Abstract
The prefrontal cortex (PFC), especially the medial sector, plays a crucial role in emotional processing. Damage to this region results in impaired processing of emotional information, perhaps due to an inability to initiate and maintain attention toward emotional materials, a process that is normally automatic. Childhood onset damage to the PFC impairs emotional processing more than adult-onset PFC damage. The aim of this work was to study the involvement of the PFC in attention to emotional stimuli, and to explore how age at lesion onset affects this involvement. To address these issues, we studied both the emotional and attentional modulation of the startle reflex. Our sample was composed of 4 patients with childhood-onset PFC damage, 6 patients with adult-onset PFC damage, and 10 healthy comparison participants. Subjects viewed 54 affective pictures; acoustic startle probes were presented at 300 ms after picture onset in 18 pictures (as an index of attentional modulation) and at 3,800 ms after picture onset in 18 pictures (as an index of emotional modulation). Childhood-onset PFC patients did not show attentional or emotional modulation of the response, in contrast to adult-onset PFC damage and comparison participants. Early-onset damage to the PFC results, therefore, in more severe dysfunction in the processing of affective stimuli than adult-onset PFC damage, perhaps reflecting limited plasticity in the neural systems that support these processes.
Keywords: Emotion, Attention, Prefrontal cortex, Childhood-onset brain damage, Startle reflex, Prepulse inhibition
Introduction
The prefrontal cortex (PFC) is a component of a neural circuit involved in the processing of emotions, especially the medial and ventral sectors. The PFC sends projections to the medial temporal lobe, basal forebrain, hypothalamus, and brainstem regions involved both in the behavioural and autonomic components of the emotional response (Ongür & Price, 2000). The PFC in cooperation with other regions, such as the amygdala (e.g., Amaral, Price, Pitkanen, & Carmichel, 1992), prepares the organism for a quick response to significant aversive signals (Kawasaki et al., 2001; Mobbs et al., 2009). In addition, the ventromedial PFC exerts inhibitory control over emotional responses (Etkin, Egner, & Kalisch, 2011; Salzman & Fusi, 2010), uniquely situating this region to exert fine control over emotional expression and autonomic responses.
In light of this emotional regulation role, studies of patients with acquired brain lesions have shown that ventromedial PFC damage can produce a wide range of emotional and behavioural disturbances, including impulsivity, hypo- and hyper-emotionality, social disturbances, and poor decision making, in the context of relatively preserved intellectual functioning (Anderson, Barrash, Bechara, & Tranel, 2006; Barrash, Tranel, & Anderson, 2000; Eslinger & Damasio, 1985). In this regard, Damasio and colleagues have proposed that ventromedial PFC damage can disrupt the processing of somatic (or emotional) signals that guide advantageous decision making (Damasio, 1996; Damasio, Tranel, & Damasio, 1991). Consistent with this notion, patients with ventromedial PFC damage do not exhibit anticipatory skin conductance responses prior to making risky decisions and also show a lack of sensitivity to future consequences (Bechara, Tranel, & Damasio, 2000; Bechara, Tranel, Damasio, & Damasio, 1996), which may contribute to many of the disturbances in real-world functioning commonly observed in these individuals. An interesting set of findings have related the severity of these emotional impairments to the age of ventromedial PFC lesion onset, with childhood-onset lesions leading to more severe alterations than lesions with an adult onset (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Anderson, Damasio, Tranel, & Damasio, 2000; Anderson, Barrash, Bechara, & Tranel, 2006; Anderson, Wisnowski, Barrash, Damasio, & Tranel, 2009).
In addition to its role in regulating emotional information, the PFC also plays a role in directing and maintaining attention (see Fuster, 2008; Posner & Petersen, 1990). In concert with parietal regions, the PFC is active during a host of tasks requiring attentional demands, and this activity increases proportionally with attentional effort (Posner & Petersen, 1990). Affective stimuli are capable of eliciting an automatic and obligatory deployment of attentional resources, and the PFC may specifically be involved in this affective capture of attention (Kawasaki et al., 2001; Vuilleumier & Huang, 2009). Evidence from studies using functional magnetic resonance imaging (fMRI) and electrophysiological recordings suggests that emotional stimuli can modulate the allocation of attentional resources, and this effect may depend on the medial prefrontal cortex (Carretié, Hinojosa, Martin-Loeches, Mercado, & Tapia, 2004; Yamasaki, LaBar, & McCarthy, 2002).
A large body of research conducted in both animals and humans has established that the startle reflex can be used to objectively study attention and emotion (Bradley, Codispoti, & Lang, 2006). Attentional modulation of the reflex is reflected by prepulse inhibition (PPI), which is an attenuation of the startle reflex to the startle probe (e.g., an abrupt noise) when the probe is immediately preceded by a non-startling, lead stimulus (e.g., a pure tone). This effect is the result of a mechanism (sensorimotor gating) that protects the processing of the lead stimulus from disruption by a subsequent stimulus (Blumenthal, 1999; Graham, 1980; Graham & Hackley, 1991). In humans, when pictures are used as prepulse stimuli (presented before the startle probe), the PPI effect is larger (i.e., greater attenuation) for emotional pictures (pleasant and unpleasant) than for neutral pictures (Bradley, Cuthbert, & Lang, 1993; Bradley, Codispoti, & Lang, 2006). This effect reflects the greater resources necessary to process the emotionally salient picture contents compared to emotionally neutral stimuli, or capture of attention by emotion (Bradley et al., 1999; Vuilleumier & Huang, 2009).
