells and the generation of a chemokine gradient within the draining lymph node (Castellino et al., 2006; Kumamoto et al., 2011). Intriguingly, CD4+ T cells are required for the migration of effector CD8+ T cells into the female reproductive tract, but not the skin, following viral infection (Jiang et al., 2012; Nakanishi et al., 2009). They are also not required for a robust primary influenza-specific CD8+ T cell response in the lung airways, although the maintenance and recall response of memory CD8+ T cells are impaired in the absence of CD4+ T cells (Ballesteros-Tato et al., 2013; Belz et al., 2002). In this study, we shed new light on how CD4+ T cells promote CD8+ T cell immunity to influenza by demonstrating a critical role for CD4+ T cells in the formation of lung resident CD103+ CD8+ Trm cells that localize to the airway epithelium. In the absence of CD4+ T cell help, antiviral CD8+ T cells failed to properly express CD103 and to localize to the lung airways, and were impaired in their ability to mediate protection upon influenza virus rechallenge.
RESULTS
Lung Resident Memory CD8 T Cells Form after Influenza Virus Infection
To study the formation of lung CD8+ Trm cells, mice were infected with a recombinant strain of the H3N2 influenza virus in which the GP33–41 epitope from lymphocytic choriomeningitis virus (LCMV) was inserted into the NA stalk region (X31-GP33). X31-GP33 infection induces three immunodominant CD8+ T cell populations (DbNP366, DbGP33, and PA224) that can be tracked using MHC class I tetramers. At memory time points (day 30–60 postinfection [p.i.]), mice were injected intravascularly with CD8β-PE antibody and sacrificed 5 min later to distinguish between lung-localized and blood-borne cells (Anderson et al., 2012). Using this technique, a population of polyclonal influenza NP366-specific CD8+ T cells within the blood circulation and accordingly CD8β+ were identified, as well as a population of cells that were protected from in vivo labeling and were CD8β− (Figure 1A). In line with previous studies, we found that the influenza-specific CD8β− cells (Trm cells) had enhanced expression of CD103 and CD69, as well as reduced expression of KLRG1, Ly6C, and CD62L relative to CD8β+ circulating cells (Tcirc) (Mackay et al., 2013; Wakim et al., 2012). Additionally, CD8+ Trm cells displayed increased CXCR3 expression and reduced expression of CD127, T-bet, and granzyme B compared to their circulating counterparts (Figure 1B). These data showed that lung Trm and Tcirc memory cells are phenotypically and anatomically distinct and provided resolution for dissecting the signals that regulate the formation and function of these different memory T cell subsets.
Figure 1. Intravascular Staining Allows for Identification of Resident and Circulating Influenza-Specific Memory CD8+ T Cell Populations.
Analysis of the NP366+ T cell response 40 days after X31-GP33 infection. Mice were intravenously (i.v.) injected with CD8β antibody prior to sacrifice to distinguish tissue parenchyma versus vascular-associated influenza-specific CD8+ T cells.
(A) Representative plots of the NP366+ T cells in the CD8+ T cell population and the CD8β+ T cells in the NP366+ T cell population.
(B) Representative histograms of CD103, CD69, KLRG1, Ly6C, CD62L, CD127, CXCR3, T-bet, and GzmB expression in CD8β+ (gray filled) and CD8β− (black) NP366+ T cells. Representative of four independent experiments with four or five mice per group carried out 30–60 days following X31-GP33 infection. Statistical analyses were performed using Prism GraphPad software v6.0. (*p < 0.05; **p < 0.01; ***p < 0.001).
CD4 T Cell Help Is Important for the Formation of CD103hi CD8+ Trm Cells
While CD4+ T cell help is important for the generation of functional CD8+ T cell memory (Sun and Bevan, 2003) following acute infection, little is known about its role in the differentiation and survival of CD8+ Trm cells. To investigate whether CD4+ T cell help was important for the formation of CD8+ Trm cells following influenza infection, we treated mice with CD4-depleting antibody (GK1.5) or PBS 1 day prior to, and at the time of, X31-GP33 infection to transiently deplete CD4+ T cells. This approach allows CD4+ recent thymic emigrants to repopulate the CD4+ T cell compartment approximately 2 weeks following GK1.5 treatment, thus creating a situation in which the role of CD4+ T cell help in the formation of CD8+ Trm cells can be determined without the additional complication of long-term CD4+ T cell loss. At effector (day 8 p.i.) and memory (day 30 p.i.) time points, the two groups of mice were intravascularly injected with anti-CD8β prior to sacrifice and the CD8+ T cell response to the immunodominant NP366-374 epitope was compared. At day 8 p.i., there was a modest reduction in the amount of CD103 expressed on the cells in the lung parenchyma (CD8β− cells) (Figure S1A-D). At memory time points (day 30–60 p.i.), both the frequency and number of NP366+ CD8 T cells in the lung, or more specifically in the lung parenchyma (CD8β− cells), were similar between the two groups of mice (Figures 2A and 2B). However, there was a marked decrease in the percentage of CD103+ as well as CD69+ and CXCR3+ NP366+ CD8+ T cells that formed in the absence of CD4+ T cell help (Figure 2C). The decline in CD103 expression on the unhelped memory CD8 T cells became even more apparent when the CD8β− NP366+ cells in the lung parenchyma were examined directly (Figure 2D). There also was a global impairment in the ability of circulating CD8β+ NP366+ T cells to upregulate CXCR3 (Figure 2D). Thus, while CD4+ T cells were not required for the expansion or migration of memory CD8+ T cells into the lung parenchyma, they were necessary for proper CD103, and to a lesser extent CD69, expression on these cells within this tissue.
Figure 2. CD4+ T Cell Help Critical for the Formation of CD103hi CD8+ Trm Cells.
Analysis of the NP366+ T cell response 40 days after X31-GP33 infection in PBS (helped) and GK1.5 (unhelped) treated mice. Analysis of the NP366+ T cell response 40 days after X31-GP33 infection.
(A) Percentage and (B) numbers of NP366+T cells and resident and circulating NP366+ T cells. Intravenously administered anti-CD8β antibody was used to distinguish NP366+ T cells in tissue parenchyma versus vascular associated.
(C) Percentages of NP366+ cells, which are CD103+, CD69+, and CXCR3+.
(D) CD103 and CD69 expression in NP366+ CD8β− T cells and CXCR3 expression in NP366+ CD8β+ T cells in helped (black) and unhelped (gray filled) mice. Representative of four independent experiments with four or five mice per group carried out 30–60 days following X31-GP33 infection. See also Figure S1.
