Abstract
Hematopoietic cell transplantation (HCT) is effective in the treatment of patients with nonmalignant diseases and for many is the only known cure. Conventional myeloablative regimens have been associated with unacceptably high early transplant-related mortality (TRM) particularly in patients with co-morbid conditions. This prospective multicenter trial was designed to determine the safety and engraftment efficacy of treosulfan-based conditioning in patients with nonmalignant diseases. Thirty-one patients received HLA-matched related (n=4) or unrelated (n=27) grafts following conditioning with treosulfan (total dose: 42 g/m2), fludarabine (total dose: 150 mg/m2), ± thymoglobulin (6 mg/kg; n=22). Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus and methotrexate. All patients engrafted. Day-100 TRM was 0%. With a median follow-up of 2 years, the 2-year survival was 90%. Three patients died of GVHD, recurrent hemophagocytic lymphohistiocytosis, and a surgical complication, respectively. The cumulative incidences of grades II–IV and III–IV acute GVHD at day 100 and chronic GVHD at 2 years were 62%, 10% and 21%, respectively. Patients who received thymoglobulin had a significantly lower incidence of grade III-IV acute GVHD (0% versus 33%; P = 0.005). These results indicate that the combination of treosulfan, fludarabine, and thymoglobulin is effective at establishing donor engraftment with low toxicity and improved survival in patients with nonmalignant diseases, and support the need for future disease-specific clinical trials.
Keywords: clinical results in inherited disorders, conditioning regimen, allo transplantation, nonmalignant diseases, reduced intensity conditioning
INTRODUCTION
The risks for mortality and late effects are the major impediments to using hematopoietic cell transplantation (HCT) as curative therapy for nonmalignant diseases. In contrast to aplastic anemia wherein intensive immune suppression without myeloablation is sufficient to ensure engraftment [1,2], the marrow space is occupied and forms an additional barrier to engraftment in patients with hemoglobinopathies and certain primary immunodeficiencies and marrow failure syndromes. The addition of the myeloablative agent busulfan to cyclophosphamide and anti-T cell serotherapy (ATG or Campath) has been successful in establishing marrow grafts in most of these disorders, with rejection rates reported to be below 15% for recipients of human leukocyte antigen (HLA)-identical sibling marrow grafts [3–7]. However, the benefit of myeloablative doses of busulfan is largely negated by potential complications, such as sinusoidal obstructive syndrome (SOS), the incidence of which depends on the disease being treated and the partner drugs given in the regimen [8]. Mitigation of liver toxicity requires pharmacokinetic monitoring of busulfan levels to guide dose adjustments [9–11]. In addition, busulfan is associated with late complications, such as infertility, pulmonary dysfunction, as well as deficiencies in growth hormone, thyroid hormone, or sex hormones [12,13]. Many patients with nonmalignant diseases are at high risk for early treatment related mortality (TRM) because of infections or organ dysfunction associated with their underlying disease. To address these problems, several groups have explored primarily immunosuppressive nonmyeloablative conditioning regimens, thereby relying on the graft-vs-host reaction to establish donor engraftment [14–19]. While the risk for regimen-related toxicity is low after nonmyeloablative conditioning regimens, there is a higher risk of mixed chimerism or graft rejection, particularly among heavily transfused patients. Donor lymphocyte infusions (DLI) have been used to increase donor chimerism; however, DLI has been associated with greater risk for graft-versus-host disease (GVHD). Taken together, experience to date suggests that the ideal regimen for achieving engraftment and minimizing GVHD in patients with nonmalignant disorders would include a myeloablative agent that has reduced toxicity.
Treosulfan is a pro-drug of an alkylating agent structurally related to busulfan; however, treosulfan has a different mode of alkylation, is not activated by the liver enzymes, and has highly predictable pharmacokinetics. In pre-clinical studies, treosulfan was shown to have anti-leukemia, myelosuppressive, and immunosuppressive properties [20]. Several European groups have studied treosulfan in combination with fludarabine for conditioning of patients with hematologic malignancies [21,22]. These studies have shown efficacy in establishing allogeneic hematopoietic stem cell grafts with a low incidence of regimen-related toxicity. Retrospective analyses by European groups also suggest improved outcomes among patients with nonmalignant disorders [23]. The current study was designed as a multi-center prospective open-label trial to evaluate the safety and engraftment efficacy of treosulfan combined with fludarabine as a conditioning regimen for patients with nonmalignant diseases given a HLA-matched related or unrelated graft.
METHODS
Patient eligibility included: diagnosis of a nonmalignant disorder other than acquired aplastic anemia or Fanconi anemia treatable by allogeneic HCT; age <55 years at time of HCT; adequate organ function, defined as cardiac ejection fraction ≥35%, pulmonary DLCO ≥50%, creatinine ≤2 times the upper limit of normal for age, and absence of liver synthetic dysfunction or severe liver cirrhosis; no evidence for uncontrolled infection or previous HIV infection; and identification of a related or unrelated donor either fully matched or mismatched for a single allele at HLA-A, -B, -C, -DRB1, or -DQB1. The protocol was approved by the Institutional Review Boards of all participating institutions and monitored by an independent Data Safety Monitoring Board (DSMB). Patients or their legal guardians provided written consent.