In contrast, when startle stimuli are delivered at longer lead intervals during picture viewing, an emotional modulation of the startle reflex is observed (Lang, Bradley, & Cuthbert, 1990). This emotional modulation is achieved through the presentation of aversive or pleasant stimuli, which leads to an increase or decrease in the magnitude of the startle reflex, respectively (Lang, Bradley, & Cuthbert, 1990). Lang and collaborators (Lang, 1995; Lang et al., 1990, 1997) have proposed that the emotional modulation of the startle reflex is the result of motivational priming: the startle reflex is enhanced when the aversive motivational system is engaged by unpleasant pictures, and is attenuated when the appetitive system is engaged by pleasant pictures (Lang, 1995; Lang et al., 1990). Previous work using this paradigm in humans has found a deficit in this emotional modulation after lesions involving either the amygdala (e.g., Buchanan, Tranel, & Adolphs, 2004) or frontal cortex (Sanchez-Navarro, Martinez-Selva, & Roman, 2005).
Several lines of research have also attempted to identify the neural mechanisms supporting the attentional modification of the startle reflex. Studies conducted in rats have found regulatory effects of the prefrontal cortex on sensorimotor gating (e.g., Bubser & Koch, 1994; Koch & Bubser, 1994; Zavitsanou, Cranney, & Richardson, 1999). Particularly, Swerdlow, Geyer, & Braff (2001) have pointed out the participation of the medial prefrontal cortex in PPI regulation. Functional neuroimaging studies suggest the involvement of the human PFC in the prepulse inhibition effect, consistent with previous results in rats (Campbell et al., 2007; Hazlett et al., 1998; Neuner et al., 2010). These findings, along with reported deficits in attention following PFC damage (Stuss & Benson, 1986) and prefrontal activity during the performance of tasks requiring attention (Fuster, 2008), suggest that the PFC is necessary for effective regulation of attention. In spite of these findings, the effects of prefrontal cortex lesions on PPI have not been addressed in humans. In addition, little is known about the dependence of these deficits on the maturation level of the PFC. Indeed, this latter question is a key one as early brain damage involves a disruption of the maturation process of the brain, which could lead to a greater impact on brain function compared to lesions occurring after the brain has normally developed. Clinical observations, for example, have often noted that PFC damage occurring early in life leads to a persistent pattern of deficits, perhaps due to the alteration of the normal development of PFC functions and connectivity (e.g., Anderson et al., 1999). Particularly severe disruption of emotional processing has been observed in individuals with early onset damage to PFC (Boes et al., 2011). Thus the current study addresses a key question that presents a challenge to the neuroscientific dogma on brain plasticity and recovery of function demonstrated in many other neurological systems.
This research was aimed at clarifying the effects of childhood-onset PFC damage on both attentional and emotional processing, as compared to PFC lesions acquired in adulthood. For this purpose we used an acoustic startle modification paradigm including prepulse inhibition as well as emotion-modulated startle. As noted above, much previous research has focused specifically on the role of the ventromedial sectors of the PFC in emotional processing. The current research expands this focus beyond the ventromedial PFC in our childhood-onset group for the following reasons: 1) the social-emotional deficits caused by childhood-onset lesions to any neural region are not well understood, 2) it is possible that damage to the PFC, broadly construed, could exert effects in childhood-onset cases that are not seen in adult-onset cases due to interrupted connectivity in the younger cases. To address the goals of the study, we will compare performance of a group of patients with childhood-onset damage to the PFC, broadly construed, to a group of patients with adult-onset PFC damage. Following previous findings that have revealed greater deficits following childhood-onset ventromedial PFC damage (e.g., Anderson et al., 2006; Boes et al., 2011), we expected that patients with childhood-onset damage to the PFC would show reduced allocation of processing resources to affective stimuli, in the form of an absence of attenuation of the startle reflex. Further, we expected that PFC patients would show a diminished emotional modulation effect, particularly in those who have suffered PFC damage during childhood, since previous studies have found altered emotion-related behaviours in these patients (e.g., Anderson et al., 1999, 2006, 2009).
Method
Subjects
Ten adults with unilateral or bilateral focal, stable lesions were selected from the Patient Registry of the Division of Cognitive Neuroscience at the University of Iowa. Demographic, neuroanatomical, and neuropsychological characteristics are summarized for each participant group in Table 1. All lesions in these patients involved the PFC and were acquired either during childhood (n = 4) or adulthood (n = 6). Lesions included portions of the ventromedial PFC, as well as lateral orbital gyri, superior medial prefrontal cortex and superior, middle, and inferior frontal gyri. Lesion etiologies comprised vascular (n = 4), resection of a benign tumor or cyst (n = 5), and focal trauma (n = 1). All patients had no premorbid history of psychiatric disorder and no neurological disease unrelated to the focal lesion.
Table 1.
Demographic, Neuroanatomical, and Neuropsychological Characteristics.
| PFC- Adult (n = 6) |
PFC- Childhood (n = 4) |
Comparison group (n = 10) |
|
|---|---|---|---|
| Demographics | |||
| Age | 58.3 ± 7.1 | 28.3 ± 6.2 | 39.5 ± 12.8 |
| Sex | 4 F, 2 M | 3 F, 1 M | 2 F, 8 M |
| Education | 14.0 ± 1.8 | 12.0 ± 0.0 | 16.4 ± 1.8 |
| Neuroanatomical Factors | |||
| Lesion Laterality | 4 B, 0 L, 2 R | 1 B, 2 L, 1 R | - |
| Lesion Volume (mm3) | 48858 ± 25193 | 66368 ± 48802 | |
| Age of Lesion Onset | 49.2 ± 5.9 (range: 43–58) | 1.8 ± 2.8 (range: 0–6) | - |
| Chronicity (years) | 9.2 ± 6.2 (range: 3–21) | 26.5 ± 9.0 (range: 14–35) | - |
| Neuropsychological Data | |||
| WAIS Verbal IQ | 105.5 ± 11.6 | 102.3 ± 12.4 | - |
| WAIS Performance IQ | 102.0 ± 13.3 | 96.5 ± 13.5 | - |
Means and standard deviations are reported. WAIS (Wechsler Adult Intelligence Scale), F (Female), M (Male), B (Bilateral), L (Left), R (Right).