As CD4+ T cell depleted mice displayed delayed viral clearance, we next asked whether this defect contributed to the impairment in CD103 expression on CD8+ T cells in the lung (Figure S1E). To address this question, we administered sera from X31-GP33 immune animals to PBS (helped)- and GK1.5 (unhelped)-treated mice at day 6 p.i., sufficient to mediate viral control within 24 hr of treatment ((Laidlaw et al., 2013). Recipient mice were sacrificed 30 days following X31-GP33 infection and this showed that the CD103 expression was reduced on the virus-specific CD8+ T cells from both groups of CD4+ T cell-depleted mice, those treated with sera from X31-immune animals, and those treated with sera from naive animals (Figure S1F). Thus, delayed viral clearance did not appear to account for impaired CD103 expression in unhelped CD8+ Trm cells.
CD4+ T Cells Are Critical for the Localization of Influenza-Specific Trm CD8+ T Cells to the Lung Airways
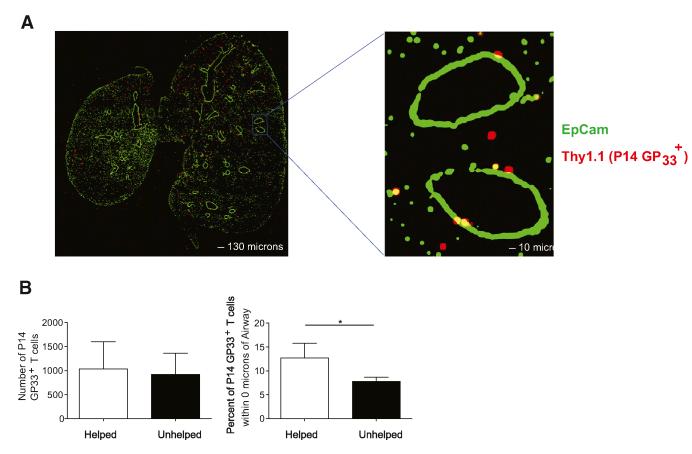
We next determined the location of influenza-specific CD8+ T cells in the lung in the absence of CD4+ T cell help. Previous work in humans and mice has found that CD8+ T cells become embedded in the airway epithelium following influenza infection, where they are ideally localized to respond to future rechallenge (Piet et al., 2011; Wu et al., 2014). CD103 is critical for this process as it binds to E-cadherin and increases adherence to epithelial cells (Lee et al., 2011), suggesting that unhelped CD8+ T cells might be impaired in their ability to localize to and be maintained in the lung airways due to reduced expression of this integrin. To examine this question, we transferred Thy1.1+ P14 CD8+ T cells into control mice or those depleted of CD4 T cells and subsequently infected with X31-GP33. P14 CD8+ T cells, which recognize the GP33–41 epitope, recapitulated the endogenous system, and showed that unhelped mice have a significant impairment in their ability to up-regulate CD103 following X31-GP33 infection (Figure S2). At a memory time point (day 45 p.i.), we used confocal microscopy to analyze the distribution of GP33+ memory CD8+ T cells by staining fixed lung sections with anti-Thy1.1 and anti-EpCAM to identify the transferred P14 Thy1.1+ T cells and airway epithelial cells, respectively (Figure 3A). At this time point, 80%–90% of the P14 Thy1.1+ T cells in the lungs were contained within the lung parenchyma based on intravascular staining (Figure S2). Using IMARIS software, the position of each P14 Thy1.1+ T cell in relationship to the EpCAM+ airway epithelial cells was determined. The percentage of P14 Thy1.1+ T cells in contact with airway epithelial cells was then compared between helped and unhelped samples. Similar to our previous findings (Figures 2A and 2B), the number of P14 Thy1.1+ T cells found within the lung were not different between the two groups of mice. However, there was a significantly decreased percentage of unhelped memory P14 Thy1.1+ T cells embedded in the airway epithelium compared to their helped counterparts (Figure 3B). Thus, the reduced expression of CD103 on unhelped memory CD8 T cells was associated with a significant impairment in their ability to localize to the airway epithelium.
Figure 3. CD4+ T Cell Help Is Important for the Localization of Influenza-Specific Memory CD8+ T Cells to Lung Airway Epithelium.
Analysis of the localization of P14 GP33+ T cells 45 days after X31-GP33 infection in PBS (helped) and GK1.5 (unhelped) treated mice. 50,000 Thy1.1+ P14 GP33+ T cells were transferred to each mouse 1 day prior to X31-GP33 infection to facilitate identification of influenza-specific CD8+ T cells.
(A) At a memory time point (day 45), the mice were sacrificed and the lungs fixed and stained for Thy1.1 (red) and EpCAM (airways, green) and analyzed with confocal microscopy. Representative staining showing presence of Th1.1+ P14 GP33+ T cells embedded in lung airways.
(B) Comparison of the total number of P14 GP33+ T cells in each image and the percent of P14 GP33+ T cells touching the lung airway between helped (PBS treated) and unhelped (GK1.5 treated) mice. Imaris suite was used for analysis of all images. Representative of two independent experiments with four or five mice per group carried out 30–45 days following X31-GP33 infection. See also Figure S2.
Unhelped CD8+ Trm Cells Have Impaired Heterosubtypic Immunity
We next sought to investigate the ability of unhelped CD8+ T cells to mediate protection upon heterosubtypic rechallenge. However, the inability of CD4+ T cell-depleted mice to form anti-influenza antibodies compromises our ability to directly compare protective immunity between GK1.5-treated and -untreated mice. To circumvent this issue, we performed experiments in MD4 mice that have a transgenic B cell receptor specific for hen egg lysozyme (Mason et al., 1992), and thus are unable to form influenza-specific B cell responses (Laidlaw et al., 2013). We infected PBS (helped) or GK1.5 (unhelped) treated MD4 immunoglobulin transgenic mice with X31-GP33 to eliminate CD4 T cells at the time of CD8 T cell priming and generate unhelped Trm CD8 T cells. However, then both groups of mice were treated with GK1.5 antibody at day 14 p.i. to prevent the formation of influenza-specific CD4+ memory T cells and at day 30 p.i., they were challenged with a lethal dose of the recombinant heterosubtypic H1N1 influenza PR8-GP33 virus. By eliminating both anti-influenza antibodies and CD4+ memory T cell cells, this approach allowed us to directly compare the secondary responses of helped and unhelped memory CD8 T cells to influenza. These experiments revealed that mice containing helped memory CD8+ T cells were better protected from heterosubtypic influenza infection than their unhelped counterparts (Figure 4A). This protection was associated with greater recruitment of virus-specific CD8+ T cells into the airways 4 days after secondary infection, as well as increased expression of granzyme B (Figures 4B and 4C). Nonetheless, GK1.5-treated mice still displayed lower viral titers compared to unimmunized controls, demonstrating that unhelped CD8+ T cells were capable of mediating a degree of heterosubtypic protection (Figure 4A).
