Abstract
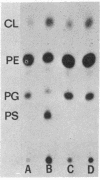
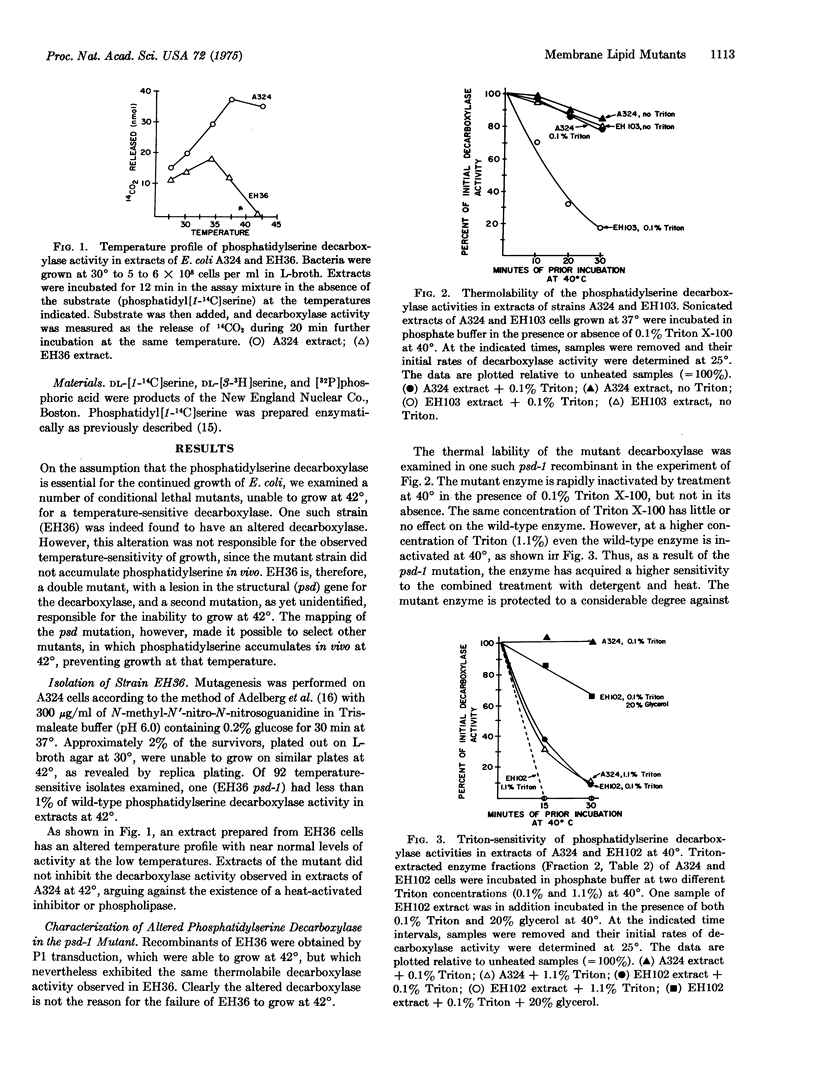
Phosphatidylserine decarboxylase catalyzes the last step in the pathway leading to phosphatidylethanolamine, the principal membrane lipid of E. coli. Mutants of E. coli have now been isolated in which this enzyme is theramolabile. The structural gene for phosphatidylserine decarboxylase (psd gene) is closely linked to the pur A locus at about 83 min on the standard map of the E. coli chromosome. When a mutant with thermolabile decarboxylase is incubated at 42 degrees, growth ceases, but only after a substantial fraction (20-40%) of the total phospholipid of the cell has been replaced by phosphatidylserine. Examination of such mutants with altered content of phospholipids may shed light on the role of specific phospholipids in membrane function.