Visualization of PFC lesions within a common anatomical space was performed using the MAP-3 technique implemented with Brainvox software (Frank, Damasio, & Grabowski, 1997; see Figure 1). Briefly, this technique involves: (1) viewing MR/CT slices of the lesioned brain and a reference brain reconstructed in three dimensions, (2) identifying and marking major sulci in both the lesioned brain and the reference brain, (3) adjusting the orientation and slicing of the reference brain to approximate the orientation and slicing of the lesioned brain, and (4) manually tracing the contours of the lesion onto each corresponding slice of the reference brain while taking into account anatomical landmarks including gray and white matter components of the lesion. Using this approach, we calculated the total lesion volume (in mm3) as well as the specific brain areas damaged for each patient (see Table 2). Detailed neuroanatomical data could not be obtained for one patient with childhood-onset PFC damage due to difficulties in clearly delineating the boundaries of the lesion.
Figure 1.
Mesial and lateral views of the lesion overlap from childhood-onset PFC damage (A) and adult-onset PFC damage (B). Areas where lesions from multiple brains overlap are reflected by more intense red color, whereas areas where lesions are from a few or a single brain are reflected by more intense blue color.
Table 2.
Total Lesion Volume (in mm3) and Regions Damaged for Each Patient.
| PFC-Adult Patients |
PFC-Childhood Patients |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0770 | 2025 | 2352 | 2391 | 2577 | 3032 | 2517 | 2748 | 2837 | |
| Lesion Volume | 33190 | 40575 | 13632 | 71033 | 52494 | 82226 | 28583 | 49054 | 121466 |
| Region | |||||||||
| alSFG | B | B | B | B | L | L | B | ||
| amSFG | B | B | B | B | L | L | B | ||
| plSFG | R | ||||||||
| vmSFG | B | R | B | B | B | B | L | L | B |
| MFGa | B | R | B | B | R | L | L | B | |
| MFGp | L | B | |||||||
| IFGpo | R | ||||||||
| IFGpt | L | B | |||||||
| IFGporb | R | R | B | R | R | L | R | ||
| lOrbG | L | B | B | R | B | L | B | ||
| mOrbG | B | B | B | B | B | B | L | L | B |
| Grec | B | B | B | B | B | B | L | L | R |
| FP | B | R | R | B | B | B | L | B | |
| Cing_sg | R | B | B | B | B | L | B | ||
| Cing_ant | L | B | R | B | L | L | B | ||
| Cing_post | R | ||||||||
| tpol | R | ||||||||
| SPL | R | ||||||||
| preCun | R | ||||||||
| asIns | L | R | |||||||
| Thalamus | R | ||||||||
| Caudate | L | R | |||||||
| Putamen | L | ||||||||
| NA | L | L | R | R | |||||
B (Bilateral), L (Left), R (Right), alSFG (anterior lateral superior frontal gyrus), amSFG (anterior medial superior frontal gyrus), plSFG (posterior lateral superior frontal gyrus), vmSFG (ventral medial superior frontal gyrus), MFGa (anterior middle frontal gyrus), MFGp (posterior middle frontal gyrus), IFGpo (inferior frontal gyrus pars opercularis), IFGpt (inferior frontal gyrus pars triangularis), IFGporb (inferior frontal gyrus pars orbitalis), lOrbG (lateral orbital gyrus), mOrbG (medial orbital gyrus), Grec (gyrus rectus), FP (frontal pole), Cing_sg (subgenual cingulate), Cing_ant (anterior cingulate), Cing_post (posterior cingulate), tpol (temporal pole), SPL (superior parietal lobule), preCun (precuneus), asIns (anterior short insula), NA (nucleus accumbens).
In addition, 10 healthy normal comparison participants with no known neurological or psychiatric history were recruited for the study to demonstrate the typical pattern of emotion and attentional modulation of the startle reflex and to serve as a contrast to potential lesion and age-of-lesion-onset differences in startle reactivity in the lesion groups. All participants provided informed consent in accordance with the Human Subjects Committee at the University of Iowa prior to their participation in this research.
Experimental stimuli
We selected 54 color pictures1 from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008). According to the normative ratings 18 pictures were unpleasant (e.g., burn victims, scenes of violence; valence Mean = 2.08, SD = .41), 18 were pleasant (e.g., romantic couples, sports; valence Mean = 7.40, SD = .37), and 18 were neutral (e.g., household objects, buildings; valence Mean = 4.84, SD = .23). Pleasant and unpleasant pictures did not differ in arousal level (Mean = 6.47, SD = .34, and Mean = 6.65, SD = .41, respectively), and neutral pictures were low in arousal (Mean = 2.49, SD = .38).
The pictures were arranged in six blocks of nine pictures per block, in such a way that three pictures of each affective valence category occurred in each block. We constructed three orders of picture presentation and randomly assigned subjects to one of the three orders.
Each picture was presented for 6 s (with random intertrial intervals of 20–30 s), and picture offset was a blank screen. Startle probes were presented during two out of three pictures of each affective category per block, at 300 ms (PPI trials) or 3,800 ms (emotional modulation trials) after picture onset. To ensure unpredictability, we delivered two startle probes per block during the intertrial intervals. Pictures were presented to the subjects on a 19-inch computer monitor.
The acoustic startle stimulus was a 50-ms, 105-dB(A) burst of white noise (20 Hz–20 KHz) with instantaneous rise- and fall-time, generated by computer and presented binaurally through Sennheiser headphones (Model HDA 200). The intensity of the acoustic startle stimulus was calibrated using a sound level meter.