Figure 4. Unhelped Influenza-Specific CD8+ T Cells Impaired in Ability to Mediate Heterosubtypic Protection.
Analysis of the ability of helped and unhelped memory CD8+ T cells to mediate heterosubtypic protection. To directly compare the ability of helped and unhelped CD8+ T cells to mediate heterosubtypic protection, PBS (helped)- and GK1.5 (unhelped)-treated MD4 mice were infected with X31-GP33. Two weeks later, both groups were treated with GK1.5 to create both helped and unhelped mice that do not have influenza-specific CD4+ T or B cells. Both groups were then rechallenged with the heterosubtypic influenza virus PR8-GP33, alongside that of naive mice as unimmunized controls.
(A) Viral load at day 2 after PR8-GP33 rechallenge.
(B) Number of P14 GP33+ T cells in lung airway at day 4 after PR8-GP33 infection.
(C) MFI of granzyme B expression of lung P14 GP33+ T cells at day 4 after PR8-GP33 infection. Representative of four independent experiments with three to five mice per group.
(D) Helped and unhelped MD4 mice were generated as described above. At a memory time point (day 30), P14 GP33+ T cells were isolated from the spleens of helped and unhelped X31-GP33 immune mice and equal numbers of congenically marked cells transferred in to helped or unhelped MD4 mice. The next day all recipients were challenged with PR8-GP33 and the number of the transferred P14 GP33+ found in the lung airways 6 days later determined. Representative of three independent experiments with three to five mice per group.
Although these data showed that CD4+ T cell help was important for the ability of CD8+ T cells to mediate heterosubtypic protection, they did not indicate which CD8+ memory T cell populations were responsible for the impaired protection. To address whether defective function of the unhelped CD103dim CD8+ Trm cells or the unhelped CXCR3dim Tcirc cells, or both, was responsible for the reduced recruitment of secondary effector cells into the airways upon rechallenge, we employed an adoptive transfer approach. MD4 X31-GP33 immune mice containing helped or unhelped influenza-specific memory CD8+ T cells were created and served as recipients One day prior to rechallenge, congenically marked (Thy1.1+) Tcirc P14+ T cells isolated from the spleens of X31-GP33-infected helped and unhelped mice were transferred in equal numbers into the recipient mice to create four groups that contained (1) helped Trm and helped Tcirc, (2) helped Trm and unhelped Tcirc, (3) unhelped Trm and helped Tcirc, or (4) unhelped Trm and unhelped Tcirc. The four groups were rechallenged with PR8-GP33 and the recruitment of the donor 2− effector P14 CD8 T cells into the lung airways was assessed 6 days after rechallenge. Compared to the control mice (group 1), the numbers of donor P14 CD8 T cells recruited into the air-ways were significantly reduced when either the circulating (group 2) or resident (group 3) memory CD8 T cell population lacked CD4+ T cell help, with the fewest number of cells recruited in mice in which both the transferred and resident populations were unhelped (group 4) (Figure 4D). Thus, CD4+ T cells are important for generating functional resident and circulating memory CD8+ T cells capable of orchestrating optimal protective recall responses to heterosubtypic influenza virus challenge.
CD4+ T Cell-Mediated Control of CD103+ CD8+ Trm Cell Development Is Interferon-γ-Dependent
We next delineated when CD4+ T cell help was required for generation of lung CD103+ CD8+ Trm cells following influenza infection. Mice were treated with GK1.5 at days 0, 3, or 7 after X31-GP33 infection and the memory CD8+ T cells were examined 30 days later. Mice depleted of CD4+ T cells at the time of infection or 3 days p.i. had a similar reduction in CD103 expression in the influenza-specific CD8β− cells. However, CD4+ T cell depletion 7 days p.i. had no effect on CD103 expression (Figure 5A). Thus, CD4+ T cells are needed during the first week of influenza infection to promote the formation of lung CD103+ CD8+ Trm cells.
Figure 5. CD4+ T Cell-Derived IFN-γ Is Important for Formation of CD103hi CD8+ T Cells.
Analysis of the temporal requirements for CD4+ T cell help for the formation of CD103hi CD8+ Trm cells. Mice were treated with GK1.5 antibody at various time points after X31-GP33 infection.
(A) CD103 MFI in CD8β− GP33+ T cells at day 45 after X31-GP33 infection. Representative of three independent experiments with four or five mice per group.
(B) 1 × 106 P14 GP33+ CD8+ T cells and 1 × 106 Smarta GP66+ CD4+ T cells were transferred to mice 1 day prior to WSN-GP33/67 infection and the kinetics of influenza-specific CD4+ and CD8+ T cell entry into the lung airways determined. Representative of two independent experiments with three to four mice per group. WT:Cd4−/− and Ifng−/−:Cd4−/− mixed bone-marrow chimeras were generated. Following reconstitution, the WT:Cd4−/− mice were treated with PBS or GK1.5 and all groups infected with X31-GP33 and sacrificed at day 40.
(C) Percentages (top) and numbers (bottom) of NP366+ T cells, and resident versus circulating NP366+ T cells. Intravenous anti-CD8β antibody was used to distinguish tissue parenchyma versus vascular-associated NP366+ T cells.
(D) Representative plots of CD103+CD69+ NP366+ T cells (top). Percentages of NP366+ cells that are CD103+, CD69+, and CD103+CD69+ (bottom). Representative of three independent experiments with four or five mice per group carried out 30–60 days following X31-GP33 infection. See also Figure S3.
To address whether CD4+ T cells might direct CD8+ T cell migration to a tissue microenvironment promoting Trm formation, such as airway epithelia, we tracked the kinetics of CD4+ and CD8+ T cell entry into the airways early after viral challenge. To enhance detection of virus-specific T cells, we transferred congenically marked Thy1.1+ P14 CD8+ and Ly5.1+ Smarta (Stg) CD4+ T cells, which recognize the LCMV GP61-80 epitope, into mice that were subsequently infected with a recombinant WSN-33/67 influenza virus strain that expresses both the LCMV GP33–41 and GP67–77 epitopes (Marsolais et al., 2009). Low, but similar frequencies of Stg and P14 T cells were present at day 4 p.i.; however, by day 5 p.i., the frequency of Stg CD4+ T cells in the airways was much larger than that of the P14 CD8+ T cells (Figure 5B, ~20% versus ~5%, respectively). At day 6 p.i., the frequency of P14 CD8+ T cells had increased to that similar to the Stg T cells. These studies suggested that entry of influenza-specific CD4+ T cells into the airways appeared to precede that of CD8+ T cells by about 1 day.
