Abstract
In Parkinson’s disease (PD) patients, alpha-synuclein (α-syn) pathology advances in form of Lewy bodies and Lewy neurites throughout the brain. Clinically, PD is defined by motor symptoms that are predominantly attributed to the dopaminergic cell loss in the substantia nigra. However, motor deficits are frequently preceded by smell deficiency or neuropsychological symptoms, including increased anxiety and cognitive dysfunction. Accumulating evidence indicates that aggregation of α-syn impairs synaptic function and neurogenic capacity that may be associated with deficits in memory, learning and mood. Whether and how α-syn accumulation contributes to neuropathological events defining these earliest signs of PD is presently poorly understood. We used a tetracycline-suppressive (tet-off) transgenic mouse model that restricts overexpression of human A30P α-syn to neurons owing to usage of the neuron-specific CaMKIIα promoter. Abnormal accumulation of A30P correlated with a decreased survival of newly generated neurons in the hippocampus and olfactory bulb. Furthermore, when A30P α-syn expression was suppressed, we observed reduction of the human protein in neuronal soma. However, residual dox resistant A30P α-syn was detected in glial cells within the hippocampal neurogenic niche, concomitant with the failure to fully restore hippocampal neurogenesis. This finding is indicative to a potential spread of pathology from neuron to glia. In addition, mice expressing A30P α-syn show increased anxiety-related behavior that was reversed after dox treatment. This implies that glial A30P α-synucleinopathy within the dentate gyrus is part of a process leading to impaired hippocampal neuroplasticity, which is, however, not a sole critical event for circuits implicated in anxiety-related behavior.
Keywords: Gliosis, Parkinson’s disease, S100B, Transgenic, A30P alpha-synuclein, Conditional, Propagation
Introduction
In Parkinson’s disease (PD), the natively unfolded protein alpha-synuclein (α-syn) aggregates into Lewy bodies (LB) and Lewy neurites (LN) through the transition from monomers into stable fibrils via intermediate oligomers (Conway et al., 2000b). Differences in fibrillization rate have been observed in relation to distinct mutations within the α-syn gene. In particular the A30P mutation appears to promote its conversion into oligomers (Conway et al., 1998; Li et al., 2002; Narhi et al., 1999) whilst exhibiting a slow propensity to fibrillize (Choi et al., 2004; Conway et al., 2000a; Li et al., 2001). Additionally, increased levels derived from impaired clearance of the protein by lysosome and/or the proteasome (Cuervo et al., 2004; Nonaka and Hasegawa, 2009) may incite both release (Jang et al., 2010; Lee et al., 2005) and seeding effects of neurotoxic α-syn species on its soluble counterpart; thus starting a vicious cycle of further α-syn conversion, overload of protein degradation systems and exocytosis that may hasten cell death. These neurodegenerative processes are typically accompanied by reactive gliosis. Contributing glial cells overexpress GFAP to form stable astrocytic processes and S100B, a neurotrophic factor that is known to stabilize calcium homeostasis and to balance oxidative stress (Rothermundt et al., 2003). The topographical pattern of the neurotoxic progression suggests a spread of α-synucleinopathy within regions of the central nervous system, probably through cell-to-cell transfer between neurons (Desplats et al., 2009) or neuron to glia (Lee et al., 2010a). Despite the detection of this pattern the underlying mechanisms of disease progression that contribute to earliest PD symptoms as smell deficit, anxiety, and depression prior to motor effects remains unclear. Changes in anxiety and/or depression related behavior however, were directly attributed to alpha-synucleinopathy in transgenic animal models (George et al., 2008; Nuber et al., 2011; Oaks et al., 2013). Lifelong adult neurogenesis is implicated in memory formation and mood disorders (Eisch and Petrik, 2012). In vitro, α-syn mutations were observed to influence the fate and differentiation of neuronal progenitors (Schneider et al., 2007) and may therefore influence the capability of adult neurogenesis (Song et al., 2002). A detrimental impact of human α-syn on adult neurogenesis has been reported in mouse models expressing wild-type (WT) and mutant (A53T, A30P) α-syn (Winner et al., 2004, 2008, 2012). Importantly, in conditional mouse models, the suppression of either WT or A30P expression is able to restore the negative influence of α-syn on neurogenesis related to the olfactory bulb (Marxreiter et al., 2009; May et al., 2012; Song et al., 2002). The functional role of hippocampal neurogenesis is matter of intensive debate, e.g. recent studies showed that depletion of adult hippocampal neurogenesis led to a prominent anxiety- and/or depression-related behavior when stress was invoked (Revest et al., 2009; Snyder et al., 2011), whereas other studies did either not observe any change or an decrease in anxiety (recently reviewed by (Petrik et al., 2012).
Using a conditional model with doxycycline (dox) controllable neuronal expression of human mutant (A30P) α-syn we demonstrate here, that A30P overexpression in the hippocampus correlates with its posttranslational modification, astrogliosis, and a reduced survival of newborn neurons. We further observed an age-dependent and site-specific increase in co-localization of A30P α-syn and S100B within astrocytes that persisted after dox treatment, although the overall transgenic protein levels were reduced. Importantly, presence of glial A30P α-syn was concomitant with the pronounced impact and inability to restore hippocampal neurogenesis while these changes were not observed in the subventricular zone/olfactory bulb (SVZ/OB) circuit. Functional analyzes showed an increased anxiety in transgenic mice that was reversed by dox treatment. Thus our findings suggest that both neuronal and glial A30P α-syn pathology lead to the severely impaired neuronal plasticity of the hippocampus, which however may not be the sole site for anxiety related networks. These data also shed some light into the progression of synucleinopathies being transmitted between distinct cell-types and may help to explain in part the spatial progression of neuropathology.
Materials and methods
Transgenic mice
Construction of tet regulated mutant A30P α-syn expressing mice has been described in detail before (Marxreiter et al., 2009). The same cohort of mice was analyzed for the present study. All experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and approved by the local governmental commission for animal health. Animals for the analysis of adult neurogenesis were 2 months of age at the beginning of the experiment. Animals for the behavioral testing were 2 months of age and received. To suppress transgenic expression, animals received 2 mg/ml doxycycline (dox) (Sigma) in a 5% sucrose solution in the drinking water for 8 weeks. Sucrose was weekly reduced by 0.1% until a final concentration of 0.2% was reached as previously published (Nuber et al., 2011).
Sequential protein extraction
For sequential extraction of cytosolic, membrane-bound and detergent-stable α-syn, hippocampi and olfactory bulb were subdivided from dissected brains on a chilled stage. Sequential extraction of α-syn was performed as previously described (Tofaris et al., 2006). Shortly, tissues were homogenized in 100 µl of TBS + [50 mM Tris–HCl, pH 7.4, 175 mM NaCl; 5 mM EDTA, protease inhibitor cocktails (Calbiochem, CA)] and spun for 30min at 120,000 ×g. Afterwards, the pellet was extracted in TBS + containing 1% of Triton X-100 (TX), and TBS+, 1 M sucrose, and RIPA buffer (TBS+, 1% NP-40, and 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate), each extraction step followed by centrifugation for 20 min at 120,000 ×g. The detergent-stable pellet was finally solubilized in 8 M urea/5% SDS.
Western blot analyses
To analyze biochemical pattern of human α-syn, S100B, GFAP and influence of dox on protein expression, we used dissected brains of n = 3 untreated and dox-treated 4 months old adult mice. For western blot analyses 25 µg of TBS, TX of mouse brain protein extracts, and 0.2 µg of mono-ubiquitinated recombinant α-syn (Hejjaoui et al., 2011) were run on 4–12% Bis-Tris gels (Invitrogen, Life Technologies, Carlsbad, CA) and transferred to nitrocellulose membranes (Millipore, Bedford, MA). After washing in phosphate-buffered saline (PBS) each membrane was blocked for 30 min in PBST (phosphate-buffered saline with 0.2% Tween-20) containing 5% of bovine serum albumine (BSA) at room temperature and subsequently incubated with either human-specific antibody syn211 (1:600; Sigma, Saint Louis, MO; named hSYN), human and rodent specific antibody syn1 (1:1000; clone 42, BD Bioscience, San Diego, CA; named h + mSYN), or an antibody that recognizes mouse α-syn (1:1000; #4179, Cell Signaling Technology, Danvers, MA; named mSYN), or antibodies against astroglial markers S100B (1:500; Sigma) or GFAP (1:1000; Abcam, Cambridge, MA) in PBST containing 5% of BSA over night. After washing with PBST, membranes were probed with corresponding secondary antibodies (1:5000, American Qualex, CA), visualized with enhanced chemiluminescence (ECL, PerkinElmer, Boston, MA), and analyzed with the VersaDoc gel imaging system (BioRad). Proteins were normalized to β-actin (1:3000), used as a loading control. Quantification of signal intensities was performed as described previously (Nuber et al., 2008).
Analysis of neurogenesis
Single transgenic mice CaMKIIα-tTA served as control (named ctl, n = 4) for A30P α-syn transgenic mice (named A30P, n = 5). In an additional group, α-syn expression was suppressed starting at the age of 2 months for 8 weeks via dox application in the drinking water (named A30P + dox, n = 5). In order to label newly generated cells, all groups received intraperitoneal injections of bromodeoxyuridine (BrdU, 50 mg/kg) for 5 consecutive days at the age of 2–3 months and were killed 32 days after the first BrdU injection (see also Fig. 1A). All mice were kept in normal light/dark cycle (12 h light/12 h dark) and had free access to food. The animals water intake and weight was monitored daily. Animal perfusion and histological processing were performed as previously described (Marxreiter et al., 2009). Brains were cut sagitally into 25 µm sections using a sliding microtome (Leica, Bensheim, Germany) on dry ice. Sections were then stored in cryoprotectant (ethylene glycol, glycerol, PB, pH 7.4, 1:1:2 by volume) at − 20 °C until further processing.