Physiological Data Collection
Acquisition, amplification, and filtering of the physiological signals were conducted with a Biopac MP150 system (Biopac Systems, Inc., Goleta, CA). This system converted analog signals to digital by means of a 16-bit A/D converter. Acquisition system control, recording parameters, and data storage were performed with AcqKnowledge software (Biopac Systems, Inc., Goleta, CA).
Startle blink reflex was measured by recording electromyographic (EMG) activity from the orbicularis oculi muscle beneath the left eye, through bipolar placement of 4-mm Ag/AgCl surface electrodes (Fridlund & Cacioppo, 1986). The raw EMG signal was amplified, and frequencies below 28 Hz and above 500 Hz were filtered out (Blumenthal et al., 2005).
Procedure
All sensors were attached once subjects were accommodated in a comfortable chair, located 0.75 m in front of a computer monitor. After sensor attachment, subjects were instructed that a series of pictures would be displayed on the screen and that they should pay attention to each picture for the entire time it was on the monitor. Subjects were also instructed that at times a brief noise would be heard over the headphones and that they should ignore it. Before the experimental task, subjects were exposed to four example trials, using four startle stimuli delivered during the presentation of four neutral pictures. After psychophysiological recording, electrodes were removed and subjects were asked to view the pictures again for as long as they liked, and to rate affective valence and arousal of each picture, using the Self-Assessment Manikin (pencil and paper version; Hodes, Cook, & Lang, 1985). This instrument is a 9-point rating scale for each dimension, such that 9 represents a high rating (i.e., high pleasure, high arousal), and 1 represents a low rating (i.e., low pleasure, low arousal). Participants were instructed that once they pushed a keyboard button, a picture would appear on the screen and that when they pushed it again, the picture would disappear, leaving a blank screen. They would then rate the picture. For each subject, the presentation order of the pictures was the same as in the psychophysiological session.
Data Reduction
The EMG signal was reduced offline. Peak detection and analysis were performed using the A.D. Instruments Chart v6.1 software. The raw signal was full-wave rectified and integrated offline with a time constant of 10 ms (Blumenthal, 1994). Startle blink magnitude was scored as the difference (in microvolts) between the peak appearing within 21–150 ms following startle probe onset, and the mean EMG activity (baseline) recorded within 1–20 ms after startle probe onset (Blumenthal et al., 2005). To decrease variability and to establish a common metric across subjects, the raw blink magnitudes elicited during the picture viewing trials were z-score standardized and expressed as T scores (Mean = 50, SD = 10) individually for each subject. For statistical analyses, magnitude was computed as zero in those trials in which no response was detected.
Data Analysis
In order to ensure that subjects showed the same probability of response, we studied the percentage of startle responses between groups using an analysis of variance (ANOVA). We also compared the response magnitude in absence of pictures between groups to ensure that the three groups did not differ in the non-modulated startle response.
Since previous research has revealed possible differences in startle reflex related to age (e.g., Ellwanger, Geyer, & Braff, 2003) startle reflex data were analyzed by a 3 (Group: healthy comparison subjects, adult-onset PFC damage, and childhood-onset PFC damage) × 2 (Zone: PPI vs. Affect) × 3 (Picture category: unpleasant, neutral, and pleasant), mixed model ANCOVA with Group as a between-subjects factor, Zone and Picture category as within-subjects factors, and Age as a covariate.
The analyses of subjective ratings (affective valence and arousal) were conducted using 3 (Group) × 3 (Picture category) mixed model ANOVAs separately for valence and arousal, with Group as a between-subjects factor and Picture category as a within-subjects factor.
The statistical analyses were performed with the PASW package (version 19; Chicago, IL). For the main statistical tests, we also report a measure of effect size, partial eta-squared (η2). When appropriate, a Huynh-Feldt adjustment to the degrees of freedom was used in repeated measures tests in order to correct any potential inflation of the reported probability values, and epsilon values (ɛ) are reported. Paired comparisons were performed with a Bonferroni correction to control the overall level of significance (Keselman, 1998).
Results
Demographics and Neuroanatomical Data
The average age of the groups differed significantly, F(2,17) = 11.09, p = .001. Paired comparisons showed that adult-onset patients were older than both childhood-onset patients (p = .001) and healthy controls (p = .008). We did not find differences in gender distribution between groups, χ2 (2) = 5.12, p = 0.077.
Preliminary analyses conducted on lesion laterality did not reveal significant differences in startle magnitude between subjects with bilateral or unilateral brain damage. Hence, according to the aim of this research, we collapsed across the variable of lesion laterality and focused our analyses on groups determined by age of lesion onset. In addition, there was no difference in total lesion volume between the childhood-onset and adult-onset PFC groups (Mann-Whitney U = 8, p = .91).
Startle Reflex
The groups did not differ in the probability of showing a startle reaction to the startling noise stimuli, F(2,17) = .135, p = .87 (Mean proportion of startle stimuli that resulted in a measurable startle response across participant groups: Comparisons: Mean = .82, SD = .27; PFC-adult: Mean = .85, SD = .23; PFC-childhood: Mean = .90, SD = .12).
Similarly, the three groups did not differ in the startle magnitude obtained in absence of pictures, F(2,17) = 1.84, p = .19 (Comparisons: Mean = 50.24, SD = 3.32; PFC-adult: Mean = 47.28, SD = 2.79; PFC-childhood: Mean = 48.41, SD = 2.68).
The analysis of the startle reflex depending on the Zone and Picture category revealed a significant Zone × Picture category interaction, F(2,32) = 3.30, p = .050, η2 = .171, indicating that the pattern of startle reflex across picture categories was different in the PPI zone and the Affect zone. However, this result was qualified by a near significant Group × Zone × Picture category interaction, F(4,32) = 2.64, p = .052, η2 = .248, significant linear contrast, F(2,16) = 4.63, p = .026, η2 = .366. Since Age did not yield any statistical effect, for each group we conducted separate 2 (Zone) × 3 (Picture category) ANOVAs.