The conditioning regimen consisted of treosulfan 14 g/m2 given once daily IV on days −6 through −4 (total dose 42 g/m2), fludarabine 30 mg/m2 given once daily IV on days −6 through −2 (total dose 150 mg/m2). To prevent GVHD, patients were given tacrolimus and 4 doses of methotrexate as previously described [24]. Due to a higher than anticipated incidence of acute grades III-IV GVHD in the first 9 patients, thymoglobulin [rabbit anti-thymocyte globulin (rATG)] was added to the regimen and given once daily IV on days −4 through −2 (total dose 6 mg/kg) [25]. Bone marrow was the preferred stem cell source, infused on day 0; however, unmodified G-CSF mobilized peripheral blood stem cells (PBSC) were allowed if marrow was not available. Patients were given supportive care, including antibiotic prophylaxis, intravenous immunoglobulin, intravenous nutrition, and weekly polymerase chain reaction (PCR) monitoring for reactivation of cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus, according to institutional practices.
The pre-HCT co-morbidity score was assessed by the HCT co-morbidity index (HCT-CI) [26,27]. Diagnosis, clinical grading, and treatment of acute and chronic GVHD were performed according to established criteria [28,29]. Toxicities were defined by the National Cancer Institute’s Common Toxicity Criteria, version 2.0, excluding hematologic toxicities [30]. Disease response was evaluated by disease-specific clinical parameters and cellular functional assays. Specifically, in patients with a diagnosis of primary immune deficiency, immune reconstitution was assessed by lymphocyte immunophenotype analysis, lymphocyte proliferation to mitogen and anti-CD3 antibody, and quantitative serum immune globulin production. Additional immunologic assays included flow cytometry for forkhead box P3 (FoxP3) in patients with immune dysregulation polyendocrinopathy enteropathy X-linked (IPEX), neutrophil oxidative burst in patients with chronic granulomatous disease (CGD), and natural killer (NK) cell function in patients with hemophagocytic lymphohistiocytosis (HLH). In patients with bone marrow failure syndromes, hematopoietic recovery was assessed by peripheral blood counts and/or bone marrow aspiration or biopsy. Flow cytometry of peripheral blood for glycosylphosphatidyl inositol (GPI)-anchored extracellular proteins was used to assess paroxysmal nocturnal hemoglobinuria (PNH), and hemoglobin gel electrophoresis was used to assess sickle cell disease. Procedures for immunophenotype analyses and sorting of peripheral blood cell subsets by flow cytometry have been described previously [16]. Donor chimerism levels were assessed in flow cytometry sorted CD33+, CD3+, CD19+, and CD56+ subsets by PCR-based analyses of polymorphic microsatellite regions, using methods previously described [31–34].
Study endpoints and statistical analysis
The primary endpoint of the study was engraftment, defined as an absolute neutrophil count ≥ 500 cells/mL for 3 consecutive days with documented donor CD3+ chimerism. Stopping rules were set for graft failure > 10% or day-200 TRM > 25%. Donor chimerism was calculated based on the percent of CD3+ cells of donor origin, and defined as full chimerism if ≥ 95%, high-level mixed chimerism if ≥ 50% but < 95%, low-level donor chimerism if ≥ 5% but < 50%, and graft rejection if < 5% of donor cells were present. The main secondary endpoints were TRM at day +200, acute GVHD grades II–IV, chronic GVHD, and overall survival. TRM and incidence of GVHD were calculated using cumulative incidence estimates, treating death due to disease as a competing risk event for TRM, and second HCT, death, and DLI as a competing risk for GVHD. Discontinuation of systemic immune suppression was calculated using cumulative incidence estimates, with death on systemic immune suppression as a competing risk event. Survival curves were calculated using the Kaplan and Meier method. Cox regression was used to evaluate risk factors for survival. Donor chimerism was analyzed with the Wilcoxon rank-sum test.
RESULTS
Patient characteristics
Between October 24, 2009 and July 3, 2013, 31 consecutive patients meeting eligibility criteria were enrolled. rATG was added to the regimen for all patients enrolled after June 1, 2011 (n = 22). Patient characteristics at the time of HCT are shown in Table 1. The diagnoses included primary immune deficiency disorders (n=13; 45%), HLH (n=6; 19%), bone marrow failure syndromes (n=6; 19%), and red blood cell disorders (n=6; 19%). At least one co-morbidity per the HCT-CI scoring system was present in 68% of the patients, and 29% had HCT-CI scores ≥ 3. Eighteen patients (58%) had at least one of these risk factors: HCT CI ≥ 3, failure to thrive (FTT), or previous HCT. Unrelated donor grafts were matched for HLA-A, -B, -C, -DRB1, and -DQB1 in 26 patients and mismatched for one C allele in one patient. Two patients received unrelated PBSC grafts with cell doses of 20.29 and 11.04 × 106 CD34+ cells/kg, respectively; all other patients received marrow grafts, with a median cell dose of 5.6 (range 1.6–13.2) × 106 CD34+ cells/kg.
Table 1.