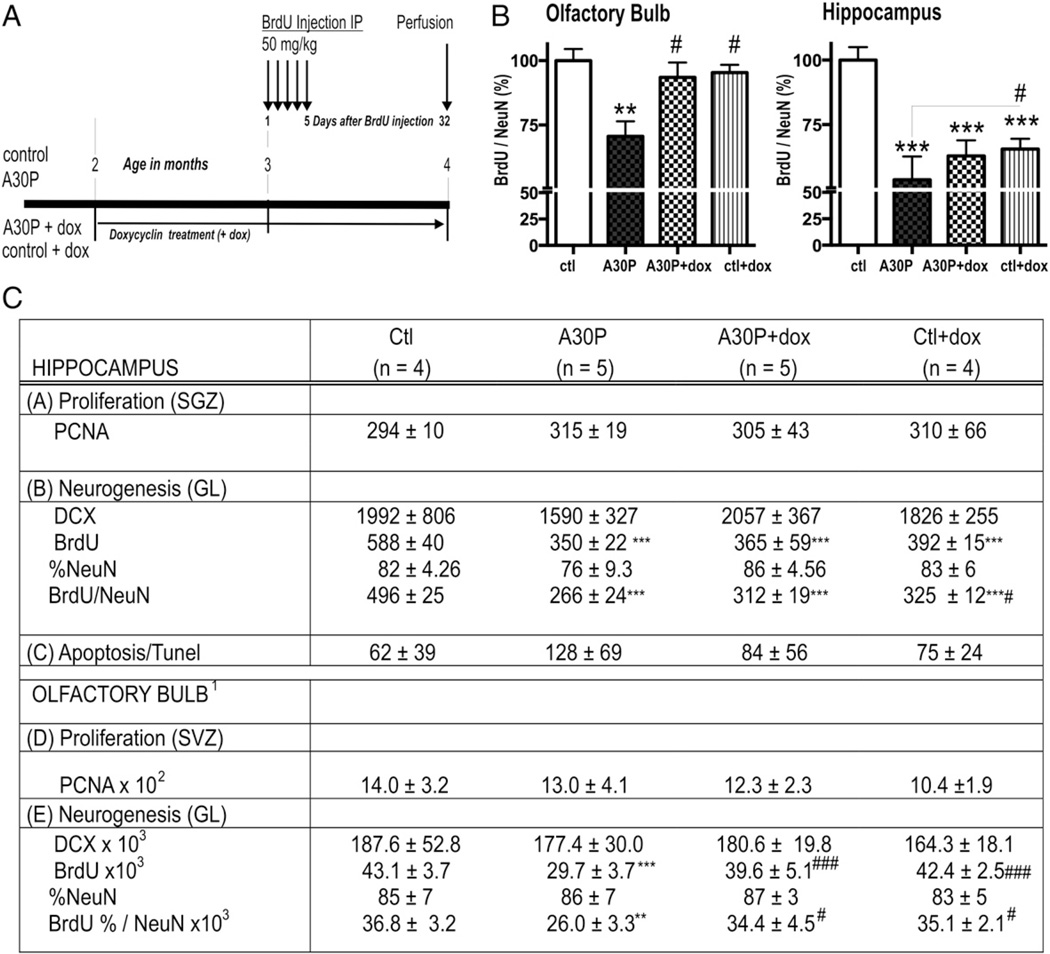
Fig. 1.
Stereological quantification and analysis of adult hippocampal neurogenesis. In (C) numbers and percentages are given as mean ± SD. (A) BrdU injection paradigm. (B) Respective graphs of stereological BrdU/NeuN counts in OB and HC. (C) The number of PCNA-positive cells is presented for the subgranular cell layer without significant difference. DCX total cell numbers reflect the neuroblast population with no difference between groups (p > 0.05). Quantification of neurogenesis is shown by the number of newly generated BrdU-positive cells, the percentage of NeuN-positive cells and the calculated number of new neurons (BrdU/NeuN = BrdU × %NeuN). ***™p < 0.001, **p < 0.01 versus ctl; # p < 0.05 and ## for p < 0.01, #### p < 0.001 versus A30P. (C) TUNEL-positive profiles were unchanged (p > 0.05).1 OB cell counts as previously published (Marxreiter et al., 2009.)
Antibodies, immunohistochemistry and confocal microscopy
For evaluation of neurogenesis, the following primary antibodies and final dilutions were used: mouse monoclonal anti-proliferating cell nuclear antigen (PCNA), 1:500 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), rat monoclonal anti-BrdU, (1:500, Oxford Biotechnology, Oxford, UK), goat polyclonal anti-doublecortin C18 (DCX), (1:500, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), mouse monoclonal anti-neuronal nuclei (NeuN) (1:500, Chemicon, Temecula CA, USA), rat monoclonal anti-human α-syn syn211, (1:1000, Sigma), rabbit anti-S100B (1:500, Sigma), mouse anti-glial fibrillary acidic protein (GFAP; 1:500, Abcam), rabbit anti-Calbindin 1:500 (Sigma). BrdU and PCNA labelling techniques were performed as previously described (Marxreiter et al., 2009). Donkey anti-mouse, -rat or -goat biotinylated, 1:500 (Jackson Immuno Research, West Grove, PA, USA) were used as well as the avidin-biotin peroxidase complex 1:100 (Vectastain Elite, Vector Laboratories, Burlingama, CA, USA).
Secondary antibodies for immunofluorescence were: donkey anti-rat fluorescein isothiocyanate (FITC), 1:500 and donkey anti-mouse CY5, 1:500 (Jackson Immuno Research, West Grove, PA, USA). For colocalization analyses sections were double labeled with antibodies against human α-syn (1:2500; syn 211) detected with the Tyramide Signal Amplification TM-Direct (Red) system (1:100, NEN Life Sciences, Boston, MA) and antibodies against GFAP (1:500, Abcam) and S100B (1:300, Sigma). Sections were imaged with a Zeiss 35 microscope (Zeiss, Germany) with an attached MRC 1024 LSCM system (Bio-Rad, Hercules, CA).
Counting procedures
All counting procedures were performed on blind-coded slides. For quantification, a systematic unified random counting procedure, similar to the optical dissector (Gundersen et al., 1988), was used with a semiautomatic stereology system (Stereoinvestigator, MicroBrightField, Colchester, VT, USA). Briefly, to count PCNA-positive cells in the subgranular zone (SGZ) and BrdU-positive, DCX-positive and TUNEL-positive cells in the granular cell layer, every 6th section per hemisphere was analyzed by exhaustively counting the region on each section under exclusion of the uppermost focal plane. Absolute cell numbers per anatomical region of one hemisphere were obtained by multiplication with 6. To obtain total numbers for the whole brain, values were doubled.
For differentiation analysis, on average 50 BrdU-positive cells were analyzed in the granular cell layer of the hippocampus by using a confocal scanning laser microscope (Leica TCS-NT, Bensheim, Germany). Newborn cells (BrdU positive) were determined for co-expression of neuronal (BrdU/NeuN) markers in the granular cell layer.
Thresholding analysis with Image J software
Digital confocal images (×60 magnification, 512 × 512 pixel area) of immunostained brain sections of the HC dentate gyrus, the GL and GR of the OB were taken (10 Images from each of three sections per mouse per region of interest, n = 3 mice per group). Images were converted to 8-bit grayscale and thresholding function was used to set a black and white threshold corresponding to the respective immunostaining. For quantification of GFAP and GLT-1, “Analyze Particles” function was used to sum up the total area of positive staining and calculate the fraction of the total area. The co-localization of images was analyzed with ImageJ software (National Institutes of Health) and the Colocalization Colormap plug-in (Adam Gorlewicz; http://rsbweb.nih.gov/ij/notes.html) as previously described (Zhou et al., 2011). The percent (%) area fraction of co-localization between α-syn and GLT-1 was performed using the plug-in “colocalization highlighter”. Co-localized points images were converted into 8-bit greyscale and quantified adjusted to a common threshold as previously described (Lee et al., 2012).
Behavioral studies
Open field analysis
General explorative behavior was conducted by an open field test. 2 months old male animals (A30P: n = 9; ctl: n = 6) were placed individually in a black plastic arena (50 × 50 × 50 cm) illuminated with 150 lx. Animal movement was tracked for 10 min using the VideoMot2 system (TSE Systems, Bad Homburg, Germany). For evaluation of motor and emotional behavior the box was virtually divided in a 15 × 15 cm center zone, and a surrounding 8 cm border belt.
TMT-induced fear behavior
2,4,5-trimethylthiazoline (TMT) is a synthetic fox feces odor and known to elicit fear behavior as avoidance reactions (Buron et al., 2007) and risk assessment in rodents (Hebb et al., 2004). TMT was dropped on a filter paper (4 cm × 5 cm) and placed into a corner of the open field arena and mice were tested as previously described (Nuber et al., 2011). Briefly, the corner with the odor was changed in a pseudo-randomized matter and movements of animals were tracked for 10 min. Time spent in the center and the corner (expressed as the percent of total time) was used as behavioral parameter. Avoidance score was calculated as the substracted difference between times spent in corner with and without TMT exposure.
Cliff avoidance test
The cliff avoidance test is commonly used to analyze risk assessment in mice (Brandewiede et al., 2005; Glynn et al., 2003; Lione et al., 1999). 2 months old A30P (n = 9) and ctl littermates (n = 6) were tested before and after 8 weeks of dox treatment. Cliff avoidance was tested in a box consisting of 4 non-transparent walls placed on bench side upon tape-secured glass plates (46 × 49 cm). Plates were positioned on the edge of a bench, so that one plate covered the bench, whereas the other plate suspended over the edge of the bench, 90 cm above the floor, creating the appearance of a visual cliff (see also Fig. 4A). A 40-watt lamp was positioned 60 cm below the open side, to further highlight the edge of the bench (“visual cliff”). Mice were placed in a central 5 × 5 cm area at the bench side at the edge of the cliff and the time spent by the mouse on the bench side and on the open side was measured during the 180 s testing period.
Fig. 4.