In the healthy comparison group, a significant Zone effect was found, F(1,9) = 12.78, p = .006, η2 = .587, indicating that startle magnitude was greater in the Affect trials than in the PPI trials (see Figure 2). This effect was qualified by a significant Zone × Picture category interaction, F(2,18) = 6.34, p = .008, η2 = .413. Analyses conducted in the PPI zone revealed a significant quadratic trend, F(1,9) = 10.84, p = .009, η2 = .546, indicating that startle magnitudes were lower while viewing affective pictures in comparison to startle magnitudes while viewing neutral pictures (see Figure 3). In the Affect zone we found a significant effect of Picture category, F(2,18) = 6.24, p = .009, η2 = .409. Startle reflex varied depending on the affective valence of the pictures, significant linear trend, F(1,9) = 22.45, p = .001, η2 = .714, with greater startle magnitudes during unpleasant pictures than during pleasant pictures (p = .003).
Figure 2.
Startle magnitudes for each individual adult onset (A1-A6) and childhood onset (CH1-CH4) PFC patient, as well as mean of the startle magnitude (error bars show standard error of the mean) for each group in the PPI zone and Affect zone. Startle magnitude was greater in the Affect zone than in the PPI zone in both the comparison group and the adult-onset damage group, but not in the childhood-onset damage group.
Figure 3.
Startle magnitudes for each individual adult onset (A1-A6) and childhood onset (CH1-CH4) PFC patient, as well as mean startle magnitude in the PPI zone as a function of the picture type (error bars show standard error of the mean). Only the comparison group showed greater startle magnitudes while viewing neutral pictures in comparison to the affective pictures (unpleasant and pleasant).
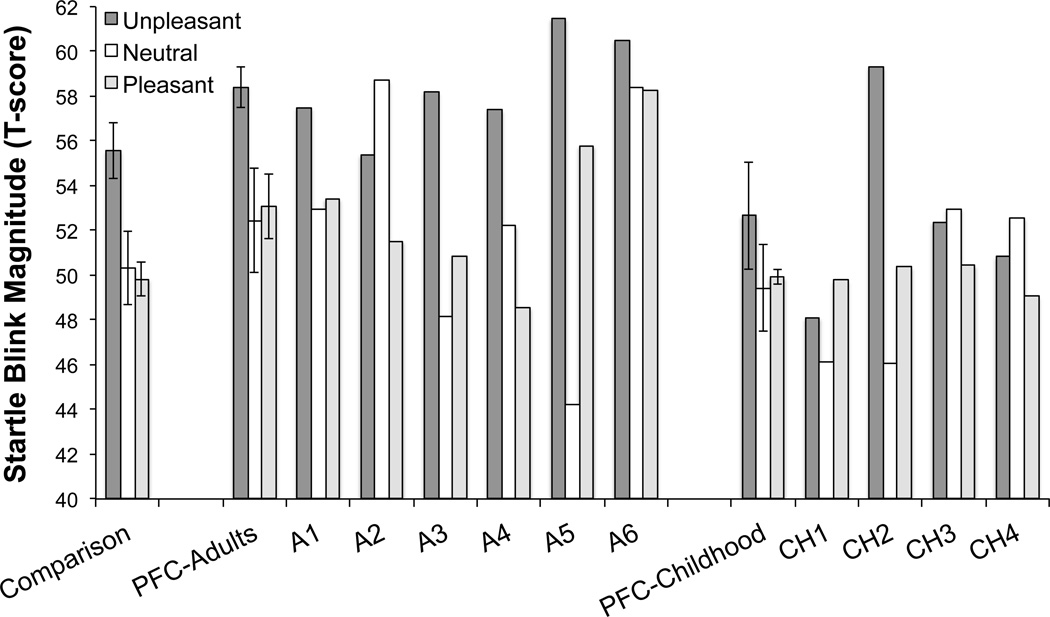
In the adult-onset PFC damage group, a significant main effect of Zone was found on startle magnitude, F(1,5) = 18.39, p = .008, η2 = .786, due to greater startle magnitudes in the Affect zone than in the PPI zone (see Figure 2). Although the Zone × Picture category interaction did not reach statistical significance, F(2,10) = 2.38, p = .14, η2 = .322, this result might be due to the small simple size, since pairwise comparisons revealed large effect sizes in the response difference between picture categories, especially in the Affect trials. We conducted additional separate analyses for each Zone (PPI and Affect). In the PPI zone, we did not find any effect of the Picture category on startle magnitude, F(2,10) = .023, p = .98, η2 = .005 (see Figure 3). In the Affect zone, however, a marginal effect was found for Picture category, F(2,10) = 4.04, p = .052, η2 = .447, significant linear trend, F(1,5) = 28.9, p = .003, η2 = .853 (see Figure 4). Pairwise comparisons revealed greater startle magnitudes while viewing unpleasant pictures compared to pleasant pictures (p = .009).
Figure 4.
Startle magnitudes for each individual adult onset (A1-A6) and childhood onset (CH1-CH4) PFC patient, as well as mean (and standard error of the mean) startle reactivity in the Affect zone as a function of the Picture category. Startle magnitudes during unpleasant pictures were greater than during pleasant pictures in the comparison group and adult-PFC damage group, but not in the childhood-onset group.
In the case of the childhood-onset PFC damage group, we did not find any effect of Zone, F(1,3) = .060, p = .82, η2 = .020, Picture category, F(2,6) = 1.94, p = .22, η2 = .393, or interaction among these factors, F(2,6) = 1.21, p = .362, η2 = .287. As in the case of the adult-onset PFC damage group, we also conducted additional analyses for each Zone. Results did not yield statistical significance in either PPI zone, F(2,6) = 2.28, p = .18, η2 = .432, or Affect zone, F(2,6) = .87, p = .47, η2 = .23 (see Figure 3 and Figure 4).