Patient and Transplant Characteristics
| Characteristic | Number |
|---|---|
| Total number of patients | 31 |
| Sex (M:F) | 22:9 |
| Age, (yrs) at HCT (median, range) | 10.7 (0.4–30.5) |
| Diagnosis | |
| IPEX | 6 (19%) |
| CGD | 5 (16%) |
| Other PID | 2 (10%) |
| HLH | 6 (16%) |
| DBA | 3 (10%) |
| SDS | 3 (10%) |
| SCD | 4 (10%) |
| PNH | 2 (13%) |
| HCT CI | |
| 0 | 10 (30%) |
| 1 | 7 (22%) |
| 2 | 5 (16%) |
| 3–6 | 9 (29%) |
| FTT | 11 (35%) |
| Previous HCT | 3 (10%) |
| Cell Source | |
| MSD marrow | 4 (13%) |
| URD marrow | 25 (81%) |
| URD PBSC | 2 (6%) |
| CMV donor/recipient serology | |
| D−/R− | 12 (39%) |
| D−/R+ | 6 (19%) |
| D+/R− | 5 (16%) |
| D+/R+ | 8 (26%) |
| ABO match | |
| Match | 12 (39%) |
| Major mismatch | 12 (39%) |
| Minor mismatch | 7 (22%) |
Abbreviations: D, donor; DBA, Diamond-Blackfan anemia; CGD, chronic granulomatous disease; CMV, Cytomegalovirus; HCT, hematopoietic cell transplantation; F, female; FTT, failure to thrive; HCT-CI, hematopoietic cell transplantation comorbidity index; HLH, hemophagocytic lymphohistiocytosis; IPEX, immune dysregulation polyendocrinopathy enteropathy X-linked syndrome; M, male; MSD, matched sibling donor; PBSC, peripheral blood stem cells; PID, primary immunodeficiency disorder; PNH, paroxysmal nocturnal hemoglobinuria; R, recipient; SCA, sickle cell disease; SDS, Shwachman-Diamond syndrome; URD, unrelated donor; yrs, years
Engraftment and chimerism
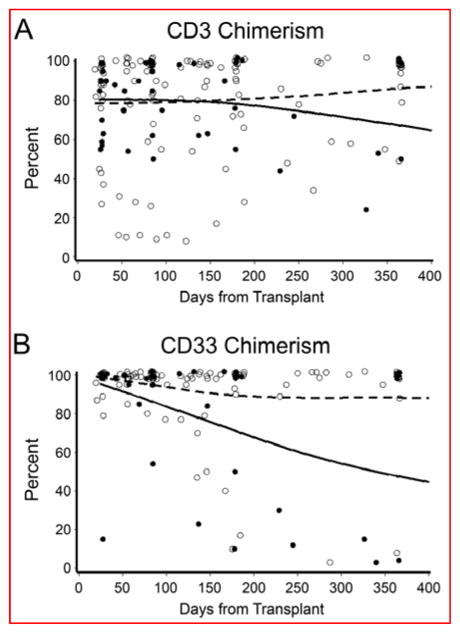
Primary engraftment was observed in all patients, with a median time to neutrophil engraftment of 21 (range, 12–46) days. Secondary graft failure in the setting of high-level donor chimerism (75% CD3+, 100% CD33+ donor cells) was observed at day +76 in one patient with sickle cell disease. This patient received a marrow graft with a low cell dose of 0.7 × 108 total nucleated cells/kg. Excluding this patient, the median time to platelet recovery, defined as >50 × 109/L for 5 days without transfusion support, was 23 (range, 10–102) days. Donor chimerisms at the most recent evaluation are shown in Table 2. Among the 9 patients not given rATG, one had secondary graft failure (described above) and two patients (one with HLH and one with IPEX) had low-level mixed donor chimerism. Among the 22 patients who received rATG, 19 (86%) had full or high-level mixed donor CD3+ chimerism and 3 (14%) had low-level mixed donor CD3+ chimerism at the most recent evaluation. The CD3+ and CD33+ donor chimerism levels over the first year after HCT are shown in Figure 1. In general, CD3+ donor chimerism remained stable over time, and there was no statistically significant difference in donor chimerism levels between patients given rATG and those not given rATG at any of the assessed time points (day 28, day 84, 6 months, 1 year, p>0.25). Two patients received a second HCT, one for treatment of secondary graft failure (described above) and one for treatment of recurrent HLH in the setting of mixed chimerism (36% CD3+, 3% CD33+ donor cells). Both of these patients had originally received a marrow graft from a HLA identical sibling, with CD34+ cell dose/kg of 2.4 and 10.2 × 106, respectively, and neither had received rATG.
Table 2.
Donor chimerism and disease response according to diagnosis
| Diagnosis | Percent Donor | Response | Follow-up (yrs) | |
|---|---|---|---|---|
| CD3+ | CD33+ | |||
| Immune Deficiency Syndromes | ||||
| IPEX* | 9 | 0 | Normal FOXP3 expression | 3.7 |
| IPEX* | 100 | 100 | Normal FOXP3 expression | 3.0 |
| IPEX | 98 | 100 | Normal FOXP3 expression | 2.3 |
| IPEX | >95 | 100 | Normal FOXP3 expression | 2.6 |
| IPEX | 100 | 93 | Normal FOXP3 expression | 0.5 |
| IPEX | 37 | 3 | Normal FOXP3 expression | 1.6 |
| CGD* | 100 | 100 | Normal neutrophil oxidative burst | 4.0 |
| CGD | 100 | 100 | Normal neutrophil oxidative burst | 2.4 |
| CGD | 100 | 100 | Normal neutrophil oxidative burst | 2.2 |
| CGD | 100 | 100 | Normal neutrophil oxidative burst | 2.0 |
| CGD | 90 | 97 | Normal neutrophil oxidative burst | 2.0 |
| Other PID | 100 | 100 | Clinical remission of EBV | 2.2 |
| Other PID | 100 | 100 | Clinical remission, off IVIG | 2.0 |
| Hemophagocytic Lymphohistiocytosis | ||||
| HLH* | 36 | 3 | Recurrent disease | 1.4** |
| HLH | 80 | 70 | Recurrent disease | 0.6† |
| HLH | 100 | 100 | Normal NK function Clinical remission |
2.3 |
| HLH | 100 | 100 | Normal NK function Clinical remission |
1.7 |
| HLH | 99 | 100 | Normal NK function Clinical remission |
1.4 |
| HLH | 100 | 100 | Clinical remission | 0.4† |
| Bone Marrow Failure Syndromes | ||||
| DBA | 100 | 100 | Normal hemoglobin, non-transfused | 2.1 |
| DBA | 58 | 68 | Normal hemoglobin, non-transfused | 1.6 |
| DBA | 94 | 100 | Normal hemoglobin, non-transfused | 1.0 |
| SDS* | 100 | 100 | Normal blood count Normal bone marrow cellularity |
2.6 |
| SDS | 100 | 100 | Normal blood count Normal bone marrow cellularity |
2.0 |
| SDS | 100 | 100 | Normal blood count Resolution of cytogenetic abnormality in the marrow |
1.0 |
| Red Cell Disorders | ||||
| SCD* | 75 | 100 | HbS <5% | 0.3** |
| SCD* | 100 | 100 | HbS <5% Resolution of symptoms |
3.0 |
| SCD | 100 | 100 | HbS 30% (donor with HbS trait) Resolution of symptoms |
1.4 |
| SCD | 55 | >95 | HbS 40% (donor with HbS trait) Resolution of symptoms |
1.0 |
| PNH* | 100 | 100 | Normal blood counts Normal GPI expression |
3.4 |
| PNH* | 100 | 100 | Normal blood counts Normal GPI expression |
0.4† |
No rATG
Last evaluation point before a second HCT.