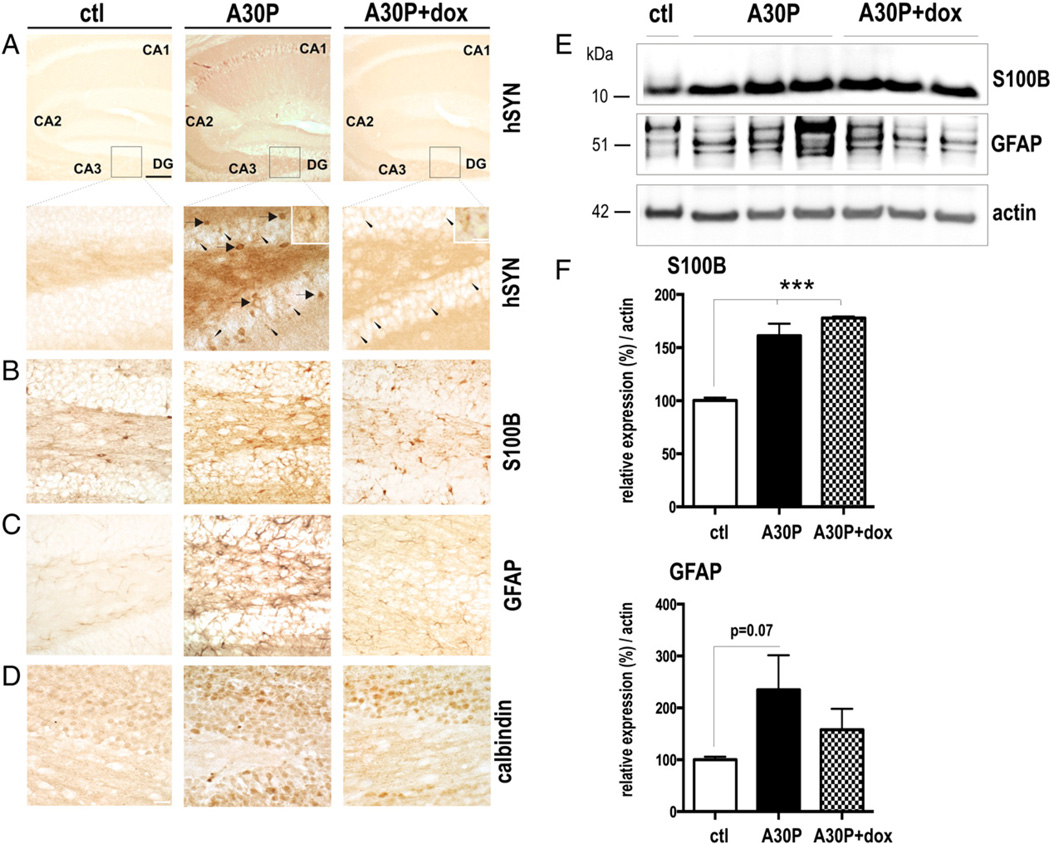
Human A30P α-syn overexpression increases glial markers. Vibratome sections of the hippocampal area from untreated and dox treated A30P mice (n = 3) were stained with antibodies against human α-syn (hSyn) (A), glial markers (B,C) and calbindin (D). Representative staining of hSyn a neuropil and cellular soma (arrow) was found in granule cells of the dentate gyrus. Higher power microphotographs additionally revealed labeled cell bodies with delicate processes resembling astrocyte-like cells (arrowheads; insets). Dox treatment cleared human α-syn staining of the neuronal cell-soma. Residual staining of the smaller and star-shape astrocyte-like structures were detected after dox treatment (arrows; higher power inset). (B) Vibratome sections of the indicated brain areas were stained with antibodies against astrocytic marker, anti-GFAP antibody and (C) anti-S100B antibody. Dox treatment revealed numerous S100B immunoreactive cells in the dentate gyrus also seen in mice without treatment (D) Calbindin staining of adjacent sections showed an overlapping increase of immunoreactivity in the indicated brain regions. (E) Representative immunblots of hippocampal brain extracts displaying tendentious upregulation of GFAP and significant increase of S100B levels in A30P and dox-treated A30P mice. (F) Quantification of expression levels of 3 independent blots. ***™p < 0.001 (One-way ANOVA post-hoc LSD). Scale bar: 60 µm in A 10 µm in insets and 20 µm in B-D.
Statistical analysis
Data are expressed as mean values ± standard error (SEM). Statistical analysis of neurogenesis and cliff avoidance test was performed using a one-way analysis of variance (ANOVA) followed by Bonferroni’s Multiple Comparison Test. Statistical analysis of immunoreactivity was performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests or Fisher’s LSD for pairwise comparisons. For quantitative analysis of immunoblots we used two-tailed student’s t-test (PRISM 6, Graph Pad software, CA).
Results
Survival and restoration of newly built neurons is more severely affected in HC than in OB of A30P mice
In our previous study we found a significant reduction of newborn neurons in the olfactory bulb [Fig. 1 (Marxreiter et al., 2009)] inversely correlating with strong α-syn overexpression in conditional transgenic mice. These mice express human mutant A30P α-syn directed by the neuron-specific CaMKIIα promoter (see also Figs. 2A,B). We therefore asked, whether this phenotype also applies for the hippocampus and leads to an associated behavioral alteration. We first stereologically analyzed newly generated, BrdU positive cells in the hippocampal dentate granule layer (GL) and observed a highly significant decrease of BrdU cell numbers in A30P when compared to controls (ctl) (BrdU, Fig. 1C, one-way ANOVA p = 0.04; post hoc Bonferroni, p < 0.001; see also Suppl. Fig. 1). To characterize the differentiation and survival of the newly generated cells in detail we analyzed co-labeling of BrdU with NeuN via confocal microscopy. We first analyzed whether overexpression of α-syn interfered with maturation of newly generated neurons using PCNA and DCX antibody as markers of developing neurons. We did not find a significant difference in the detected neuroblast population (Fig. 1C p > 0.05). Differentiation as revealed by the percentage of BrdU co-labeling with NeuN, was unchanged between A30P and the ctl group (% NeuN, Fig. 1C, p > 0.05).
Fig. 2.
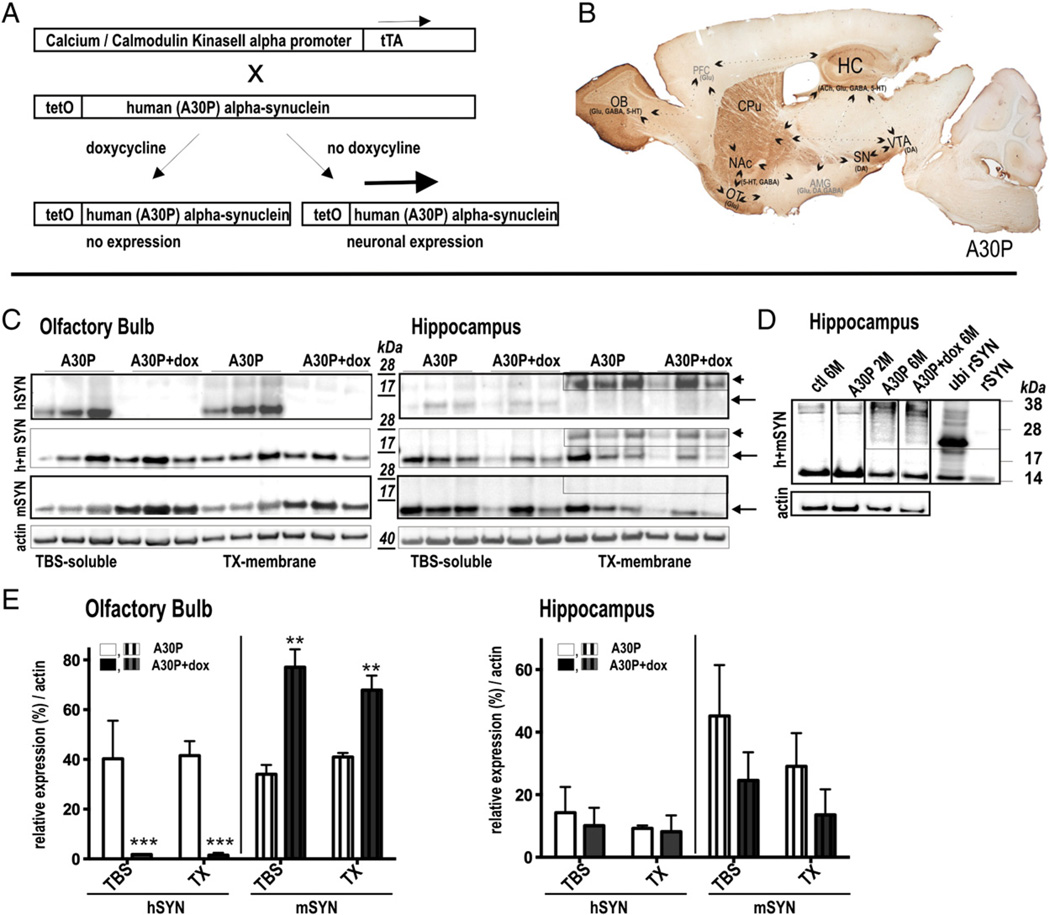
Expression pattern and regulatability of human A30P α-syn in subregions of conditional mouse brain (A) In double transgenic mice, expression of human A30P α-syn gene is under control of the tetracycline-controlled transactivator (tTA) responsive promoter (tetO) and tTA expression under the control of the CaMKIIa promoter, leading to neuron-specific expression. Expression is abolished by tetracycline derivates (doxycycline; dox) as it binds to tTA rendering it incapable of binding to tetO sequence. (B) Sagittal overview showing strong expression of human A30P α-syn in the (OB), the substantia nigra (SN), the ventral tegmental area (VTA), the nucleus accumbens (NAc), the caudate–putamen (CPu), the olfactory tubercle (OT) and the hippocampus (HC). These brain regions are implicated in resilience to anxiety disorders and are reported to regulate stress response [modified from (Russo et al., 2012) with the OB circuitry added in accordance to its function in depression (Song and Leonard, 2005)]. (C) Immunoblots of olfactory and hippocampal brain extracts using antibodies against human α-syn (hSYN), mouse α-syn (mSYN) and mouse and human α-syn (h + mSYN). Ultracentrifugation and sequential extraction steps were used to obtain TBS-sol-uble and TX-membrane fractions of α-syn in A30P mice with and without treatment of dox. Tight regulation of 14 kDa soluble and membrane bound α-syn was detected in the OB whereas in the HC ∼25 kDa dox-resistant α-syn species accumulated in the membrane fraction. Note that in the OB the endogenous α-syn was opposingly expressed. (D) HC α-syn and ubiquitinated recombinant α-syn expression pattern. (E) Quantification of human and murine α-syn expression levels (n = 3 per group). Results are expressed as mean + SEM. **p < 0.01; ***p < 0.001 (two-tailed t-test).