It is possible that the early entry of CD4+ T cells into the airways conditions the epithelia to recruit CD8+ T cells. For example, prior work has shown CD4+ T cells are critical for controlling the migration of effector CD8+ T cells into the female reproductive tract following herpes simplex virus infection, through the secretion of IFN-γ and the induction of local chemokines (Nakanishi et al., 2009). Therefore, we assessed whether IFN-γ-producing CD4+ T cells might be playing a similar role in the context of influenza infection. To this end, chimeras were generated in which wild-type (WT) or IFN-γ-deficient bone marrow was mixed with CD4+ T cell-deficient bone marrow and transferred into irradiated CD4+ T cell-deficient mice (Figure 5C). This created two groups, in which all CD4+ T cells did (WT:CD4+ T cell-deficient chimeras) or did not (IFN-γ-deficient:CD4+ T cell-deficient chimeras) produce IFN-γ. Following reconstitution, the WT:CD4+ T cell-deficient mice were treated with either PBS or CD4-depleting mAb, and all groups were challenged with X31-GP33. At a memory time point (45 days), the mice were intravascularly labeled and sacrificed. Similar to our previous findings (Figures 2A and 2B), we saw no statistically significant differences in the percentage or number of the NP366-specific CD8+ T cells or in the localization of these cells to the blood (CD8β+) or lung parenchyma (CD8β−) (Figure 5C). However, there was a significant decrease in the percentage of CD103+CD69+NP366+ cells in the IFN-γ-deficient CD4+ T cell-deficient and the control CD4-depleted WT:CD4+ T cell-deficient groups relative to the PBS-treated WT:CD4+ T cell-deficient group (Figure 5D). These data showed that mice containing IFN-γ-deficient CD4+ T cells recapitulate the defective development of CD103+ CD8+ Trm cells observed in CD4-depleted mice. Polyclonal virus-specific CD8+ T cells lacking IFN-γ receptor (IFN-γR) expression displayed no defect in CD103 expression following X31-GP33 infection, indicating the requirement for CD4-derived IFN-γ was CD8+ T cell extrinsic (data not shown). Moreover, CD4+ T cell help did not affect the ability of lung-resident CD8+ Trm cells to respond to TGF-β-mediated induction of CD103 because both helped and unhelped GP33+ T cells isolated from the mediastinal lymph nodes (MLN) on day 5 after X31-GP33 infection could upregulate CD103 after overnight stimulation with TGF-β (Figures S3A and S3B). This result suggested that unhelped CD8+ T cells are capable of expressing high amounts of CD103 after their exit from the MLN and that the impaired CD103 expression on unhelped cells might stem from inadequate exposure to tissue-dependent signals in the lung. But interestingly, there was no global defect in TGF-β or chemokine production in the lung airways in X31-GP33 infected CD4+ T cell-depleted mice (Figure S3C). Altogether, these data suggest that CD4+ T cells direct the localization of CD8+ T cells to the airways through fine-tuning of the surrounding chemokine gradient, rather than gross chemokine production, thereby regulating the degree of exposure to signals, such as TGF-β, that induce CD103 expression.
Unhelped CD8+ Trm Cells Display Enhanced T-bet Expression
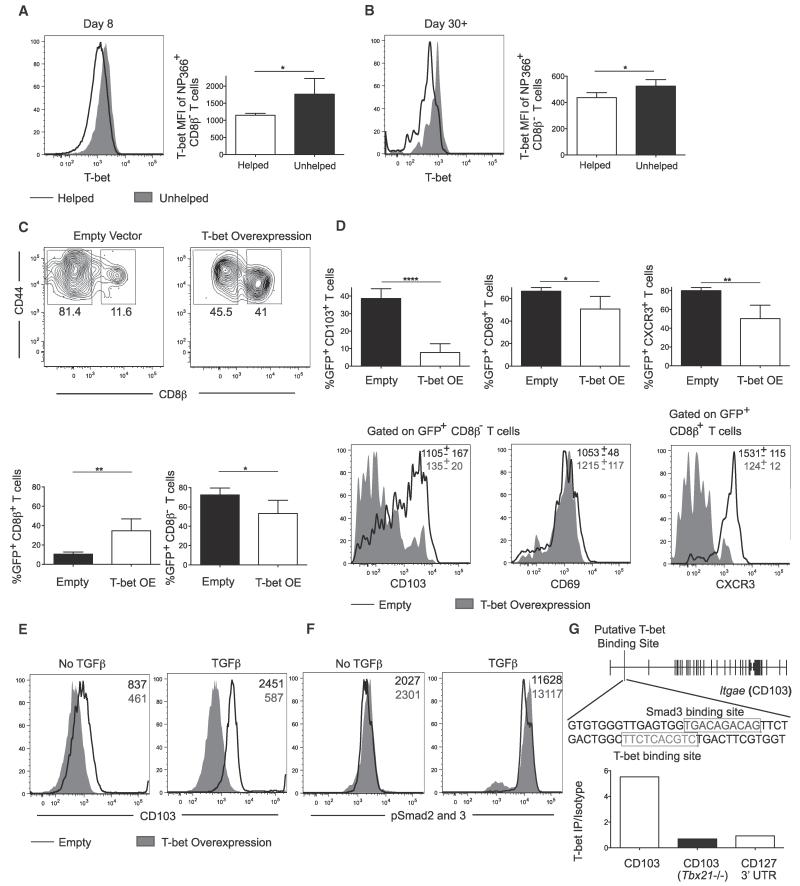
CD4+ T cells can regulate the inflammatory environment, leading to differences in the transcriptional profile of CD8+ T cells (Castellino et al., 2006; Intlekofer et al., 2007). Therefore, we sought to evaluate the transcriptional mechanism by which CD4+ T cells might regulate the formation of CD8+ Trm cells. A major regulator of effector CD8+ T cell fates is T-bet, which operates in a graded manner to promote differentiation of memory precursor cells when expressed at lower amounts and terminal effector cells when higher (Joshi et al., 2007; Takemoto et al., 2006). T-bet was differentially expressed in influenza-specific memory CD8+ T cells, with Trm CD8+ T cells having less expression relative to Tcirc memory CD8+ T cells at day 8 p.i. and at memory time points (day 30-60) (Figures 1 and S4A). Furthermore, unhelped NP366-specific effector and memory CD8+ T cells in the lung (CD8β−) expressed more T-bet at days 8 and 30 p.i. than their helped counterparts (Figures 6A and 6B). These data suggest that lower amounts of T-bet might promote the formation of CD103+ Trm cells in the lung, whereas higher concentrations promote Tcirc memory cell formation.