Died
Abbreviations: AIHA, autoimmune hemolytic anemia; CGD, chronic granulomatous disease; DBA, Diamond-Blackfan anemia; EBV, Epstein Barr Virus; HbS, hemoglobin S; HLH, hemophagocytic lymphohistiocytosis; IPEX, immune dysregulation polyendocrinopathy enteropathy X-linked syndrome; IVIG, intravenous immune globulin; NK, Natural Killer cell; PID, primary immunodeficiency disorder; PNH, paroxysmal nocturnal hemoglobinuria; rATG, rabbit anti-thymocyte globulin (thymoglobulin); SCA, sickle cell disease; SDS, Shwachman-Diamond syndrome.
Figure 1. CD3+ and CD33+ donor chimerism in 31 patients conditioned with treosulfan and fludarabine.
Shown are the percent donor chimerism of sorted peripheral blood CD3+ (panel A) and CD33+ (panel B) subsets according to day after transplant. The open circles represent percent donor chimerism measured for patients who were given thymoglobulin (n = 22), and the closed circles represent percent donor chimerism measured for patients who were not given thymoglobulin (n = 9) in the regimen. Spline smoothed regression lines reflect the trend in mean chimerism over time, for patients given thymoglobulin (dashed line) and for patients not given thymoglobulin (solid line). Overlapping points at day 28, day 84, day 180, and 1 year, and at 100% chimerism, are jittered slightly to improve visibility.
Graft-versus-host disease
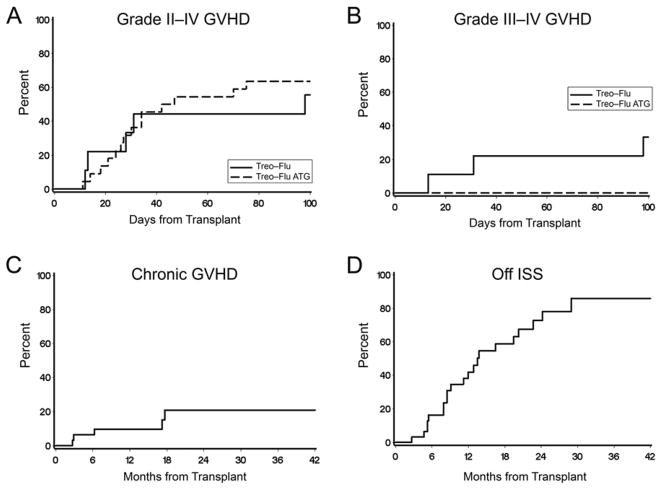
Grades II-IV acute GVHD occurred in 19 patients for an overall cumulative incidence of 62%; most were grade II (9 grade IIa, 7 grade IIb). Severe acute GVHD grades III-IV occurred in three patients (10%), none of whom had received rATG (Figure 2). The incidence of acute GVHD grades III-IV was statistically significantly reduced in recipients of rATG (0% versus 33%; p=0.005), but was not associated with age or donor type. Late acute GVHD was observed in 4 patients. The 2-year cumulative incidence of chronic GVHD was 21% (Figure 2) and was not statistically significantly different between patients who received and did not receive rATG (p=0.50). Seventy-eight percent of patients were off systemic immunosuppression by 2 years after HCT (Figure 2).
Figure 2. Acute and chronic graft-vs.-host disease (GVHD).
Shown is the cumulative incidence of acute GVHD grades II-IV (panel A) and acute GVHD grades III-IV (panel B) according to whether patients were given thymoglobulin (dashed line, n = 22) or not given thymoglobulin (solid line, n = 9) in the regimen. Panel C shows the cumulative incidence of chronic GVHD. Panel D shows the cumulative incidence of discontinuation of systemic immune suppression following HCT.
Toxicities and infections
Safety of the regimen was assessed by measurement of the toxicities observed from the start of conditioning through day 30 after HCT. In total, 13 patients (42%) developed one or more grade ≥ 3 toxicities during this period. Toxicities commonly associated with myeloablative regimens occurred infrequently, specifically grade 3 mucositis developed in three patients (10%) and grade 3 skin rash not attributable to infection or GVHD occurred in three (10%); no patient developed liver toxicity. In addition, one patient developed transient hypoxia (grade 3). Two patients, ages 5.6 and 23 months, respectively, had a single episode of a focal seizure that occurred at day +8 and day +25, respectively. In addition, 3 patients developed grade 3 pancreatitis which resolved in all cases. Two patients also developed a severe allergic reaction to rATG (grade 3), resulting in its discontinuation after the second dose; both events resolved. In the 3 patients who had received a prior HCT there were no significant toxicities.