The total number of newly generated BrdU/NeuN positive cells (BrdU × %NeuN) represents the survival of newly generated neurons. In the A30P group, multiplication of newly generated cells and neuronal differentiation resulted in a marked decreased survival in the A30P group compared to the ctl group (55% of ctl level; Figs. 1B,C; one-way ANOVA, post hoc Bonferroni, p < 0.001). Interestingly, the reduction of hippocampal BrdU/NeuN positive cells exceeded the relative loss detected in OB (∼29%) in A30P mice (p < 0.05, pairwise comparison). Whereas OB neurogenesis was fully restored after dox treatment, we found only a partially increase (∼17%, p < 0.05) in the hippocampal DG of the identical animal (Figs. 1B,C). Our results also show that dox treatment had a general negative effect on hippocampal neurogenesis, which was however less pronounced in ctl group when compared to A30P mice (Figs. 1B,C, p < 0.05).
α-Syn accumulates as dox-resistant structures in hippocampus of A30P mice
The detected site-specific difference in neurogenesis impairment and restoration prompted us to analyze α-syn pattern and regulatability in the olfactory bulb (OB) and hippocampus (HC) in A30P transgenic mice. Inducible expression of human mutant (A30P) α-syn in double transgenic mouse brain depends on the expression of the doxycycline (dox) modulated tet transactivator (tTA) (Mayford et al., 1996) (Fig. 2A). In our previous publication (Marxreiter et al., 2009) and as displayed in Fig. 2C, we observed an intense and tightly controllable human α-syn expression in e.g. the olfactory bulb (OB) of double transgenic A30P mice (p < 0.001, Fig. 2C). Immunohistochemical staining showed an additional immunoreactivity in the striatum (CPu), the substantia nigra (SN), the ventral pallidum (VP), the ventral tegmental area (VTA), the olfactory tract (OT), the nucleus accumbens (NA) and the hippocampus (HC) (Fig. 2B). Immunopositive neurons were detected prominently in regions of the mesolimbic and striatal DA pathway, i.e. the ventral striatum and the midbrain VTA, that are also innervating several limbic structures as the amygdala and prefrontal cortex (PFC), although the latter regions were devoid of transgenic expression. A specific function of these immunopositive subregions is not unitary and they may interact in terms of memory, addiction, stress and plasticity, involving the serotonin (5-HT), dopamine (DA), gamma-aminobutyric acid (GABA), glutamate and acetylcholine (ACh) neurotransmitter systems (Fig. 2B). Biochemical characterization of subdivided mouse brain extracts of the OB and the HC confirmed transgenic expression in untreated animals. Immunoblotting using the human α-syn specific antibody syn211 or an antibody recognizing mouse and human α-syn revealed that a significant proportion of the human α-syn migrated at approximately 25 kDa at the membrane fraction of the HC. Such a discrepancy may indicate toward either a dimerization process or post-translational modification. Recent studies (Hasegawa et al., 2002) (Anderson et al., 2006) reported (mono-, di, tri-) ubiquitinated α-syn species at the size of approximately 22–25, 29–32, 36–42 kDa, respectively. We found a similar migration pattern when using mono-ubiquitinated recombinant α-syn (courtesy of H. Lashuel, Ecole Polytechnique Fédérale de Lausanne, Switzerland), indicating that this posttranslational modification could account for the migration shift observed in the polyacrylamide gel (Fig. 2D). In contrast, dimers, an entity allowing formation of insoluble fibrils, are less stable under denaturating conditions (Tsika et al., 2010). After dox treatment we found an opposing reduction of rodent α-syn specific for the OB (p < 0.01) that might compensate for the detected high transgenic protein levels, which was not detected in the HC (Figs. 2C, E). Treatment with dox further downregulated the monomeric α-syn in the OB, whereas in the HC, the more prominent higher molecular signals were not cleared after dox treatment (Figs. 2C, E). As the higher molecular form was detected with antibodies against human α-syn but not with the antibody against murine α-syn it may account for modification of the human α-syn.
A30P α-Syn overexpression is associated with increased anxiety in A30P mice, which is improved after suppression with dox
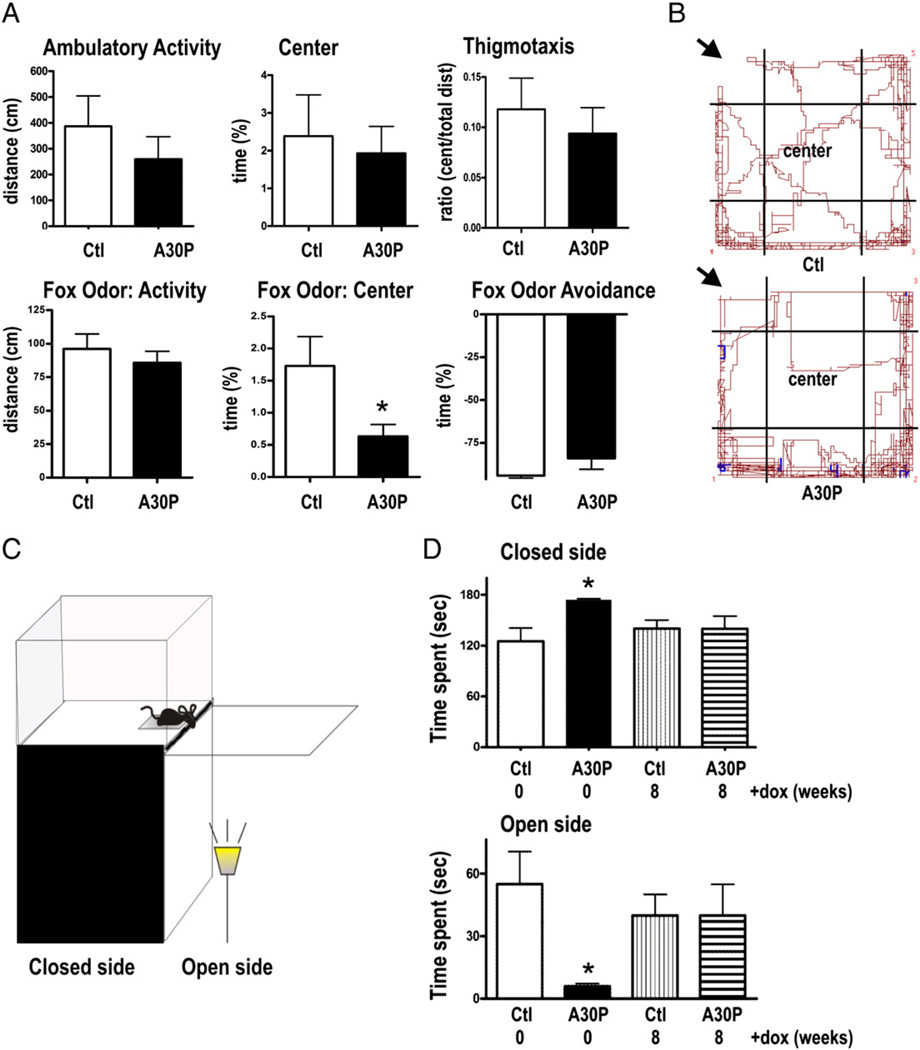
Impaired neurogenesis and pathological α-syn accumulation in respective brain regions may lead to a phenotype relating to dysfunctional mesolimbic circuitries, which play major roles in reward, the vigilance state, and in regulation of hippocampal activity (Morgane et al., 2005). Thus we first performed open field analysis to test for general differences in behavioral motivation for novelty and locomotor activity. In the open field the 2 months old A30P mice showed a non significant trend towards a decrease in ambulatory locomotor activity [386.5 ± 118.4 cm (ctl), 259 ± 86.4 cm (A30P), Fig. 3A, p > 0.05], and novelty seeking behavior as indicated by percentage of time spent in the center [2.4 ± 1.1 (ctl), 1.9 ± 0.7 cts (A30P), Fig. 3A, p > 0.05] and the ratio of time spent in the center to the total distance [0.12 ± 0.03 (ctl), 0.09 ± 0.03 (A30P); Fig. 3A; p > 0.05; two-tailed t-tests). Since decreased neurogenesis may correlate with increased anxiety and stress (Crupi et al., 2010; Revest et al., 2009), potentially triggered by a predatory odor (Mirescu and Gould, 2006; Tanapat et al., 1998), we further exposed A30P mice to a component of fox feces, 2,4,5-trimethyl-3-thiozialin (TMT), to add a fear-inducing stimulus to the open field test. Pairing the open field with this olfactory fear stimulus, we observed that A30P mice avoided the center of the open field arena as shown by the significant decrease in percentage of time spent in the center when compared to ctl group [1.7 ± 0.5 (ctl), 0.6 ± 0.2 (A30P), Fig. 3A, p < 0.05, two-tailed t-test). A30P and ctl mice showed no difference in avoidance behavior of the odor corner [94% ± 1.4% (ctl), 89% ± 3% (A30P), Fig. 3A, p > 0.05] displaying normal sensory function to smell this emotionally active odor. The performance was visualized as shown by representative tracking-paths of mice (Fig. 3B). There was no difference in overall locomotor activity between both groups (p > 0.05). Also, weights of the animals did not significantly differ (data not shown).
Fig. 3.
Suppression of α-syn reverses anxiety-related behavior in A30P mice. (A) Spontaneous locomotor behavior was analyzed over a 10 min period by using video-tracking software. Although mice did not show significant differences in locomotor behavior, A30P mice (n = 9) revealed tendency to visit the center area less frequently when compared to respective controls (ctl, n = 6). (B) Next, A30P mice were exposed to the fox-like odor TMT to stimulate fear related behavior. Decreased time spent in the center implies enhanced anxiety (p < 0.05). Aversive behavior to the TMT odor was unchanged as both tested groups significantly avoided the corner with TMT (p< 0.05). (B) Note decrease in center paths of an A30Pmouse exposed to TMT (arrows indicate odor corner) when compared to ctl in representative track panels. (C) Visual cliff avoidance of 2 months-old A30P mice and ctl was measured by estimating time spent on the closed bench side and brightly lid open side of the elevated visual cliff held. The cartoon in (C) shows set-up of apparatus used. (D) As expected, both ctl (p < 0.01) and A30P mice (p < 0.001) avoided the open side when compared to bench side; however there was a significant effect of genotype, showing that A30P spent more time at the bench side (p < 0.01) and less time at the open side (p < 0.01) when compared to ctl. Suppression of α-syn transgene expression reversed the anxiety-like behavior, as no significant difference was detected between dox-treated groups. Data are displayed as mean + SEM (*p < 0.05 One-way ANOVA with Tukey’s multiple comparison post-hoc analysis).