Full text
PDF
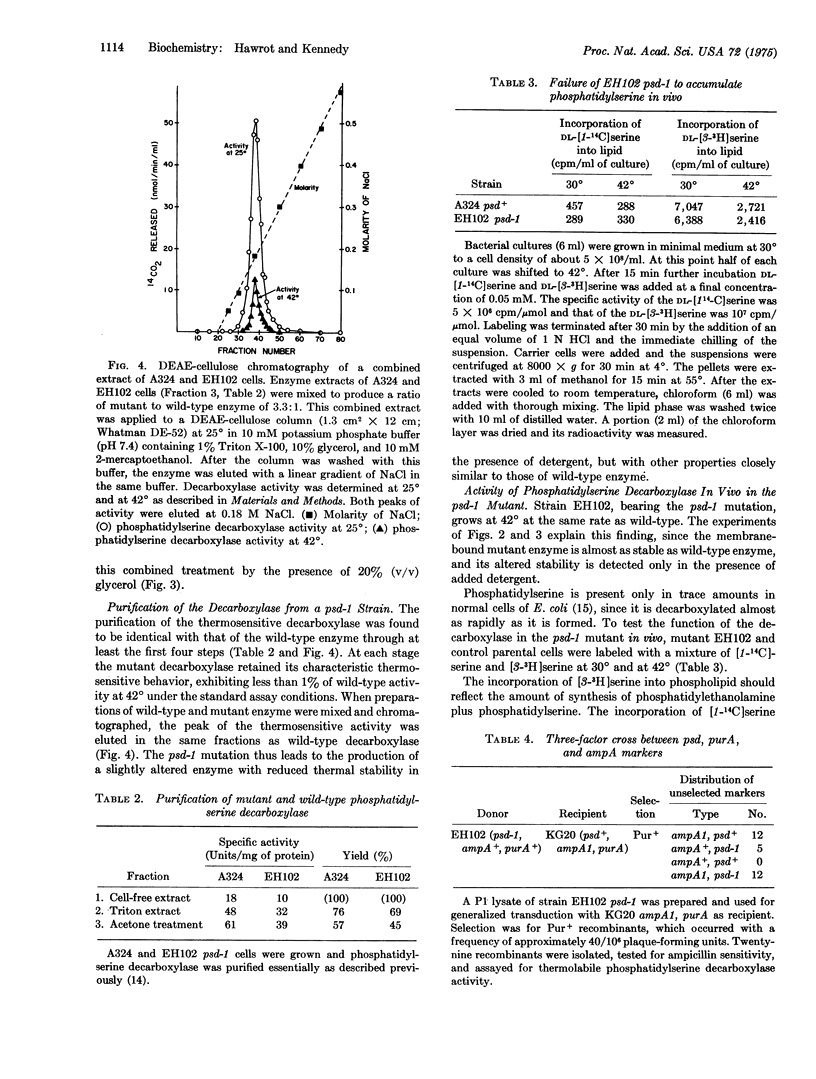


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr A new method for selection of Escherichia coli mutants defective in membrane lipid synthesis. Nat New Biol. 1972 Nov 1;240(96):21–22. doi: 10.1038/newbio240021a0. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Birge C. H., Vagelos P. R. Evidence for two genes specifically involved in unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1969 Nov;100(2):601–604. doi: 10.1128/jb.100.2.601-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Dowhan W., Wickner W. T., Kennedy E. P. Purification and properties of phosphatidylserine decarboxylase from Escherichia coli. J Biol Chem. 1974 May 25;249(10):3079–3084. [PubMed] [Google Scholar]
- Glaser M., Bayer W. H., Bell R. M., Vagelos P. R. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kito M., Lubin M., Pizer L. I. A mutant of Escherichia coli defective in phosphatidic acid synthesis. Biochem Biophys Res Commun. 1969 Feb 21;34(4):454–458. doi: 10.1016/0006-291x(69)90403-3. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota A., Shibuya I., Maruo B., Ishinaga M., Kito M. An extremely labile phosphatidylserine synthetase of an Escherichia coli mutant with the temperature-sensitive formation of phosphatidylethanolamine. Biochim Biophys Acta. 1974 Jun 26;348(3):449–454. [PubMed] [Google Scholar]
- Pizer L. I., Merlie J. P., De Leon M. P. Metabolic consequences of limited phospholipid synthesis in Escherichia coli. J Biol Chem. 1974 May 25;249(10):3212–3224. [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Cronan J. E., Jr, Beacham I. R., Harder M. E. Proceedings: Genetic engineering of membrane lipid. Fed Proc. 1974 Jun;33(6):1725–1732. [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]