Life-threatening (grade 4) toxicities occurred in two patients (6%), which resolved. One of these patients (who had diaphragmatic hemiparesis that preceded transplant) developed hypoxia and non-infectious pulmonary infiltrates on day −4 and required continuous positive airway pressure support for 8 days. The other patient who had herpes stomatitis developed mucosal bleeding and pulmonary infiltrates requiring ventilatory support for 17 days. We observed no apparent difference in toxicity scores according to patient age or BSA.
Within the first 100 days after HCT, viral reactivation was detected by PCR in 11 of 22 patients who received rATG and in 4 of 9 patients not given rATG. All patients with CMV reactivation (n=9) received pre-emptive antiviral treatment, and no patient developed CMV disease. Reactivation of EBV was detected in four patients, adenovirus in two, BK virus in two, human herpes virus 6 (HHV6) in two, and herpes simplex virus in one. Two patients acquired RSV infection on day +4, and +59, respectively and were treated successfully with ribavirin. All viral infections resolved either spontaneously or with antiviral therapies. One patient with chronic granulomatous disease (CGD) developed a probable pulmonary fungal infection which resolved following treatment with voriconazole. No clinically significant bacterial infections were observed.
Overall survival and transplant-related mortality
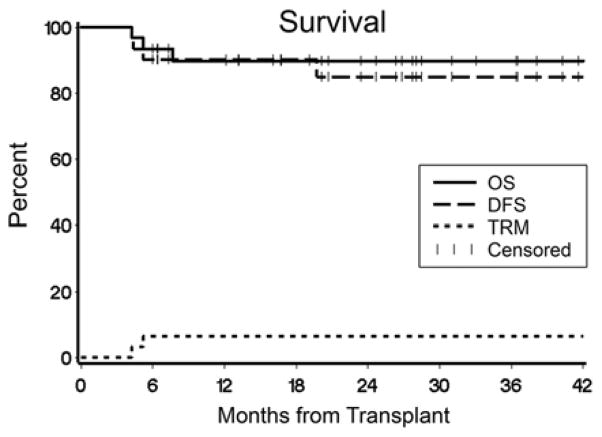
Twenty-eight patients survive at a median of 26.1 (range 7–48.3) months after HCT for a 2-year projected overall survival of 90% (Figure 3). Day 100 TRM was 0%. There were 3 deaths. One patient died of recurrent CNS HLH on day +233 in the setting of high level mixed chimerism (CD33+ 70%, CD3+ 80% donor). One patient with PNH in complete remission died of GVHD on day +158; this patient had not received rATG. One patient with HLH in complete remission died on day +129 after a surgical complication unrelated to HCT.
Figure 3. Overall survival, disease-free survival, and transplant-related mortality (n=31).
Shown are the Kaplan Meir estimates of overall survival (solid line) and disease-free survival (dashed line), and the cumulative incidence of transplant-related mortality (dotted line).
Disease response
Complete disease responses were observed in 28 patients (90%; Table 2), as measured by clinical symptoms and laboratory assays. All patients with a diagnosis of a primary immune deficiency disorder or a bone marrow failure syndrome had complete resolution of disease. Among the six patients treated for HLH, 4 achieved complete remissions and 2 had recurrent disease. Specifically, one patient with a history of CNS disease pre HCT experienced CNS relapse at day +212 despite high-level mixed donor chimerism and died of disease, and one with low-level mixed chimerism was given a second HCT for treatment of recurrent HLH and survives in remission. The two patients with PNH experienced complete disease responses with normalization of blood counts and normal expression of GPI-anchored extracellular proteins, although one died of GVHD. Among the four patients with sickle cell disease, three survive without evidence of sickle cell disease; one was given a second HCT for treatment of secondary graft failure despite high level donor chimerism and hemoglobin S <5%, and survives without disease.
DISCUSSION
The objective of this study was to establish the safety and engraftment efficacy of treosulfan and fludarabine as conditioning for patients with nonmalignant disorders in a prospective multi-center clinical trial. The results demonstrate that the combination of treosulfan and fludarabine provides sufficient myeloablation to achieve engraftment in a broad range of nonmalignant disorders without the potentially serious complications associated with traditional myeloablative regimens such as busulfan and cyclophosphamide. Specifically, there were no deaths within the first 100 days, and no patient developed SOS, multi-organ failure, or fatal infections. Of the three deaths, one was caused by GVHD in a patient who did not receive rATG and was considered transplant related. The other causes of death included recurrent CNS HLH and a surgical complication unrelated to HCT. Grade 4 toxicities were limited and the low incidence of regimen-related mortality compared to historical results observed with busulfan and cyclophosphamide-based regimens was quite encouraging, especially given the fact that more than 50% of the patients in this study had risk factors for poor outcome. For high-risk patients in particular, a less toxic yet sufficiently myeloablative HCT regimen such as treosulfan and fludarabine may provide considerable benefit and perhaps will encourage HCT earlier in the disease course before the development of significant comorbidities.
The incidence of viral reactivation did not appear to be higher in the rATG cohort, although the small number of patients in the comparator group limits the interpretation of this observation. Importantly, no patients died from infectious complications. The use of rATG correlated with a lower incidence of severe acute GVHD. The relatively high incidence of grades II-IV acute GVHD reported here may be explained in part by the predominance of unrelated donor grafts in this study, as well as by an increase in diagnostic sensitivity for GVHD of the upper intestinal tract, reflecting the early use of endoscopic biopsies to detect GVHD in patients with anorexia, as previously described [35].