We next conducted an elevated visual cliff avoidance task that is used to test risk assessment and anxiety in mice based on the hypothesis that there is greater stress from stepping over a visual cliff onto a brightly lit area versus the dark and closed bench side (Brandewiede et al., 2005; Glynn et al., 2003; Lione et al., 1999) (Fig. 3C). Importantly, A30P mice spent significantly less time exploring the open glass side [55 ± 16 s (ctl), 6 ± 1 s (A30P)] and more time at the closed bench side [125 ± 16 s (ctl), 174 ± 1 s (A30P)], respectively (Fig. 3D; one-way ANOVA p = 0.03, post hoc Tukey p < 0.01;). In order to analyze reversibility of the detected anxious phenotype, mice were treated for 8 weeks with dox and re-tested in the cliff avoidance task. Treatment significantly increased time spent at the open side in a process over time selectively in A30P (one-way ANOVA p = 0.03, post hoc Tukey p < 0.05) whilst this behavior of ctl mice did not differ over the tested period (p > 0.05). Importantly, after 8 weeks of dox treatment time spent at bench and on the open side was comparable to those seen in healthy controls [40 ± 10 sec (ctl + dox), 40 ±15 sec (A30P + dox) p > 0.05, post hoc Tukey, Fig. 3D], suggesting that neuronal clearance of A30P by suppression of α-syn expression resulted in an improvement of the anxiety-like behavior.
A30P α-syn overexpression increases glial reactivity in the HC
Convergent evidence indicates that interconnections between the HC, amygdala (AMG), and prefrontal cortex (PFC) are implicated in the regulation of fear responses [reviewed by (Herry et al., 2010)]. We therefore hypothesized that the observed biochemical and functional alterations were due to pathological changes in the HC. We used immunohistochemical stainings against glial fibrillary acidic protein (GFAP) and S100B to identify astrogliosis, since it is a common cellular response surrounding neuropathology in brain of α-syn tg mice (Cabin et al., 2005; Lee et al., 2002; Lim et al., 2011; Neumann et al., 2002; Nuber et al., 2011; Tofaris et al., 2006). We found that neurons in the granular layer (GL) of the DG and the CA1 region accumulated human α-syn in the cellular soma (Fig. 4, overview panel). Higher magnification of the DG area showed additional numerous darker A30P accumulations in distinct cell types of smaller shape, resembling glial cells. Using antibodies against GFAP and S100B we observed a strong increase of the immunoreactive signal in regions that also displayed strong accumulation of transgenic protein (Figs. 4B,C). After dox treatment some of the smaller patches of α-syn persisted whereas adjacent neuronal soma that was strongly stained in the untreated condition was devoid of α-syn immunoreactivity (Fig. 4A). We further observed a high amount of S100B positive cells localized in the DG after dox treatment (Fig. 4B) whereas the GFAP staining was strongly reduced (Fig. 4C). Excess of expressed or released S100B was demonstrated to result in a sustained increase of intracellular Ca2+ levels concomitant with cell death (Mariggio et al., 1994), that might be buffered by an upregulation of Ca2+ binding protein, calbindin. As expected, an upregulation of calbindin was detected in DG. After dox treatment increase in calbindin immunoreactivity was still detected in the DG (Fig. 4D).
Immunoblotting using antibodies against S100B and GFAP (Fig. 4E) matched the increase of astroglial markers in HC of A30P transgenic mice on structural level (Figs. 4B,C). We observed a tendentiously increased GFAP protein expression in A30P that was less pronounced in dox treated group (one-way ANOVA, p = 0.33; two-tailed t-test: ctl vs A30P: p = 0.07). Importantly, S100B was significantly higher expressed in both untreated and dox-treated A30P mice (p < 0.001, post hoc Tukey).
Taken together these data suggest, that α-syn accumulation was associated with dox resistant pathological changes in the hippocampal DG.
A30P α-syn leads to gliosis and accumulates as dox-resistant deposit in HC S100B glial cells
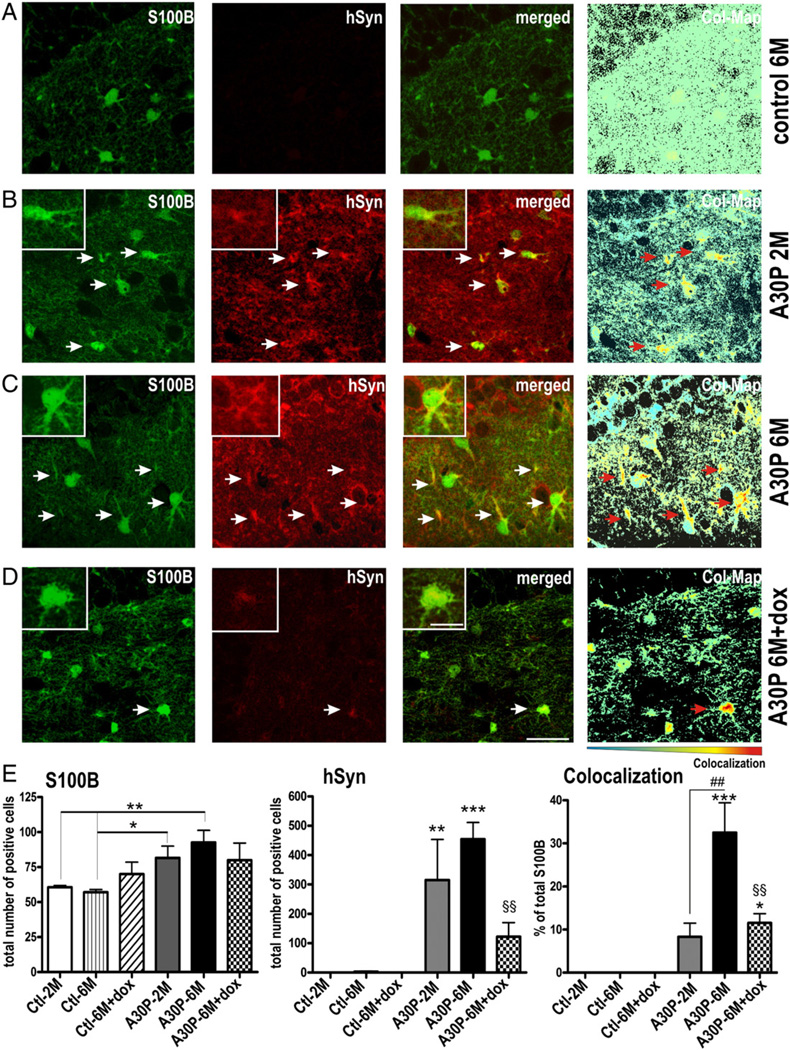
Our finding, that α-syn accumulated within distinct cellular structures in hippocampal DG inducing a high immunoreactivity against S100B lead us to investigate, whether human α-syn directly co-localizes with glial markers (Fig. 5). Using confocal microscopy, we indeed observed a strong co-localization of human α-syn with numerous S100B positive cells (Figs. 5B–D). Since human α-syn was expressed under the regulative control of the CaMKIIα promoter, that has been shown to be specific for neuronal expression (Chandrasekaran et al., 2006; Erondu and Kennedy, 1985; Mayford et al., 1997; Wang et al., 2008) these data suggest that a spread of human α-syn from neuron to glia may occur as a specific pathogenic feature in the DG of A30P mice. In PD spread of α-syn pathology positively correlates with age in both transgenic mice (Mougenot et al., 2012) and human disease (Kordower et al., 2008; Li et al., 2008). Therefore, we performed stereological quantification of S100B and human α-syn double-labeled DG cells at the age of 2 and 6 months (Fig. 5E). We first quantified total numbers of S100B positive cells and observed overall increased levels by overexpression of trans-gene protein (one-way ANOVA, p = 0.05). Using planned comparisons to study a possible age effect, we found a profound increase of S100B cell numbers at 6 months in A30P mice (∼37%; p < 0.01). When analyzing double labeling, we found that in 6 months old mice approximately 33% (32.6% ± 6.8% SEM) of S100B cells displayed α-syn immunoreactive patches. Importantly, co-localization events at 6 months were significantly higher than at 2 months of age, that showed less than 10% (8.3% ± 3.1% SEM; p < 0.01) of S100B cells harboring α-syn; the percentage of the latter was non-significant when compared to control mice at both time points (p > 0.05; Fig. 5B). Interestingly, S100B staining was detected in the cell body and in occasional processes in all groups but was more clearly detected in glial processes in older A30P animals (compare insets of Fig. 5). To estimate whether the α-syn pattern is dox resistant, we analyzed co-localization events in 6 months old mice that were treated for additional 8 weeks with dox. We detected both a reduction of approximately 73% of α-syn positive cell numbers and co-localization of α-syn in S100B positive cells after dox treatment (11.5% ± 2.2% SEM, p < 0.01) when compared to the untreated 6 months old A30P mice. However, when directly compared to treated and untreated control mice, the co-localization events were still significantly higher in dox-treated A30P condition (planned comparison; p < 0.05), whilst this was statistically not observed for 2 months-old A30P mice. Taken together, this indicates an accumulation of dox-resistant glial α-syn as an age-dependent effect. It also favors the hypothesis that the detected glial α-syn originates from neurons.
Fig. 5.
Abnormal accumulation of human A30P α-syn in S100B expressing glial cells. Sagittal sections from A30P single-tg ctl (6 months old, 6 M) and A30P double-tg animals (2 M and 6 M), and dox treated A30P and ctl mice (6 M) were immunostained with S100B and an antibody against human α-syn (hSyn) to label neuronal and glial α-syn co-localization. (B, C) Confocal images of 2 M and 6 M A30P mice showed strong somatic and punctuate staining of hSyn in the neuropil. α-Syn was occasionally detected as small granules in S100B positive glial cells. Note the increase of hSyn and higher branching of S100B-positive glia in 6 M A30P mice. Residual patches of human α-syn were still detected in S100B positive glia after 8 weeks of dox treatment, measured by percentage of S100B with α-syn. To better visualize the co-localization, the Colocalization ColorMap (Col-Map) plugin was used. (E) Data are expressed as means + SEM of n = 3 per group. * p < 0.05, ** p < 0.01, *** p < 0.001 versus ctl, ## p < 0.01 versus A30P-2 M, §§ p < 0.01 versus A30P-6 M. Scale bars: 25 µm, and 5 µm in insets.