Figure 6. T-bet Suppresses Formation of Trm Cells and Is Capable of Directly Binding to Itgae Locus.
Analysis of T-bet expression in NP366+ CD8β− T cells in PBS (helped) and GK1.5 (unhelped) treated mice.
(A) Representative histogram of T-bet expression and MFI at day 8 after X31-GP33 infection in helped and unhelped mice.
(B) Representative histogram of T-bet expression and MFI at day 45 after X31-GP33 infection in helped and unhelped mice. P14 GP33+ T cells were transduced with T-bet overexpressing or an empty vector and transferred to mice infected with X31-GP33 the prior day. Mice were sacrificed 30 days after X31-GP33 infection.
(C) Representative plot (top) and percentage (bottom) of resident versus circulating transduced (GFP+) T cells. Intravenous anti-CD8β antibody was used to distinguish tissue parenchyma versus vascular-associated GFP+ T cells.
(D) Percentage of GFP+ cells which are CD103+, CD69+, and CXCR3+ (top). CD103 and CD69 expression in GFP+ CD8β− T cells and CXCR3 expression in GFP+ CD8β+ T cells in empty vector control (black) and T-bet overexpressing (gray filled) cells (bottom). Representative of three independent experiments with four or five mice per group.
(E) Representative histogram of CD103 expression in empty (black) or T-bet overexpressing (gray filled) cells upon skewing in absence or presence of TGF-β. Representative of three independent in vitro culture experiments.
(F) Representative histogram of pSmad2/3 expression in empty (black) or T-bet overexpressing (gray filled) cells following 1 hr culture in the absence or presence of TGF-β. Representative of three independent in vitro culture experiments.
(G) Determination of the ability of T-bet to bind to Itgae locus in CD8+ T cells at a site in intron 1 in which T-bet is known to bind in CD4+ T cells. P14 GP33+ T cells were isolated at day 8 following LCMV Armstrong infection and ChIP PCR was performed to assess T-bet enrichment in intron 1 relative to an isotype control. Binding of T-bet to the CD127 3′ UTR and binding of T-bet in intron 1 of the Itgae locus in T-bet−/− P14 GP33+ T cells were used as negative controls. The red text indicates a putative Smad3 binding site and the green text indicates a putative T-bet binding site. Representative of three independent experiments with five to ten pooled mice per group. See also Figure S4.
T-bet Overexpression Impairs Trm Formation through Modulation of TGF-β Responsiveness
To investigate the importance of T-bet in Trm formation, we next evaluated the formation of CD103+ Trm cells following overexpression of this transcription factor. P14+ cells were activated in vitro, transduced with a T-bet-expressing or an empty retroviral vector, and transferred into mice infected 1 day prior with X31-GP33. The recipient mice were allowed to rest until a memory time point (day 30) when they were intravascularly labeled with CD8β mAb and sacrificed. T-bet overexpression caused a significant increase in the percentage of circulating (CD8β+) GP33-specific cells in the vasculature, accompanied by a corresponding decrease in the percentage of CD8β− Trm cells (Figure 6C). We also observed an impairment in the ability of GP33-specific CD8 T cells to upregulate CD103 upon T-bet overexpression, even in the CD8β− cell population (Figure 6D, lower panels). Furthermore, CXCR3 expression, especially in the Tcirc (CD8β+) memory CD8+ T cells, was significantly reduced by T-bet overexpression, in line with previous findings (Slütter et al., 2013a) (Figure 6D, lower panels). Similar to our observation in unhelped memory CD8+ T cells (Figure 2D), T-bet overexpression had only a minimal effect on CD69 expression. Thus, T-bet appears to play a critical role in regulating the ability of CD8+ T cells to upregulate CD103 and become resident in the lung.
We next tested whether T-bet-mediated repression of CD103 was due to its effects on the migration of CD8+ T cells, and hence their exposure to the surrounding inflammatory milieu, or whether T-bet could directly inhibit CD103 upregulation in response to TGF-β signaling. To examine this question, we transduced activated P14+ cells with a T-bet-expressing or empty control retroviral vector, and then cultured with TGF-β or left untreated. Relative to the cells transduced with the empty vector, those overexpressing T-bet displayed a modest reduction in CD103 expression even without the addition of TGF-β (Figure 6E, left plots). TGF-β induced CD103 upregulation in the empty vector-transduced P14+ CD8 T cells, in a Smad3-dependent manner (Mokrani et al., 2014, data not shown); however, this upregulation was abrogated in cells overexpressing T-bet (Figure 6E, right plots). T-bet overexpressing cells displayed no defect in pSmad2 and pSmad3 induction following TGF-β stimulation, indicating there was no effect on TGF-β receptor activation (Figure 6F). Together, these results indicated that T-bet repressed TGF-β-mediated induction of CD103 in antigen-specific CD8+ T cells.
T-bet can directly bind to the Itgae (CD103) locus in the first intron in CD4+ T cells (Nakayamada et al., 2011). Therefore, we assessed whether it can also bind to Itgae in virus-specific CD8+ T cells by isolating P14+ effectors from day 8 after LCMV-Armstrong infection, which induces a large quantity of virus-specific CD8+ T cells with robust T-bet expression (Joshi et al., 2007). Chromatin immunoprecipitation (ChIP) using anti-bodies to T-bet was performed on these cells followed by qPCR. This demonstrated enrichment in T-bet binding in the first intron of Itgae, at the same site bound in CD4+ T cells (Figure 6G). Controls showed no enrichment for T-bet binding at this site in T-bet-deficient P14 effector cells or at the 3′ untranslated region (UTR) of Il7ra, a site at which this factor does not bind (Figure 6G). These data indicated that T-bet could bind to Itgae in virus-specific CD8+ T cells and intriguingly, computational analysis indicated that there is a putative Smad3 binding site overlapping the T-bet binding site. Together with the T-bet overexpression data in Figure 6E, this suggests that T-bet likely interferes with pSmad2 and pSmad3 transcriptional activation of Itgae down-stream of TGF-β signaling, possibly through direct competition for binding.
Because TGF-β is capable of suppressing T-bet expression in CD4+ T cells (Gorelik et al., 2002), it is possible that this also occurs in CD8+ T cells. To test this hypothesis, P14+ CD8+ T cells lacking TGF-β receptor II (TGFβRII) were generated by inter-crossing Tgfbr2f/f Lck-Cre and P14 mice. Then P14+ Tgfbr2f/f Lck-Cre+ or Tgfbr2f/f Lck-Cre− (littermate controls) CD8+ T cells were transferred to mice 1 day prior to infection with X31-GP33. At day 15 p.i., these mice were intravascularly injected with CD8β-PE mAb and the CD8 T cells were analyzed for CD103 and T-bet expression. As expected, Tgfbr2f/f Lck-Cre+ P14+ cells were unable to express CD103 following infection and displayed a profound reduction in the percentage of Trm cells (Figures S4B and S4C). Additionally, the Tgfbr2f/f Lck-Cre+ P14+ cells displayed a significant increase in T-bet expression (Figures S4D and S4E). These data demonstrate a dual function for TGF-β in both inducing CD103 and suppressing T-bet expression in virus-specific CD8+ T cells.