Lastly, the Group × Zone interaction did not yield statistical significance, F(2,16) = .77, p = .48, η2 = .088.
Affective Valence and Arousal Ratings
A significant main effect of Picture category was found for Affective valence, F(2,34) = 115.24, p < .0001, ɛ = .735, η2 = .871, as well as significant linear and quadratic trends, F(1,17) = 134.41, p < .001, η2 = .888, and F(1,17) = 21.32, p < .0001, η2 = .555, respectively. As can be seen in Table 3, paired comparisons revealed differences between all the picture categories (all P’s < .0001).
Table 3.
Mean (and Standard Deviation) for the Affective Ratings.
| Comparison group | PFC-Adult | PFC-Childhood | |
|---|---|---|---|
| Affective valence ratings | |||
| Pleasant | 7.26 (0.57) | 6.68 (0.80) | 5.53 (2.40) |
| Neutral | 5.08 (0.29) | 5.38 (0.87) | 4.39 (1.64) |
| Unpleasant | 1.78 (0.58) | 2.59 (1.35) | 1.65 (0.16) |
| Arousal ratings | |||
| Pleasant | 5.72 (0.90) | 5.22 (0.76) | 4.49 (1.97) |
| Neutral | 1.92 (0.97) | 2.90 (1.77) | 1.26 (0.21) |
| Unpleasant | 6.95 (1.13) | 5.27 (2.25) | 5.49 (2.85) |
Lastly, the Group factor did not reach statistical significance, F(2,17) = 3.30, p = .062, η2 = .280, as well as the Group × Picture category interaction, F(4,34) = 1.80, p = .174, ɛ = .735, η2 = .175.
Regarding the arousal ratings, we also found a significant main effect of Picture category, F(2,34) = 38.09, p < .0001, ɛ = .756, η2 = .691. A significant quadratic trend, F(2,17) = 58.74, p < .0001, η2 = .776, revealed that affective pictures were rated higher in arousal than neutral pictures (see Table 3). Unpleasant and pleasant pictures were rated with higher arousal than neutral pictures (both P’s < .0001), whereas there was no difference in arousal ratings between the pleasant and unpleasant pictures (p = .232).
The Group factor and the Group × Picture category interaction did not yield statistical significance, F(2,17) = 2.19, p = .145, η2 = .205 and F(4,34) = 1.74, p = .183, ɛ = .756, η2 = .170, respectively.
Discussion
The PFC is involved in the processing of affective stimuli. Damage to the PFC alters emotional processing and also affects cognitive processes such as decision making that are influenced by emotion. One way that the PFC may be involved in emotional processing is in the allocation of processing resources driven by incoming affective stimuli (Vuilleumier & Huang, 2009). The current findings support this contention by showing that PFC damage disturbs the inhibition of the startle reflex caused by affectively engaging pictures. In addition, our data show an age-of-onset dissociation in this deficit: patients with childhood onset of PFC damage did not show the PPI effect, whereas patients with adult onset damage showed this effect (greater startle magnitude in the affect trials than in the PPI trials). These data, along with the absence of any other modulation (attentional and emotional) of the startle reflex in the childhood-onset PFC patients, are in agreement with findings demonstrating more severe behavioral, cognitive, and emotional deficits when PFC lesions occur in childhood compared to adult-onset PFC lesions (e.g., Anderson et al., 1999, 2009).
In contrast to the childhood-onset PFC patients, those with adult-onset PFC lesions showed the typical PPI effect. However, these patients did not show a greater startle inhibition during the emotional pictures than during the neutral pictures, in contrast to healthy subjects. Since it has been suggested that the startle inhibition to affective pictures reflects the greater demand on resources necessary to process these pictures as compared to neutral pictures (Bradley & Lang, 2001; Bradley et al., 1999, 2006), our results suggest that the PFC is involved in the analysis and recognition of the motivational significance of affective stimuli (Lang et al., 1997) and that damage to this region may lead to undifferentiated processing of these stimuli. Similar results have also been found in psychiatric populations, like psychopathic subjects (e.g., Levenson et al., 2000), and previous research has also found a relationship between PFC lesions and the development of maladjustment social behaviors (e.g., Anderson et al., 1999; Damasio, Tranel & Damasio, 1990; Saver & Damasio, 1991). An alternative interpretation of this effect could be related to emotional arousal instead of attentional engagement, that is, after the attentional resources have been captured by affective pictures, these pictures could promote a lower physiological response than in comparison subjects.
The finding that childhood-onset PFC lesions disrupt the very early attenuation of the startle response (i.e., PPI effect), whereas adult-onset PFC lesions do not, suggests that the PFC, along with other neural structures, is a necessary region for the development of a protective mechanism for the initial processing/encoding of salient stimuli. It may be that this process depends on the development of mechanisms within the PFC that have a long developmental time course. Hence, the PFC might be an important region for the development of a sensory gating mechanism, together with other brain structures, such as the thalamus, hippocampus and amygdala (for a review see Li, Du, Li, Wu, & Wu, 2009, and Swerdlow et al., 2001).