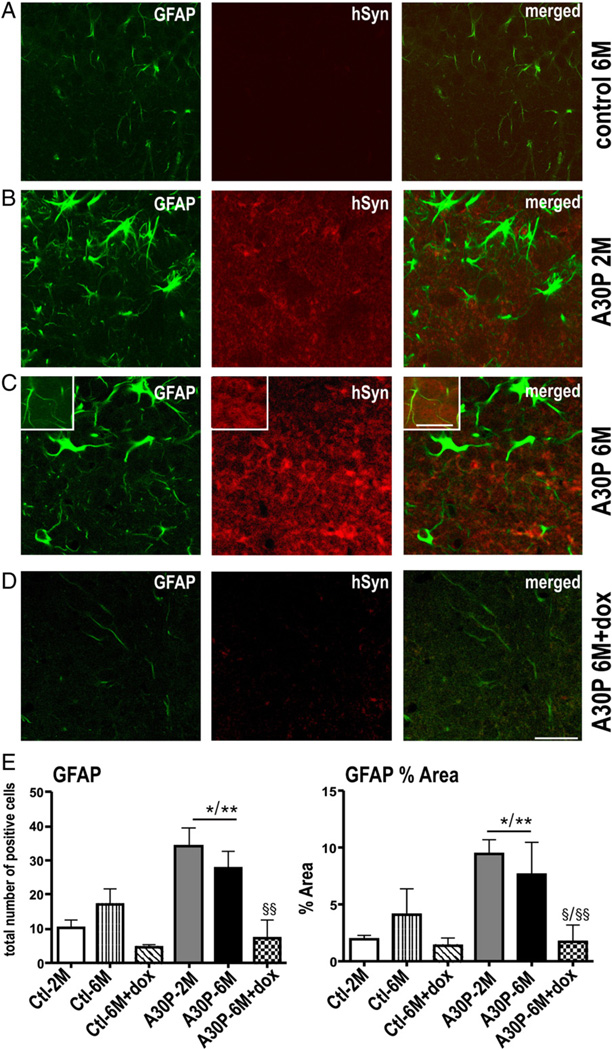
We next analyzed human α-syn and GFAP double labeling in the DG (Fig. 6). We first evaluated increase in astrogliosis by counting GFAP positive round-structured cell bodies (total cell number, one-way ANOVA p < 0.001) and its processes (% area, one-way ANOVA, p = 0.02). Independent of age, A30P mice showed a significant increase in both cell bodies (∼44%; p < 0.05, post hoc Tukey) and stained surface area (∼35%; p < 0.05, post hoc Tukey) when compared to controls (Fig. 6E). Dox treatment reduced the GFAP immunoreactivity (∼7%, p < 0.01) to levels of control mice. However, although closely associated with A30P positive neurons, the human α-syn protein was not co-detected with GFAP in astroglial cells. Therefore, the dox-resistant α-synucleinopathy detected in S100B positive glia may depend on either compartment or the astrocytic subtype as previously described in course of amyloid pathology in AD (Wilcock et al., 2009).
Fig. 6.
Human A30P α-syn overexpression increases GFAP-expressing astrocytes, which is suppressed by dox. Double-fluorescence confocal photomicrographs showed hypertrophic astrocytes immunostained with GFAP associated with overexpression of A30P but without col-localizing with human α-syn (B,C and inset) when compared to dox-treated (D) or crl (A). (E) Cell bodies and processes were quantified as total number and area fraction per 10 sight-fields and data are present as mean + SEM. *p < 0.05, **p < 0.01 versus ctl; Scale bar: 25 µm and 10 µm in insets.
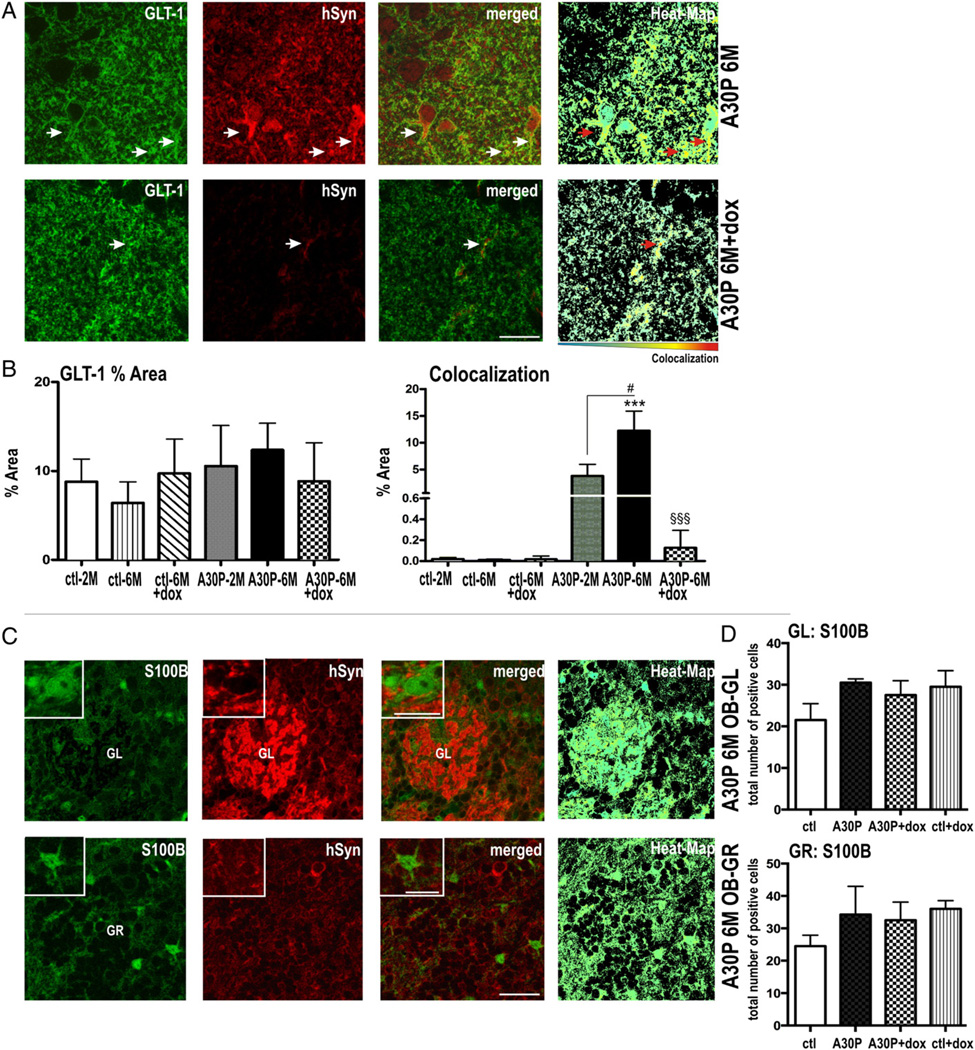
In order to more clearly define the co-localization of human α-syn within astrocytes, we next analyzed sections labeled with the astroglial membrane marker GLT-1 (Fig. 7). The expression pattern of GLT-1 was similar in the DG of all tested mice (Figs. 7A,B). When analyzing double-labeling, we found that a small number of α-syn positive puncta co-localized with GLT-1 at 2 months (3.9% ± 2.2%). In 6 months old A30P mice, the number of co-localization events was significantly increased (12.3% ± 3.6%, p < 0.05), thus further confirming an age-dependent effect of glial α-syn accumulation. Most likely owing to the relative rare localization of α-syn at the astrocytic membrane, the level of co-localization, albeit detectable, did not reach significance in dox-treated A30P mice (one-way ANOVA, p > 0.05).
Fig. 7.
Site and compartment specific co-localization of human A30P α-syn assessed by S100B and GLT-1 co-staining in A30P mice. (A) Representative immunostaining for GLT-1 and human α-syn in the DG area of the hippocampus and (B) quantitated by percent of immunoreactive area Co-localization of GLT-1 (green) and α-syn (red) is shown in yellow fluorescence (arrows in merged) were also visualized by Col-Map plugin (Heat-Map). (C) Double labeling astrocytes with S100B (green) and α-syn (red) and (D) quantitation by stereological counts (per 10 sight-fields) did not show glial accumulation in OB. Data are present as mean + SEM. #p < 0.05 versus A30P-2 M, ***p < 0.001 versus ctl: §§§ p < 0.001 versus A30P-6 M. Scale bar: 25 µm in A, 30 µm in B and 10 µm in insets.
Since OB neurogenesis and the behavioral phenotype were reversed after α-syn suppression, we speculated that the α-syn induced gliopathy might be site-specific for the HC in our A30P tg mice. We therefore further analyzed the OB glomerular (GL) and granular (GR) cell layer for co-localization of human α-syn within astrocytes (Fig. 7C). We did not find a significant co-localization of human α-syn in S100B positive cells (as exemplarily shown via Heat-Maps in Fig. 7C). Also, S100B positive cell numbers were not significantly upregulated by A30P α-syn overexpression in the OB (Fig. 7D, one-way ANOVA, p > 0.05).