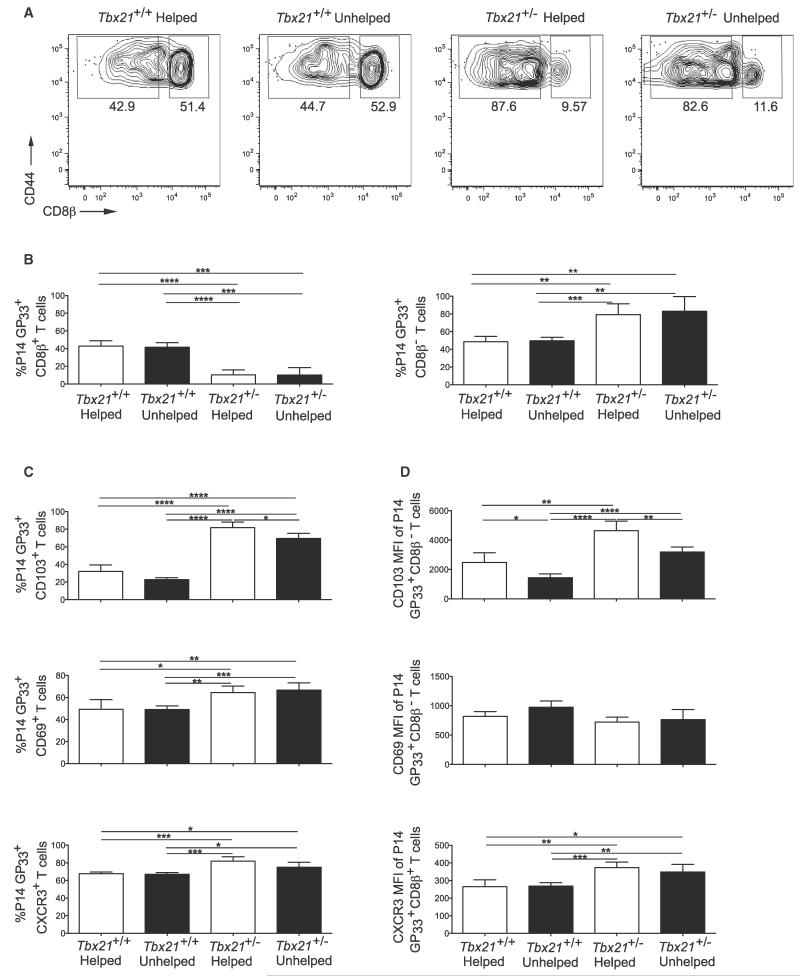
Genetic Reduction of T-bet Is Sufficient to Rescue CD103 Expression in Unhelped Trm Cells
Given that unhelped CD8+ Trm cells expressed more T-bet than helped cells and that its overexpression could repress that of CD103 downstream of TGF-β, we next determined whether genetic reduction of T-bet expression could restore CD103 expression in unhelped Trm cells. Tbx21+/+ or Tbx21+/− P14+ CD8+ T cells were transferred into mice that were treated with a CD4-depleting mAb or PBS 1 day prior to infection with X31-GP33. At a memory time point (day 40 p.i.), the mice were intravascularly injected with CD8β mAb and sacrificed. This showed that reducing T-bet by one-half significantly decreased the percentage of CD8β+ circulating cells and, conversely, increased the percentage of CD8β− Trm cells in both the helped and unhelped Tbx21+/− P14 GP33-specific CD8 T populations (Figures 7A and 7B). Furthermore, there was a marked increase in CD103 expression in both the helped and unhelped Tbx21+/− GP33-specific CD8 Trm cells with CD103 expression on unhelped Tbx21+/− Trm cells being restored to expression comparable to wild-type Trm cells (Figures 7C and 7D). The Tbx21+/− P14+ CD8+ T cells also displayed a modest but significant increase in the percentage of CD69+ and CXCR3+ cells (Figure 7C). These results showed that enhanced T-bet expression in the unhelped Trm cells contributes to their impaired CD103 expression, and likely airway localization. They also indicated that T-bet acts like a rheostat to control the differentiation of resident or circulating memory CD8 T cells following respiratory viral infection.
Figure 7. Genetic Modulation of T-bet Rescues the Ability of Unhelped Trm Cells to Upregulate CD103.
50,000 Tbx21+/+ and Tbx21+/− P14 GP33+ T cells were transferred to mice treated with PBS (helped) or GK1.5 (unhelped) 1 day prior to X31-GP33 infection. Mice were sacrificed at day 40 postinfection.
(A) Representative plot of the percentage of resident versus circulating helped and unhelped Tbx21+/+ and Tbx21+/− P14 GP33+ cells. Intravenous anti-CD8β antibody was used to distinguish tissue parenchyma versus vascular-associated GFP+ T cells.
(B) The percentage of resident versus circulating helped and unhelped Tbx21+/+ and Tbx21+/− P14 GP33+ cells.
(C) Percentage of P14 GP33+ cells that are CD103+, CD69+, and CXCR3+.
(D) MFI of CD103 and CD69 expression in P14 GP33+ CD8β− T cells and CXCR3 MFI in GP33+ CD8β+ T cells. Representative of three independent experiments with four or five mice per group carried out 30–60 days following X31-GP33 infection.
DISCUSSION
It is becoming increasingly appreciated that Trm cells are essential in providing protection against mucosal infection (Cauley and Lefrançois, 2013). Our study has demonstrated that CD4+ T cells play an important role in the formation of protective CD8+ Trm and Tcirc cells following influenza infection. In the absence of CD4+ T cell help, CD8+ Trm cells have reduced CD103 expression and are impaired in their ability to properly embed within the airways, with CD8+ Tcirc cells displaying reduced CXCR3 expression. Upon rechallenge, unhelped CD8+ T cells have an impaired ability to upregulate granzyme B and are compromised in their migration to the lung airways, due to defects in both the Trm and Tcirc populations, leading to suboptimal heterosubtypic protection. Our data suggest that CD4+ T cells act through an IFN-γ-dependent mechanism to elicit signals necessary for the migration and airway retention of CD103+ CD8+ T cells. These findings are consistent with previous reports regarding the role of CD4+ T cells in the female reproductive tract following HSV infection (Nakanishi et al., 2009), and together these studies illustrate a critical role for CD4+ T cells in directing effector CD8+ T cell migration into a tissue microenvironment where they are poised to mediate protection upon recall.