Our data also show a different pattern of emotional modulation of the startle reflex depending on the time of lesion onset. Like comparison subjects, adult-onset PFC lesion patients showed greater startle magnitudes during unpleasant than during pleasant pictures, whereas this effect did not appear in the childhood-onset group. These data give further support to previous clinical findings showing greater emotional dysfunction in patients with childhood-onset ventromedial PFC damage compared to those with adult-onset lesions (Anderson et al., 2006), and extend these findings by demonstrating that this damage need not be circumscribed to the ventromedial sector of the PFC in childhood-onset cases to exert these effects. While adult-onset lesions disrupt normally developed abilities, childhood-onset lesions may prevent the development of these abilities (Anderson et al., 1999, 2000). Hence, PFC lesions occurring in childhood may disrupt development of the prefrontal circuitry related to emotion (Anderson et al., 2000; Boes et al., 2011). The emotional modulation of the startle reflex depends on the integrity of several brain structures, including the amygdala and PFC (Buchanan et al., 2004; Sanchez-Navarro et al., 2005). The PFC, as well as the ventral portion of the ACC, may serve a regulatory function on emotional responses generated by limbic regions, such as the amygdala (Etkin et al., 2011; Salzman & Fusi, 2010). For instance, there is evidence that the PFC exerts an inhibitory control on the amygdala during emotional regulation (see Blair, 2008, and Quirk & Beer, 2006). The differences found between patients with childhood- and adult-onset PFC lesions in the emotional modulation of the startle reflex might reflect different stages in the interplay between these structures (see Durston & Casey, 2006). From this point of view, it could be hypothesized that patients with childhood-onset PFC damage have not had the opportunity to acquire the abilities to appropriately regulate their emotions, though they have acquired knowledge about the affective valence of complex affective stimuli, which likely depends on different neural regions (e.g., Phan et al., 2004). On the other hand, the lack of difference in the emotional modulation of the startle reflex between patients with adult-onset PFC damage and the healthy comparison group could be explained by the integrity in the development of the PFC together with other structures critical for this role (like the amygdala), and their reciprocal connections. The lesion of the PFC would not result, therefore, in a dramatic effect on the function of such structures related to the emotional modulation of the defensive reflexes.
Overall, these findings suggest that there may be limited plasticity in the neural systems that support the regulation of attentional and emotional processing. Further, these findings add to a growing literature suggesting that developmental disruption of the neural systems that support attentional and emotional regulation may underlie certain neuropsychiatric disorders, such as conduct disorder, psychopathy, and attention-deficit hyperactivity disorder (ADHD) (Bauer & Hesselbrock, 2001; Blair, 2008; Boes et al., 2011; Castellanos & Tannock, 2002; Rubia, 2010). In brain-injured populations, impaired allocation of attention, decision-making, and emotion regulation can lead to loss of independence and delayed rehabilitation, perhaps even more profound than impairments in language or movement. In spite of this, we lack a comprehensive survey of which lesions cause which specific defects and when spontaneous recovery occurs. This study broadens our understanding of the recovery from impairments in emotion processing caused by damage of the PFC.
Several limitations must be noted in this study. First, due to the scarcity of patients with damage restricted to the PFC region, the number of available participants was small. We cannot ensure the absence of effects on our dependent variables depending on sex, lesion laterality, lesion location, or brain damage etiology due to the insufficient sample sizes. These effects should be further evaluated since, for example, previous research has found differences in several processes, including emotion, depending on the laterality of PFC damage (Tranel, Bechara, & Denburg, 2002; Tranel, Damasio, Denburg, & Bechara, 2005). Secondly, although lesion volume did not differ significantly between the two PFC lesion groups, we acknowledge that differences in lesion coverage between the two groups may have contributed to our results to some degree. The small number of patients further precludes conclusions regarding the involvement of specific PFC regions in attention and emotion.
Future studies should evaluate whether the PFC is involved in PPI sensory gating by using a shorter inter-stimulus interval (e.g., 120 ms) as well as an experimental design that includes trials with either pure tones as lead stimuli and affective pictures. In addition, following the parametric studies conducted by Bradley et al. (1999, 2006) showing timing effects on PPI and the emotional modulation of the startle reflex, further lesion studies are needed to clarify what brain regions are responsible for mediating these effects. Another important direction for future research will be to clarify the relationship between laboratory indices of attention and emotion and disturbances in real-world functioning that are commonly associated with PFC damage.
Acknowledgements
We would like to thank Joel Bruss in the Department of Neurology, University of Iowa for his expertise and assistance in lesion analysis and figure preparation. This work was supported by National Institute of Neurological Disorders and Stroke Program Project Grant P01 19632, National Institute of Mental Health Grant R03 MH076815, and the Spanish Ministerio de Ciencia e Innovación Grant PSI2008-04394.
Footnotes
Footnotes
IAPS codes for the pictures employed in the study. Pleasant: 4608, 4660, 5470, 5621, 5626, 5629, 8030, 8034, 8080, 8170, 8180, 8190, 8200, 8300, 8370, 8470, 8490, 8501. Neutral: 7006, 7009, 7010, 7025, 7030, 7031, 7035, 7040, 7060, 7080, 7175, 7185, 7187, 7217, 7224, 7233, 7235, 7950. Unpleasant: 3100, 3102, 3140, 3150, 3170, 3400, 3500, 6230, 6250, 6260, 6313, 6350, 6360, 6530, 6570, 9405, 9570, 9921.