Discussion
α-Syn is the major component of Lewy bodies and Lewy neurites and its contribution to the etiology of sporadic and familial Parkinson’s disease is evident. It is post-translationally modified in vivo and can adopt several oligomeric states when converting from its soluble state into mature fibrils. Accumulating evidence suggest that in course of its pathological conversion α-syn may be transferred, seeding α-syn pathology in recipient cells (Desplats et al., 2009; Hansen et al., 2011; Luk et al., 2012a,2012b); a process that may account for α-syn pathology progressively affecting distinct regions of the central nervous system of PD patients in accordance to hierarchical pattern of neuropathological Braak stages (Braak et al., 2003). Beside the occurrence of neuronal pathology, astroglial deposition has been reported to associate intraneuronal inclusions in the brain of PD and was hypothesized to be due to a “slightly alterated α-syn molecule that escapes from terminal axons (…) and is taken up by astrocytes” (Braak et al., 2007). Here we show, that in A30P mouse brain human α-syn accumulates in S100B glial cells although the expression is regulated by the CaMKIIα promoter, which specificity for neurons has been well established by numerous studies (Chandrasekaran et al., 2006; Erondu and Kennedy, 1985; Mayford et al., 1997; Wang et al., 2008). A pathological spread is further supported by our biochemical and structural analyses demonstrating that (i) A30P α-syn patches are detected with specific astroglial markers, (ii) accumulating over the experimental period of 6 months, and (iii) displaying dox resistance, despite its controllable and neuron-specific expression in other brain regions as exemplarily shown for the OB. However, since these mice carry two transgenic constructs, we are unable to fully exclude an artifact of transgene expression leading to an accumulation of this protein in a specific cell-type and brain region over time. The present study was built on our previous finding, that A30P α-syn led to impaired neurogenesis of the SVZ/OB circuit, associated with ubiquitylation that was reversed after suppression of transgenic expression (Marxreiter et al., 2009). The proportion of the posttranslationally modified α-syn band that co-migrated with ubiquitinated recombinant α-syn was increased and dox stable when compared to signals seen in OB protein lysates. Similarly, astrogliosis, S100B positive cell numbers, co-localization within astrocytes, and the HC neurogenesis deficit exceeded the observed pathology in the OB, rendering the hippocampal DG a more susceptible region in regard to α-syn induced pathology. The impact of ubiquitinated α-syn in aggregate formation is yet incompletely defined, but ample evidence suggest, that it directly correlates with a dysfunction of clearance pathways, including the ubiquitin proteasome system (UPS) (Rideout et al., 2001; Snyder et al., 2003; Tofaris et al., 2001) and autophagy (Ebrahimi-Fakhari et al., 2011; Rott et al., 2011) but also mitochondrial pathology and an increase of α-syn oligomers (Martins-Branco et al., 2012). Previous studies reported that the A30P mutation per se fortifies dysfunction of UPS (Petrucelli et al., 2002), impairs chaperone-mediated autophagy (Cuervo et al., 2004) and its oligomerization process (Conway et al., 1998; Li et al., 2002; Narhi et al., 1999) further accelerating the pathology. Although it is not clear how A30P is released or taken up by HC S100B cells, its secretion through cell membranes into the extracellular space might be a protective strategy to avoid soluble oligomeric α-syn toxicity. The observed dox resistance of glial A30P α-syn may relate to an ineffective degradation of the transgenic protein. Indeed, mono-ubiquitination and de-ubiquitination implicates in protein sorting from endosomes to lysosomes (Marchese et al., 2008). This would be also in line with previous observations revealing that glial cells with either high α-syn protein levels or lysosomal inhibition failed to clear α-syn aggregates concomitant with an increased neurotoxic inflammatory response (Gu et al., 2010; Lee et al., submitted for publication). Interestingly, we observed co-localization of α-syn with S100B and GLT-1, but not GFAP in hippocampal astrocytes. In parallel, dox-resistant α-syn was detected with a steady high level of S100B positive cells whilst GFAP was downregulated by dox treatment. Astrocytes are known to express GFAP, S100B or both in relation to their function: GFAP positive astrocytes were shown to be extensively coupled via gap junctions within their local environment whereas S100B astrocytes are discussed to be less coupled and the ratio of their appearance relate to the specific hippocampal region (Walz, 2000). Thus, it may be speculated that a possible deprivation of gap junction communication of the S100B positive phenotype accentuated the observed α-syn accumulation. A shift in the astrocytic phenotype in favor to S100B was reported in course of amyloid pathology of a neurovascular model of AD (Wilcock et al., 2009). The detected continuous high expression level of S100B and calbindin may also point toward an increase in Ca2+ level, which is buffered by mitochondria under physiological conditions (Parekh, 2003). In general, S100B is thought to have a neuroprotective function at low concentrations, but was shown to impair neurogenesis at higher micromolar concentrations (Lu et al., 2011). Secondly, reactive astrogliosis inhibits neurogenesis in the adult hippocampus (Ekdahl et al., 2003; Monje et al., 2003) and inflammation promotes progenitors to preferentially differentiate into astrocytes, which may secrete S100B (Chen et al., 2008; Tatsumi et al., 2008). This sequence of events would further decrease neurogenesis and may account for the strong reduction of NeuN/BrdU cells detected in DG of A30P mice and the detected difference in site-specific severity. However, it is unclear at this point, whether the high level of S100B in A30P animals is based on differences in glial expression patterns or the proliferation state.
Impaired neurogenesis is discussed to either implicate with anxiety in mice whereas other studies did not find an associated anxiogenic response (Petrik et al., 2012). Recent studies show that treatment with antidepressant/anxiolytic drugs increased hippocampal neurogenesis in α-syn tg mice (Kohl et al., 2012; Ubhi et al., 2012). An increase in anxiety behavior observed in our mice correlates with the detected accumulation of α-syn in forebrain areas, especially the OB and HC. The CaMKIIa promoter is known to induce a phenotype that attributes to pathology of mesolimbic circuitries. In particular, HC α-synucleinopathy was shown in relation to contextual fear memory and retention in conditional CaMKIIα and α-syn double transgenic mice (Lim et al., 2011; Nuber et al., 2008). Interestingly, anxiety, anhedonia, and fatigue are early stage non-motor symptoms in PD (Hodgson et al., 1999; Owen et al., 1993; Pillon et al., 1996) and defects in adult hippocampal neurogenesis have been observed in PD patients (Hoglinger et al., 2004; Winner et al., 2012). However, after eight weeks of dox treatment we observed an amelioration of the anxiety-like behavior whereas the detected glial α-synucleinopathy and the negative impact in HC neurogenesis were not fully restored. Thus, the observed behavioral effects are therefore likely to recruit different forebrain circuitries. Moreover, there is accumulating evidence that other brain regions including the amygdala, thalamus, prefrontal cortex and olfactory bulb are also involved in anxiety behavior (Russo et al., 2012; Wang et al., 2007). Importantly, studies that compromise olfactory function in rodents lead to anxiety-like behavioral changes both in anosmic models (Brunjes et al., 1992; Song and Leonard, 2005) and mice that are still able to detect and discriminate odors (Glinka et al., 2012). Since OB neurogenesis and anxiety were impaired by transgenic expression of A30P and restored after dox-treatment in our transgenic mice, these findings suggest a positive correlation of OB induced α-synucleinopathy with the anxiogenic response. However, since A30P α-syn is also expressed in other forebrain regions that may implicate with an increase in anxiety, we cannot completely rule out other possible circuits affected by transgenic overexpression.
Conclusion
Here, we show that A30P α-syn lead to a severe HC gliopathy, including glial accumulation and impaired neuronal plasticity, which could not be regulated by dox suppression of the transgenic construct. Dox related restauration of OB neurogenesis and a possible related anxiogenic response demonstrated site-specific vulnerability of the HC region, additionally segregating its circuits from anxiety related networks.
The glial accumulation of transgenic α-syn in mice with expression directed by a neuronal promoter further suggests, that a glial phenotype may contribute to effects of neuronal α-snucleinopathy, including decreased adult neurogenesis. The selective vulnerability of the DG and its astrocytic subtype, the molecular mechanisms underlying glial α-syn accumulation, the impact of protein mutations and protein clearance mechanisms are follow up questions that need clarification. The usage of conditional transgenic mice will be of advantage to further delineate whether the underlying pathogenic events can be blocked or restored and will also help us to unveil regional susceptibility by exposing residual α-syn patterns after reduction of transgenic overexpression at different time points.
Supplementary Material
Acknowledgments
This study was supported by the Bavarian State Ministry of Sciences, Research and the Arts, ForNeuroCell (J.K., J.W.; Erlangen, Germany), the Albert-Raps Foundation and the Elite Network Bavaria (F.M., J.W.) and the German Research Foundation (Ri 682/6–1/2/3 to O.R.). F.M.is supported by the Josef-Stanglmeier Foundation. E.M. is supported by AG18440, AG022074, NS044233. B.W. is supported by the Interdisciplinary Center for Clinical Research, the Bavarian Ministry of Sciences, Research and the Arts in the framework of the Bavarian Molecular Biosystems Research Network, and the German Federal Ministry of Education and Research (BMBF, 01GQ113). S.N. is a fellow of the German Parkinson society. We thank N. Casadei for help with behavioral tests.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2013.07.004.
Conflict of interest: neither author has to declare a conflict of interest.