The combination of treosulfan and fludarabine was first reported by Casper et al as a reduced-toxicity regimen for adult patients with hematologic malignancies who were ineligible for standard myeloablative regimens because of co-morbidities [36]. In this and subsequent studies of patients with hematologic malignancies, treosulfan was dosed at 30–42 g/m2, resulting in a very low incidence of regimen related mortality, SOS, and multi-organ failure, with engraftment rates exceeding 90% [21,22,37–39]. The most common toxicities associated with treosulfan were skin rash, mild mucositis, and transient elevation of hepatic transaminases. The few reported studies of treosulfan-based conditioning in pediatric patients have been retrospective analyses. Greystoke et al. reported outcomes for 32 children with nonmalignant diseases who received treosulfan (36–42 g/m2) alone or in combination with anti-T cell serotherapy, fludarabine or cyclophosphamide [23]. Graft sources included HLA-matched or mismatched related or unrelated marrow or peripheral blood, or HLA-mismatched unrelated umbilical cord blood. Skin toxicity was reported for 46% of patients, whereas fatal SOS was observed only once and the overall survival was 84%. Slatter et. al. reported similar results for 70 patients with nonmalignant disorders given treosulfan, 36–42 g/m2, combined with fludarabine or cyclophosphamide with or without anti-T cell serotherapy conditioning for HLA matched or mismatched grafts [40]. Overall survival was 81% and deaths were mainly from late complications of HCT. Seizures were noted in 4 infants. More recently, Beier et. al. reported the outcomes of 109 pediatric patients with hematologic malignancies or nonmalignant disorders [41]. Patients were conditioned with treosulfan (21–42 g/m2) combined with various agents including fludarabine, cyclophosphamide, thiotepa, melphalan, total body irradiation, or anti-T cell serotherapy followed by HLA-matched or mismatched marrow or cord blood grafts. Similarly low incidences of toxicities were reported including SOS in 3%, high-grade skin toxicity in 4%, and seizures in 4% of patients. Taken together, these retrospective studies suggested that treosulfan-based regimens were associated with less toxicity compared to historical rates associated with busulfan based regimens, and we have now substantiated these findings in a prospective multi-center study.
It may be argued that a prospective study of reduced toxicity conditioning in a diverse group of patients will make it difficult to draw conclusions regarding its usefulness in specific disorders. However, each of these disorders are rare, and analysis of safety and efficacy would be protracted if such studies were limited to only a single disorder. Our results establish the overall safety of the treosulfan and fludarabine regimen and suggest that this regimen will be effective for establishing allografts in multiple disorders. Specifically, all patients with immune deficiency disorders including Immune dysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) and CGD are surviving with disease resolution, as are all patients with bone marrow failure syndromes (Shwachman-Diamond syndrome, Diamond-Blackfan anemia). Our results may also suggest that some disorders may be more resistant to engraftment with this regimen, as 2 of 6 patients with HLH experienced relapsed disease in the setting of mixed chimerism, one of whom required a second HCT and 1 of 4 patients with sickle cell disease required a second HCT for correction of secondary graft failure. While these numbers are too small to draw firm conclusions, several studies support the feasibility of intensifying the regimen without excessive toxicity through the addition of thiotepa, particularly in patients with thalassemia major and HLH [42–45].
The more predictable pharmacokinetics of treosulfan offers the advantage of dispensing with drug level determination as required for targeting busulfan. However, there remains a question about the appropriate dosing of treosulfan in very young patients, particularly infants. Glowka et al. reported results of treosulfan pharmacokinetic studies in seven patients ages 2–15 years, five of whom received three doses of treosulfan at 12 g/m2 per day, and one each who received doses of 10 g/m2 or 14 g/m2. The median AUC for those given the 12 g/m2 dose was similar to that reported for adults [46]. More recently, ten Brink et al used a limited sampling strategy to estimate treosulfan pharmacokinetics in 20 children (median age 6.2 years) given 14 g/m2 treosulfan [47]. Results showed predictable pharmacokinetics of treosulfan and limited inter-patient variability. No correlation with outcome was provided in either report. Based on these studies, and unpublished data in seven children who weighed < 10 kg, a preliminary population pharmacokinetic model was developed and dose recommendations on individual body surface area (BSA) were derived. The current dose recommendations are to use 14 g/m2/day treosulfan for patients with a BSA of >1.0 m2, 12 g/m2/day for a BSA of > 0.5 to 1.0 m2, and 10 g/m2/day for those with BSA ≤ 0.5 m2. Results of our study suggest that this dose adjustment may not be necessary, as all patients were given doses of 14 g/m2 regardless of age or BSA. We observed no apparent difference in toxicity scores or mortality according to patient age or BSA. Therefore, it is not clear that dose adaptation would offer any benefit in smaller children, and could potentially lower the myeloablative effect of treosulfan, leading to a higher incidence of graft rejection.
Further prospective studies are warranted to better understand the dose effect of treosulfan in patients with nonmalignant diseases, as well as for patients with alternative donor grafts such as umbilical cord blood. Patients in this study will need to be monitored for the potential late effects and the long-term stability of engraftment following treosulfan-based conditioning. The results of this multicenter prospective study support the use of treosulfan, fludarabine, and rATG conditioning for patients with nonmalignant disorders as a means to achieve engraftment of HLA-matched grafts with minimal risk for mortality or morbidity.
Highlights.
Treosulfan-based conditioning is effective at establishing donor engraftment.
Treosulfan-based conditioning has a low toxicity profile.
Treosulfan-based conditioning is well tolerated in infants.
Treosulfan-based conditioning results in an overall survival of 90%.
Acknowledgments
The authors would like to thank the nursing and clinical staff, the referring physicians, and the patients who participated in this trial. We also thank Joshua Latos for data management; Michelle Bouvier, research nurse; and Bonnie Larson, Helen Crawford, and Sue Carbonneau for assistance with manuscript preparation.