Our findings also reveal a role for T-bet in regulating the formation of CD8+ Trm cells. In the absence of CD4+ T cell help, we found that CD8+ T cells have enhanced T-bet expression, similar to what has been reported following LCMV Armstrong infection (Intlekofer et al., 2007). Our work suggests that graded expression of T-bet might act as a rheostat regulating Trm versus Tcirc cell development and is in agreement with the finding that Trm cells develop from KLRG1lo memory precursor cells (Mackay et al., 2013), whose formation is also promoted by low T-bet expression (Joshi et al., 2007). Our work also shows that CD103 expression is sensitive to the amount of T-bet protein or activity. T-bet directly binds to intron 1 in the Itgae (CD103) locus, which also contains a Smad3-binding site in virus-specific CD8+ T cells. Smad3 is required for TGF-β-mediated induction of CD103, suggesting potential mechanisms by which T-bet might repress Itgae transcription by competing with Smad3 binding, directly interacting with Smad3 to prevent its transcriptional activity, or through the recruitment of other transcriptional repressors to the locus. It will be imperative for future work to distinguish between these possibilities and examine whether T-bet controls Trm cell formation in other mucosal sites.
CD69 is necessary for Trm cells to reside in mucosal tissues (Lee et al., 2011; Mackay et al., 2013) including the lung, and as CD8+ T cells extravasate from blood to NLTs, downregulation of the transcription factor KLF2 and its target gene S1pr1 occurs, allowing for increased expression of CD69 (Casey et al., 2012; Lee et al., 2011; Skon et al., 2013). However, we found that T-bet plays a minimal role in regulating the expression of CD69. These data suggest a two-step model for Trm cell formation in which the downregulation of KLF2 and T-bet represent distinct stages in the developmental pathway. Downregulation of KLF2 through a PI(3)K and Akt kinase-dependent process might be required for the initial migration of activated CD8+ T cells into the mucosal site. Subsequent exposure to pro- and anti-inflammatory signals within the tissue might then fine-tune T-bet expression, with TGF-β inducing CD103 within the T-betlo CD8+ T cells and anchoring them in the tissue.
Following influenza infection, both resident and circulating memory CD8+ T cells have been suggested to be essential for early viral control upon rechallenge (Slütter et al., 2013a; Wu et al., 2014). Memory CD4+ Trm and Tcirc cells are also capable of mediating heterosubtypic protection (McKinstry et al., 2012; Teijaro et al., 2011). Our work extends these findings to show that CD4+ T cell help is critical for the formation of protective influenza-specific CD8+ Trm and TcircC cells. Trm cells guard the site of infection through direct effector function as well as through the induction of local chemokines that allow for the recruitment of Tcirc cells (Schenkel et al., 2013; Wu et al., 2014). Tcirc cells are important upon recall in allowing for early control of virus in the airways with high expression of CXCR3 important for entry into the airways (Kohlmeier et al., 2008; Slütter et al., 2013a). The respective contributions of these populations to protection upon rechallenge is currently unclear and it will be imperative for future work to parse apart the relative importance of these subsets, as well as whether CD4+ T cell help acts disproportionately on one of these populations to allow for optimal heterosubtypic immunity. Neutralizing antibodies targeting conserved regions of the influenza virus also can contribute to heterosubtypic protection following influenza virus rechallenge (Wrammert et al., 2011). Influenza-specific CD8+ T cells and cross-reactive antibodies cooperate to facilitate superior heterosubtypic protection, with CD4+ T cells also mediating improved protection in the presence of CD8+ T cells and/or B cells (Carragher et al., 2008; Laidlaw et al., 2013; McKinstry et al., 2012). Together, these findings suggest that simultaneously priming of multiple arms of the adaptive immune response against the influenza virus is a potent approach toward the generation of heterosubtypic immunity. The currently available live attenuated intranasal influenza vaccine Flumist® is capable of generating both an influenza-specific CD8+ and CD4+ T cell response with its protective effect enhanced by rapid boosting of the CD8+ T cell response (Slütter et al., 2013b; Sun et al., 2011). It is unclear whether Trm cells are induced by Flumist® vaccination or whether T cells so induced are capable of persisting long-term in the lung. Flumist® also does not appear to induce cross-reactive antibodies (Sun et al., 2011). Despite these limitations, it represents a promising platform to build upon in order to reach the eventual goal of designing a universal influenza vaccine that induces both Trm cells and humoral immunity in the respiratory mucosa. Elucidation of the signals that promote Trm cell formation, such as those described here and by others, will aide in the design of a potentially more efficacious vaccine for influenza.
EXPERIMENTAL PROCEDURES
See the Supplemental Information for details.
Mice
C57BL/6 mice were purchased from the National Cancer Institute or The Jackson Laboratory. Age- and sex-matched anti-HEL B cell receptor (BCR)-transgenic C57BL/6-TgN (IghelMD4) mice (referred to as MD4) and IFN-γ−/− mice were obtained from The Jackson Laboratory. CD4−/− mice were generated within our own colony. Thy1.1+ P14 CD8+ TCR transgenic and Smarta CD4+ TCR transgenic (Stg) mice have been described previously (Joshi et al., 2007). Tbx21−/− mice were obtained from L. Glimcher (Weill Cornell Medical College) and crossed to P14 transgenic mice. TGFβRIIf/f Lck-Cre mice were a gift from M. Bevan (University of Washington). To generate mice bearing LCMV-specific epitopes, we transferred splenocytes from P14-donor mice normalized for 50,000 naive P14 CD8+ T cells (with expected engraftment of 10%; thus, 5,000 cells) into B6 mice by intravenous (i.v.) injection. For experiments analyzing CD8+ T cells at early time points (days 4–6) p.i., 1 × 106 naive Thy1.1+ P14 CD8+ TCR transgenic T cells were transferred into B6 mice by i.v. injection. To assess the virus-specific CD4+ T cell response at early time points post infection, 1 × 106 naive Thy1.1+ Smarta CD4+ TCR transgenic T cells were similarly transferred. All animal experiments were done with approval of the Yale Institutional Animal Care and Use Committee.