References
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Ming K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Brandewiede J, Schachner M, Morellini F. Etiological analysis of the senescence-accelerated P/8 mouse. Behav. Brain Res. 2005;158:109–121. doi: 10.1016/j.bbr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Jazaeri A, Sutherland MJ. Olfactory bulb organization and development in Monodelphis domestica (grey short-tailed opossum) J. Comp. Neurol. 1992;320:544–554. doi: 10.1002/cne.903200411. [DOI] [PubMed] [Google Scholar]
- Buron G, Hacquemand R, Pourie G, Lucarz A, Jacquot L, Brand G. Comparative behavioral effects between synthetic 2,4,5-trimethylthiazoline (TMT) and the odor of natural fox (Vulpes vulpes) feces in mice. Behav. Neurosci. 2007;121:1063–1072. doi: 10.1037/0735-7044.121.5.1063. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Gispert-Sanchez S, Murphy D, Auburger G, Myers RR, Nussbaum RL. Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neurobiol. Aging. 2005;26:25–35. doi: 10.1016/j.neurobiolaging.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Hazelton JL, Wang Y, Fiskum G, Kristian T. Neuron-specific conditional expression of a mitochondrially targeted fluorescent protein in mice. J. Neurosci. Off. J. Soc. Neurosci. 2006;26:13123–13127. doi: 10.1523/JNEUROSCI.4191-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Miles DK, Hoang T, Shi J, Hurlock E, Kernie SG, Lu QR. The basic helix-loop-helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:10983–10989. doi: 10.1523/JNEUROSCI.3545-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett. 2004;576:363–368. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT., Jr. Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000a;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. U. S. A. 2000b;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crupi R, Cambiaghi M, Spatz L, Hen R, Thorn M, Friedman E, Vita G, Battaglia F. Reduced adult neurogenesis and altered emotional behaviors in autoimmuneprone B-cell activating factor transgenic mice. Biol. Psychiatry. 2010;67:558–566. doi: 10.1016/j.biopsych.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci. Off. J. Soc. Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, van den Buuse M, San Mok S, Masters CL, Li QX, Culvenor JG. Alpha-synuclein transgenic mice exhibit reduced anxiety-like behaviour. Exp. Neurol. 2008;210:788–792. doi: 10.1016/j.expneurol.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Glinka ME, Samuels BA, Diodato A, Teillon J, Feng Mei D, Shykind BM, Hen R, Fleischmann A. Olfactory deficits cause anxiety-like behaviors in mice. J. Neurosci. 2012;32:6718–6725. doi: 10.1523/JNEUROSCI.4287-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn D, Bortnick RA, Morton AJ. Complexin II is essential for normal neurological function in mice. Hum. Mol. Genet. 2003;12:2431–2448. doi: 10.1093/hmg/ddg249. [DOI] [PubMed] [Google Scholar]
- Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol. Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM, Trojanowski JQ, Mann D, Iwatsubo T. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J. Biol. Chem. 2002;277:49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD-1 mice. Eur. J. Neurosci. 2004;20:2415–2429. doi: 10.1111/j.1460-9568.2004.03704.x. [DOI] [PubMed] [Google Scholar]
- Hejjaoui M, Haj-Yahya M, Kumar KS, Brik A, Lashuel HA. Towards elucidation of the role of ubiquitination in the pathogenesis of Parkinson’s disease with semisynthetic ubiquitinated alpha-synuclein. Angew. Chem. Int. Ed. Engl. 2011;50:405–409. doi: 10.1002/anie.201005546. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur. J. Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hodgson TL, Dittrich WH, Henderson L, Kennard C. Eye movements and spatial working memory in Parkinson’s disease. Neuropsychologia. 1999;37:927–938. doi: 10.1016/s0028-3932(98)00151-1. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J. Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- Kohl Z, Winner B, Ubhi K, Rockenstein E, Mante M, Munch M, Barlow C, Carter T, Masliah E, Winkler J. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur. J. Neurosci. 2012;35:10–19. doi: 10.1111/j.1460-9568.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53–> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci. Off. J. Soc. Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010a;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim W, Li Z, Hall GF. Accumulation of vesicle-associated human tau in distal dendrites drives degeneration and tau secretion in an in situ cellular tauopathy model. Int. J. Alzheimers Dis. 2012;2012:172837. doi: 10.1155/2012/172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Sigurdson C, Tsigelny I, Masliah E, Lee SJ, Sigurdson C, Tsigelny I, Masliah E. Pathological propagation through cell-to-cell transmission of non-prion protein aggregates in neurodegenerative disorders (submitted for publication) 2013 [Google Scholar]
- Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- Li J, Uversky VN, Fink AL. Conformational behavior of human alpha-synuclein is modulated by familial Parkinson’s disease point mutations A30P and A53T. Neurotoxicology. 2002;23:553–567. doi: 10.1016/s0161-813x(02)00066-9. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Lim Y, Kehm VM, Lee EB, Soper JH, Li C, Trojanowski JQ, Lee VM. alpha-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:10076–10087. doi: 10.1523/JNEUROSCI.0618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lione LA, Carter RJ, Hunt MJ, Bates GP, Morton AJ, Dunnett SB. Selective discrimination learning impairments in mice expressing the human Huntington’s disease mutation. J. Neurosci. Off. J. Soc. Neurosci. 1999;19:10428–10437. doi: 10.1523/JNEUROSCI.19-23-10428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Esposito G, Scuderi C, Steardo L, Delli-Bovi LC, Hecht JL, Dickinson BC, Chang CJ, Mori T, Sheen V. S100B and APP promote a gliocentric shift and impaired neurogenesis in Down syndrome neural progenitors. PLoS One. 2011;6:e22126. doi: 10.1371/journal.pone.0022126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012a;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J. Exp. Med. 2012b;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariggio MA, Fulle S, Calissano P, Nicoletti I, Fano G. The brain protein S-100ab induces apoptosis in PC12 cells. Neuroscience. 1994;60:29–35. doi: 10.1016/0306-4522(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Martins-Branco D, Esteves AR, Santos D, Arduino DM, Swerdlow RH, Oliveira CR, Januario C, Cardoso SM. Ubiquitin proteasome system in Parkinson’s disease: a keeper or a witness? Exp. Neurol. 2012;238:89–99. doi: 10.1016/j.expneurol.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxreiter F, Nuber S, Kandasamy M, Klucken J, Aigner R, Burgmayer R, Couillard-Despres S, Riess O, Winkler J, Winner B. Changes in adult olfactory bulb neurogenesis in mice expressing the A30P mutant form of alpha-synuclein. Eur. J. Neurosci. 2009;29:879–890. doi: 10.1111/j.1460-9568.2009.06641.x. [DOI] [PubMed] [Google Scholar]
- May VE, Nuber S, Marxreiter F, Riess O, Winner B, Winkler J. Impaired olfactory bulb neurogenesis depends on the presence of human wild-type alpha-synuclein. Neuroscience. 2012;222:343–355. doi: 10.1016/j.neuroscience.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Mayford M, Mansuy IM, Muller RU, Kandel ER. Memory and behavior: a second generation of genetically modified mice. Curr. Biol. 1997;7:R580–R589. doi: 10.1016/s0960-9822(06)00287-9. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchere J, Lakhdar L, Legastelois S, Baron T. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging. 2012;33:2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J. Biol. Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J. Clin. Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Hasegawa M. A cellular model to monitor proteasome dysfunction by alpha-synuclein. Biochemistry. 2009;48:8014–8022. doi: 10.1021/bi900619j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Horsten S, Schmidt T, Boy J, Kuhn M, Nguyen HP, Teismann P, Schulz JB, Neumann M, Pichler BJ, Reischl G, Holzmann C, Schmitt I, Bornemann A, Kuhn W, Zimmermann F, Servadio A, Riess O. Neurodegeneration and motor dysfunction in a conditional model of Parkinson’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:2471–2484. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber S, Petrasch-Parwez E, Arias-Carrion O, Koch L, Kohl Z, Schneider J, Calaminus C, Dermietzel R, Samarina A, Boy J, Nguyen HP, Teismann P, Velavan TP, Kahle PJ, von Horsten S, Fendt M, Kruger R, Riess O. Olfactory neuron-specific expression of A30P alpha-synuclein exacerbates dopamine deficiency and hyperactivity in a novel conditional model of early Parkinson’s disease stages. Neurobiol. Dis. 2011;44:192–204. doi: 10.1016/j.nbd.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Oaks AW, Frankfurt M, Finkelstein DI, Sidhu A. Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function. PLoS One. 2013;8:e60378. doi: 10.1371/journal.pone.0060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Beksinska M, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Sahakian BJ, Robbins TW. Visuospatial memory deficits at different stages of Parkinson’s disease. Neuropsychologia. 1993;31:627–644. doi: 10.1016/0028-3932(93)90135-m. [DOI] [PubMed] [Google Scholar]
- Parekh AB. Mitochondrial regulation of intracellular Ca2+ signaling: more than just simple Ca2+ buffers. News Physiol. Sci. 2003;18:252–256. doi: 10.1152/nips.01458.2003. [DOI] [PubMed] [Google Scholar]
- Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucelli L, O’Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects cate-cholaminergic neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Pillon B, Ertle S, Deweer B, Sarazin M, Agid Y, Dubois B. Memory for spatial location is affected in Parkinson’s disease. Neuropsychologia. 1996;34:77–85. doi: 10.1016/0028-3932(95)00086-0. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Rideout HJ, Larsen KE, Sulzer D, Stefanis L. Proteasomal inhibition leads to formation of ubiquitin/alpha-synuclein-immunoreactive inclusions in PC12 cells. J. Neurochem. 2001;78:899–908. doi: 10.1046/j.1471-4159.2001.00474.x. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc. Res. Tech. 2003;60:614–632. doi: 10.1002/jemt.10303. [DOI] [PubMed] [Google Scholar]
- Rott R, Szargel R, Haskin J, Bandopadhyay R, Lees AJ, Shani V, Engelender S. alpha-Synuclein fate is determined by USP9X-regulated monoubiquitination. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18666–18671. doi: 10.1073/pnas.1105725108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat. Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BL, Seehus CR, Capowski EE, Aebischer P, Zhang SC, Svendsen CN. Over-expression of alpha-synuclein in human neural progenitors leads to specific changes in fate and differentiation. Hum. Mol. Genet. 2007;16:651–666. doi: 10.1093/hmg/ddm008. [DOI] [PubMed] [Google Scholar]
- Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B. Aggregated and monomeric alpha-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J. Biol. Chem. 2003;278:11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int. J. Dev. Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Takebayashi H, Manabe T, Tanaka KF, Makinodan M, Yamauchi T, Makinodan E, Matsuyoshi H, Okuda H, Ikenaka K, Wanaka A. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J. Neurosci. Res. 2008;86:3494–3502. doi: 10.1002/jnr.21862. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Layfield R, Spillantini MG. alpha-synuclein metabolism and aggregation is linked to ubiquitin-independent degradation by the proteasome. FEBS Lett. 2001;509:22–26. doi: 10.1016/s0014-5793(01)03115-5. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): implications for Lewy body disorders. J. Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsika E, Moysidou M, Guo J, Cushman M, Gannon P, Sandaltzopoulos R, Giasson BI, Krainc D, Ischiropoulos H, Mazzulli JR. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:3409–3418. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Spencer B, Rockenstein E, May V, Winkler J, Masliah E. Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of alpha-synucleinopathy. Exp. Neurol. 2012;234:405–416. doi: 10.1016/j.expneurol.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31:95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nabeshima T. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav. Brain Res. 2007;178:262–273. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wang L, Xie C, Greggio E, Parisiadou L, Shim H, Sun L, Chandran J, Lin X, Lai C, Yang WJ, Moore DJ, Dawson TM, Dawson VL, Chiosis G, Cookson MR, Cai H. The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:3384–3391. doi: 10.1523/JNEUROSCI.0185-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience. 2009;159:1055–1069. doi: 10.1016/j.neuroscience.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J. Neuropathol. Exp. Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, Couillard-Despres S, Masliah E, Winkler J. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol. Aging. 2008;29:913–925. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Regensburger M, Schreglmann S, Boyer L, Prots I, Rockenstein E, Mante M, Zhao C, Winkler J, Masliah E, Gage FH. Role of alpha-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:16906–16916. doi: 10.1523/JNEUROSCI.2723-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Masliah E, Spector SA. Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J. Infect. Dis. 2011;203:1647–1657. doi: 10.1093/infdis/jir163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.