Footnotes
Author contributions L.M.B. and A.E.W. wrote the manuscript. L.M.B., E.R.N., B.E.S., H.J.D., R.S. and A.E.W. designed the study. L.M.B., E.R.N., T.R.T., J.A.T., J.D., R.G., A.S., C.D., S.S.S., M.S.T., K.S.B., D.R., A.E.W. performed the research. L.M.B., B.E.S., A.E.W. analyzed the data. E.R.N., T.R.T., J.A.T., J.D., R.G., A.S., C.D., S.S.S., M.S.T., K.S.B., D.R., J.A.E., H.J.D., and R.S. critically reviewed the manuscript. J.A.E. identified patients. M.E.D.F. performed the grading of chronic GVHD.
Financial disclosure:
This study was supported in part by grants P01 HL036444 and K23 HL085288 from the NIH, Bethesda, MD, U.S.A., and research funding from medac, GmbH (Hamburg, Germany). In addition, medac provided Treosulfan for the study.
Conflicts of interest statement. This study was supported in part by research funding from Medac GmbH (Hamburg, Germany). In addition, Medac provided Treosulfan for the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahl C, Leisenring W, Deeg HJ, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long-term follow-up. Br J Haematol. 2005;130:747–751. doi: 10.1111/j.1365-2141.2005.05667.x. [DOI] [PubMed] [Google Scholar]
- 2.Burroughs LM, Woolfrey AE, Storer BE, et al. Success of allogeneic marrow transplantation for children with severe aplastic anemia. Br J Haematol. 2012;158:120–128. doi: 10.1111/j.1365-2141.2012.09130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi R, Ariga T, Nonoyama S, et al. Outcome in patients with Wiskott-Aldrich syndrome following stem cell transplantation: an analysis of 57 patients in Japan. Br J Haematol. 2006;135:362–366. doi: 10.1111/j.1365-2141.2006.06297.x. [DOI] [PubMed] [Google Scholar]
- 5.Tewari P, Martin PL, Mendizabal A, et al. Myeloablative transplantation using either cord blood or bone marrow leads to immune recovery, high long-term donor chimerism and excellent survival in chronic granulomatous disease. Biol Blood Marrow Transplant. 2012;18:1368–1377. doi: 10.1016/j.bbmt.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandy M, Balasubramanian P, Ramachandran SV, et al. Randomized trial of two different conditioning regimens for bone marrow transplantation in thalassemia--the role of busulfan pharmacokinetics in determining outcome. Bone Marrow Transplant. 2005;36:839–845. doi: 10.1038/sj.bmt.1705151. [DOI] [PubMed] [Google Scholar]
- 7.Henter JI, Samuelsson-Horne A, Arico M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100:2367–2373. doi: 10.1182/blood-2002-01-0172. [DOI] [PubMed] [Google Scholar]
- 8.Gaziev J, Sodani P, Polchi P, Andreani M, Lucarelli G. Bone marrow transplantation in adults with thalassemia: Treatment and long-term follow-up. Ann NY Acad Sci. 2005;1054:196–205. doi: 10.1196/annals.1345.024. [DOI] [PubMed] [Google Scholar]
- 9.Veal GJ, Nguyen L, Paci A, et al. Busulfan pharmacokinetics following intravenous and oral dosing regimens in children receiving high-dose myeloablative chemotherapy for high-risk neuroblastoma as part of the HR-NBL-1/SIOPEN trial. Eur J Cancer. 2012;48:3063–3072. doi: 10.1016/j.ejca.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Perkins JB, Kim J, Anasetti C, et al. Maximally tolerated busulfan systemic exposure in combination with fludarabine as conditioning before allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1099–1107. doi: 10.1016/j.bbmt.2011.12.584. [DOI] [PubMed] [Google Scholar]
- 11.Li CK, Yuen PM, Wong R, et al. Busulphan level and early mortality in thalassaemia patients after BMT. Bone Marrow Transplant. 1999;23:307–310. doi: 10.1038/sj.bmt.1701584. [DOI] [PubMed] [Google Scholar]
- 12.Walters MC, Hardy K, Edwards S, et al. Pulmonary, gonadal and central nervous system status after bone marrow transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2010;16:263–272. doi: 10.1016/j.bbmt.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slatter MA, Gennery AR, Cheetham TD, et al. Thyroid dysfunction after bone marrow transplantation for primary immunodeficiency without the use of total body irradiation in conditioning. Bone Marrow Transplant. 2004;33:949–953. doi: 10.1038/sj.bmt.1704456. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz ME, Barrett AJ, Brown MR, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. N Engl J Med. 2001;344:881–888. doi: 10.1056/NEJM200103223441203. [DOI] [PubMed] [Google Scholar]
- 15.Iannone R, Casella JF, Fuchs EJ, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and β-thalassemia. Biol Blood Marrow Transplant. 2003;9:519–528. doi: 10.1016/s1083-8791(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 16.Burroughs LM, Storb R, Leisenring WM, et al. Intensive postgrafting immune suppression combined with nonmyeloablative conditioning for transplantation of HLA-identical hematopoietic cell grafts: results of a pilot study for treatment of primary immunodeficiency disorders. Bone Marrow Transplant. 2007;40:633–642. doi: 10.1038/sj.bmt.1705778. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–2317. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burroughs LM, Torgerson TR, Storb R, et al. Stable hematopoietic cell engraftment after low-intensity nonmyeloablative conditioning in patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. J Allergy Clin Immunol. 2010;126:1000–1005. doi: 10.1016/j.jaci.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh RA, Kim MO, Liu C, et al. An intermediate alemtuzumab schedule reduces the incidence of mixed chimerism following reduced-intensity conditioning hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transplant. 2013;19:1625–1631. doi: 10.1016/j.bbmt.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjoo F, Hassan Z, Abedi-Valugerdi M, et al. Myeloablative and immunosuppressive properties of treosulfan in mice. Exp Hematol. 2006;34:115–121. doi: 10.1016/j.exphem.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Casper J, Wolff D, Knauf W, et al. Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol. 2010;28:3344–3351. doi: 10.1200/JCO.2009.23.3429. [DOI] [PubMed] [Google Scholar]