Infections
For primary infections, mice were inoculated intransally (i.n.) with 0.8 × 105 TCID50 recombinant X31 influenza virus expressing the LCMV GP33–41 epitope (X31-GP33), 50 TCID50 recombinant WSN influenza virus expressing the LCMV GP33–41 and GP67–77 epitopes (WSN-GP33/67), or 2 × 105 pfu LCMV Armstrong administered intraperitoneally (i.p.). For rechallenge experiments, mice were given 1,500 TCID50 recombinant PR8 influenza virus expressing the LCMV GP33 epitope (PR8–GP33) administered i.n. Prior to i.n. infections, mice were anesthetized by i.p. injection of ketamine hydrochloride and xylazine (Phoenix Scientific) in 0.2 ml Life Technologies HBSS (Invitrogen). Recombinant influenza strains containing the LCMV GP33–41 epitope inserted in the neuraminidase stalk region were obtained from Dr. Richard J. Webby (St. Jude Children’s Research Hospital) and have been previously described (Laidlaw et al., 2013). Recombinant influenza containing the LCMV GP33–41 and GP67–77 epitopes inserted in the neuraminidase stalk region were obtained from Dr. Michael Oldstone (Sripps Research Institute) and have been previously described (Marsolais et al., 2009). These viruses were propagated in eggs and stored at −80 °C. The replication and pathogenicity of these recombinant X31 and PR8 strains were not substantially different from their nonrecombinant counterparts (data not shown). Viral titers were determined by plaque assay on Vero cell monolayers (for LCMV) or on Madin-Darby canine kidney cell monolayers (for X31-GP33 and PR8-GP33).
CD4+ T Cell Depletion and Intravascular CD8+ T Cell Staining
For systemic depletion of CD4+ T cells, mice were treated i.p. with rat mAb GK1.5 (0.25mg/injection) at day −1 and 0 prior to infection unless otherwise indicated. Depletion was verified by flow cytometric analysis. For intravascular staining of CD8+ T cells, mice were injected with 3 μg of CD8β-PE antibody (BioLegend) diluted in 300 μl of sterile DPBS (Life Technologies) as previously described (Anderson et al., 2012). Mice were the sacrificed 5 min after injection.
Statistical Analysis
Results represent the mean ± SEM unless indicated otherwise. Statistical significance was determined by the paired or unpaired Student’s t test. Statistical analyses were performed with Prism GraphPad software v6.0 (*p < 0.05; **p < 0.01; ***p < 0.001).
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge all members of the Kaech and Craft laboratories for helpful discussions and critical reading of the manuscript. This work was supported by grants from the NIH (RO1AI066232 [S.M.K.], R01AI074699 [S.M.K.], R01AR40072 [J.C.], P30AR053495 [J.C.], R21AR063942 [J.C.], T32AI07019 [B.J.L.]), F31AG07777 [B.J.L.], and the Howard Hughes Medical Institute [S.M.K., B.J.L.]).
Footnotes
Supplemental Information includes seven figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2014.09.007.
AUTHOR CONTRIBUTIONS
B.J.L., J.C., and S.M.K. conceived and designed the experiments. B.J.L., N.Z., H.D.M., M.M.S., T.G., Y.H., and L.S.C. performed the experiments. B.J.L., J.C., and S.M.K. analyzed the data. B.J.L., J.C., S.M.K. wrote the manuscript.
REFERENCES
- Anderson KG, Sung H, Skon CN, Lefrançois L, Deisinger A, Vezys V, Masopust D. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Tato A, León B, Lund FE, Randall TD. CD4+ T helper cells use CD154-CD40 interactions to counteract T reg cell-mediated suppression of CD8+ T cell responses to influenza. J. Exp. Med. 2013;210:1591–1601. doi: 10.1084/jem.20130097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J. Virol. 2002;76:12388–12393. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J. Immunol. 2008;181:4168–4176. doi: 10.4049/jimmunol.181.6.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- Cauley LS, Lefrançois L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol. 2013;6:14–23. doi: 10.1038/mi.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Estimates of deaths associated with seasonal influenza — United States, 1976-2007. MMWR Morb. Mortal. Wkly. Rep. 2010;59:1057–1062. [PubMed] [Google Scholar]
- Epstein SL, Price GE. Cross-protective immunity to influenza A viruses. Expert Rev. Vaccines. 2010;9:1325–1341. doi: 10.1586/erv.10.123. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and shortlived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreijtz JHCM, Bodewes R, van Amerongen G, Kuiken T, Fouchier RAM, Osterhaus ADME, Rimmelzwaan GF. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine. 2007;25:612–620. doi: 10.1016/j.vaccine.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Kumamoto Y, Mattei LM, Sellers S, Payne GW, Iwasaki A. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc. Natl. Acad. Sci. USA. 2011;108:8749–8754. doi: 10.1073/pnas.1100567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw BJ, Decman V, Ali M-AA, Abt MC, Wolf AI, Monticelli LA, Mozdzanowska K, Angelosanto JM, Artis D, Erikson J, Wherry EJ. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 2013;9:e1003207. doi: 10.1371/journal.ppat.1003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-T, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J. Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, Kawaoka Y, Rosen H, Oldstone MBA. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc. Natl. Acad. Sci. USA. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason DY, Jones M, Goodnow CC. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/IgD transgenic mice. Int. Immunol. 1992;4:163–175. doi: 10.1093/intimm/4.2.163. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J. Clin. Invest. 2012;122:2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrani M, Klibi J, Bluteau D, Bismuth G, Mami-Chouaib F. Smad and NFAT pathways cooperate to induce CD103 expression in human CD8 T lymphocytes. J. Immunol. 2014;192:2471–2479. doi: 10.4049/jimmunol.1302192. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun H-W, Vahedi G, Hakim O, Handon R, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011;35:919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet B, de Bree GJ, Smids-Dierdorp BS, van der Loos CM, Remmerswaal EBM, von der Thüsen JH, van Haarst JM, Eerenberg JP, ten Brinke A, van der Bij W, et al. CD8+ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J. Clin. Invest. 2011;121:2254–2263. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJC, Bickham KL, Lerner H, Goldstein M, Sykes M, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slütter B, Pewe LL, Kaech SM, Harty JT. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity. 2013a;39:939–948. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slütter B, Pewe LL, Lauer P, Harty JT. Cutting edge: rapid boosting of cross-reactive memory CD8 T cells broadens the protective capacity of the Flumist vaccine. J. Immunol. 2013b;190:3854–3858. doi: 10.4049/jimmunol.1202790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J. Immunol. 2011;186:987–993. doi: 10.4049/jimmunol.1002664. [DOI] [PubMed] [Google Scholar]
- Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C, Liu X, Wang S, Liu MA, Donnelly JJ, Caulfield MJ. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J. Virol. 1998;72:5648–5653. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, Bevan MJ. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 2012;189:3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Koutsonanos D, Li G-M, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hu Y, Lee Y-T, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. Transforming growth factor-b signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.