References
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Tranel D, Damasio AR. Long-term sequelae of prefrontal cortex damage acquired in early childhood. Developmental Neuropsychology. 2000;18:281–296. doi: 10.1207/S1532694202Anderson. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Wisnowski JL, Barrash J, Damasio H, Tranel D. Consistency of neuropsychological outcome following damage to prefrontal cortex in the first years of life. Journal of Clinical and Experimental Neuropsychology. 2009;31:170–179. doi: 10.1080/13803390802360526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. CSD/BEM localization of P300 sources in adolescents 'at risk': Evidence of frontal cortex dysfunction in conduct disorder. Biological Psychiatry. 2001;50:600–608. doi: 10.1016/s0006-3223(01)01066-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD. Signal attenuation as a function of integrator time constant and signal duration. Psychophysiology. 1994;31:201–203. doi: 10.1111/j.1469-8986.1994.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. Short lead interval startle modification. In: Dawson ME, Schell AM, Böhmelt AH, editors. Startle modification: implications for neuroscience, cognitive science, and clinical science. Cambridge, UK: Cambridge University Press; 1999. pp. 51–71. [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Boes AD, Graft AH, Joshi C, Chuang NA, Nopoulos P, Anderson SW. Behavioral effects of congenital ventromedial prefrontal cortex malformation. BMC Neurology. 2011;11:151. doi: 10.1186/1471-2377-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Pictures as prepulse: attention and emotion in startle modification. Psychophysiology. 1993;30:541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell AM, Böhmelt AH, editors. Startle modification: implications for neuroscience, cognitive science, and clinical science. Cambridge, UK: Cambridge University Press; 1999. pp. 157–183. [Google Scholar]
- Bubser M, Koch M. Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology. 1994;113:487–492. doi: 10.1007/BF02245228. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Anteromedial temporal lobe damage blocks startle modulation by fear and disgust. Behavioral Neuroscience. 2004;118:429–437. doi: 10.1037/0735-7044.118.2.429. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Hughes M, Budd TW, Cooper G, Fulham WR, Karayanidis F, Schall U. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. European Journal of Neuroscience. 2007;26:2327–2333. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, Martin-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: neural correlates. Human Brain Mapping. 2004;22:290–299. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descarte’s error: emotion, reason, and the human brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Somatic markers and the guidance of behavior: theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. pp. 217–229. [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Ellwanger J, Geyer MA, Braff DL. The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biological Psychology. 2003;62:175–195. doi: 10.1016/s0301-0511(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cogntive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink: a tool for investigating early and late attentional processes. Biological Psychology. 1993;35:185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 4th ed. San Diego, CA: Academic Press; 2008. [Google Scholar]
- Graham FK. Control of blink reflex excitability. In: Thompson RF, Hicks LH, Shvyrkov VB, editors. Neural mechanisms of goal-directed behavior and learning. New York: Academic Press; 1980. pp. 511–519. [Google Scholar]
- Graham FK, Hackley SA. Passive and active attention to input. In: Jennings JR, Coles MGH, editors. Handbook of cognitive psychophysiology. New York: John Wiley; 1991. pp. 251–356. [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Germans MK, Schnur DB, Troyer BT. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–198. [PubMed] [Google Scholar]
- Hodes RL, Cook EW, Lang PJ. Individual differences in autonomic response: conditioned association or conditioned fear? Psychophysiology. 1985;22:545–560. doi: 10.1111/j.1469-8986.1985.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Adolphs R, Oya H, Kovach C, Damasio H, Kaufman O, Howard M. Analysis of single-unit responses to emotional scenes in human ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1509–1518. doi: 10.1162/089892905774597182. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Kaufman O, Damasio H, Damasio AR, Granner M, Bakken H, Adolphs R. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nature Neuroscience. 2001;4:15–16. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: an update for psychophysiological researchers. Psychophysiology. 1998;35:470–478. [PubMed] [Google Scholar]
- Koch M, Bubser M. Deficient sensorimotor gating after 6-hydroxydopamine lesion of the rat medial prefrontal cortex is reversed by haloperidol. European Journal of Neuroscience. 1994;6:1837–1845. doi: 10.1111/j.1460-9568.1994.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation, and action. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: sensory and motivational processes. Mahwah, NJ: Lawrence Erlbaum; 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instruction manual, Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neuroscience and Biobehavioral Reviews. 2009;33:1157–1167. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. Journal of Neuroscience. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner I, Stocker T, Kellermann T, Ermer V, Wegener HP, Eickhoff SB, Shah NJ. Electrophysiology meets fMRI: neural correlates of the startle reflex assessed by simultaneous EMG-fMRI data acquisition. Human Brain Mapping. 2010;31:1675–1685. doi: 10.1002/hbm.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet L. The plastic human brain cortex. Annual Review of Neuroscience. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rubin DB. r(equivalent): a simple effect size indicator. Psychological Methods. 2003;8:492–496. doi: 10.1037/1082-989X.8.4.492. [DOI] [PubMed] [Google Scholar]
- Rubia K. “Cool” inferior frontostriatal dysfunction in attention- deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biological Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cogntive Sciences. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annual Review of Neuroscience. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Navarro JP, Martinez-Selva JM, Roman F. Emotional response in patients with frontal brain damage: effects of affective valence and information content. Behavioral Neuroscience. 2005;119:87–97. doi: 10.1037/0735-7044.119.1.87. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29:1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nature Neuroscience. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric statistics for the behavioral sciences. Boston, MA: McGraw-Hill; 1988. [Google Scholar]
- Stone SP, Patel P, Greenwood RJ, Halligan PW. Measuring visual neglect in acute stroke and predicting its recovery: the visual neglect recovery index. Journal of Neurology, Neurosurgery and Psychiatry. 1992;55:431–436. doi: 10.1136/jnnp.55.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Huang YM. Emotional attention: uncovering the mechanisms of affective biases in perception. Current Directions in Psychological Sciences. 2009;18:148–152. [Google Scholar]
- Wilson BA, Davidoff J. Partial recovery from visual object agnosia: a 10-year follow-up study. Cortex. 1993;29:529–542. doi: 10.1016/s0010-9452(13)80258-4. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences USA. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavitsanou K, Cranney J, Richardson R. Dopamine antagonists in the orbital prefrontal cortex reduce prepulse inhibition of the acoustic startle reflex in the rat. Pharmacology, Biochemistry, and Behavior. 1999;63:55–61. doi: 10.1016/s0091-3057(98)00234-2. [DOI] [PubMed] [Google Scholar]