- 22.Casper J, Holowiecki J, Trenschel R, et al. Allogeneic hematopoietic SCT in patients with AML following treosulfan/fludarabine conditioning. Bone Marrow Transplant. 2012;47:1171–1177. doi: 10.1038/bmt.2011.242. [DOI] [PubMed] [Google Scholar]
- 23.Greystoke B, Bonanomi S, Carr TF, et al. Treosulfan-cointaining regimens achieve high rates of engraftment associated with low transplant morbidity and mortality in children with non-malignant disease and significant co-morbidities. Br J Haematol. 2008;142:257–262. doi: 10.1111/j.1365-2141.2008.07064.x. [DOI] [PubMed] [Google Scholar]
- 24.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 25.Deeg HJ, Storer BE, Boeckh M, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant. 2006;12:573–584. doi: 10.1016/j.bbmt.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Sorror M. How I assess comorbidities prior to hematopoietic cell transplantation. Blood. 2013;121:2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- 28.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 29.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 30.The Revised Common Toxicity Criteria: Version 2.0. DCTD, NCI, NIH, DHHS; 1999. [Google Scholar]
- 31.Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA. Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood. 1995;85:1954–1963. [PubMed] [Google Scholar]
- 32.Kasai K, Nakamura Y, White R. Amplification of a variable number of tandem repeats (VNTR) locus (pMCT118) by the polymerase chain reaction (PCR) and its application to forensic science. Journal of Forensic Sciences. 1990;35:1196–1200. [PubMed] [Google Scholar]
- 33.Boerwinkle E, Xiong WJ, Fourest E, Chan L. Rapid typing of tandemly repeated hypervariable loci by the polymerase chain reaction: application to the apolipoprotein B 3′ hypervariable region. Proc Natl Acad Sci USA. 1989;86:212–216. doi: 10.1073/pnas.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryant E, Martin PJ. Documentation of engraftment and characterization of chimerism following hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. 2. Boston: Blackwell Science; 1999. pp. 197–206. [Google Scholar]
- 35.Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 36.Casper J, Knauf W, Kiefer T, et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood. 2004;103:725–731. doi: 10.1182/blood-2002-11-3615. [DOI] [PubMed] [Google Scholar]
- 37.Nemecek ER, Guthrie KA, Sorror ML, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:341–350. doi: 10.1016/j.bbmt.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruutu T, Volin L, Beelen DW, et al. Reduced-toxicity conditioning with treosulfan and fludarabine in allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes: final results of an international prospective phase II trial. Haematologica. 2011;96:1344–1350. doi: 10.3324/haematol.2011.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wachowiak J, Sykora KW, Cornish J, et al. Treosulfan-based preparative regimens for allo-HSCT in childhood hematological malignancies: a retrospective study on behalf of the EBMT pediatric diseases working party. Bone Marrow Transplant. 2011;46:1510–1518. doi: 10.1038/bmt.2010.343. [DOI] [PubMed] [Google Scholar]
- 40.Slatter MA, Rao K, Amrolia P, et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood. 2011;117:4367–4375. doi: 10.1182/blood-2010-10-312082. [DOI] [PubMed] [Google Scholar]
- 41.Beier R, Schulz A, Honig M, et al. Long-term follow-up of children conditioned with Treosulfan: German and Austrian experience. Bone Marrow Transplant. 2013;48:491–501. doi: 10.1038/bmt.2012.188. [DOI] [PubMed] [Google Scholar]
- 42.Mathews V, George B, Viswabandya A, et al. Improved clinical outcomes of high risk beta thalassemia major patients undergoing a HLA matched related allogeneic stem cell transplant with a treosulfan based conditioning regimen and peripheral blood stem cell grafts. PLoS ONE. 2013;8:e61637. doi: 10.1371/journal.pone.0061637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernardo ME, Piras E, Vacca A, et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012;120:473–476. doi: 10.1182/blood-2012-04-423822. [DOI] [PubMed] [Google Scholar]
- 44.Choudhary D, Sharma SK, Gupta N, et al. Treosulfan-thiotepa-fludarabine-based conditioning regimen for allogeneic transplantation in patients with thalassemia major: a single-center experience from north India. Biol Blood Marrow Transplant. 2013;19:492–495. doi: 10.1016/j.bbmt.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Lehmberg K, Albert MH, Beier R, et al. Treosulfan-based conditioning regimen for children and adolescents with hemophagocytic lymphohistiocytosis. Haematologica. 2014;99:180–184. doi: 10.3324/haematol.2013.094730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glowka FK, Karazniewicz-Lada M, Grund G, Wrobel T, Wachowiak J. Pharmacokinetics of high-dose i.v. treosulfan in children undergoing treosulfan-based preparative regimen for allogeneic haematopoietic SCT (Review) Bone Marrow Transplant. 2008;42 (Suppl 2):S67–S70. doi: 10.1038/bmt.2008.287. [DOI] [PubMed] [Google Scholar]
- 47.Ten Brink MH, Ackaert O, Zwaveling J, et al. Pharmacokinetics of treosulfan in pediatric patients undergoing hematopoietic stem cell transplantation. Therapeutic Drug Monitoring. 2014 doi: 10.1097/FTD.0000000000000047. [Epub ahead of print 2014 Jan 30] [DOI] [PubMed] [Google Scholar]