Abstract
The lamina terminalis (LT) consists of the organum vasculosum of the lamina terminalis (OVLT) the median preoptic nucleus (MnPO) and the subfornical organ (SFO). All subdivisions of the LT project to the ventrolateral periaqueductal gray (vlPAG). The LT and the vlPAG are implicated in several homeostatic and behavioral functions including body fluid homeostasis, thermoregulation and the regulation of sleep and waking. By combining visualization of c-Fos protein and retrograde neuroanatomical tracer we have examined the functional correlates of LT-vlPAG projection neurons. Rats were injected with retrograde tracer into the vlPAG and following a one week recovery period, they were subjected to either, hypertonic saline administration (0.5M NaCl, 1ml/100g-i.p.), 24hrs water deprivation, isoproterenol administration (increases circulating AngII; 50μg/kg-s.c.), heat exposure (39°C for 60 mins) or permitted 180 minutes spontaneous sleep. Retrogradely labelled neurons from the vlPAG and double labelled neurons were then identified and quantified throughout the LT. OVLT-vlPAG projection neurons were most responsive to hypertonic saline and water deprivation. SFO-vlPAG projection neurons were most active following isoproterenol administration and MnPO-vlPAG projection neurons displayed significantly more Fos immunostaining following water deprivation, heat exposure and sleep. These results support the existence of functional subdivisions of LT-vlPAG projecting neurons and indicate three patterns of activity that correspond to thermal and sleep wake regulation, osmotic or hormonal stimuli.
Keywords: rat, preoptic, thermoregulation, sleep, osmotic
Introduction
The lamina terminalis (LT) and associated anteroventral third ventricle region (AV3V) is implicated in the regulation of body fluid homeostasis (Andersson et al., 1975; Johnson and Buggy, 1978). Its activation by circulating hypertonicity or circulating angiotensin II (AngII) stimulates vasopressin secretion and thirst (Aradachi et al., 1996; McKinley et al., 1982; Richard and Bourque, 1992; Simpson and Routtenberg, 1973; Thrasher et al., 1982). The ventral LT (MnPO and OVLT) is responsive to perturbations in temperature and this area expresses prostaglandin receptors supporting a role in febrile responses (Lazarus et al., 2007; Travis and Johnson, 1993). Additionally, a subpopulation of GABAergic neurons within the MnPO and possibly the OVLT are activated during sleep compared to waking (Gong et al., 2004; Gvilia et al., 2006; Uschakov et al., 2006).
The LT and preoptic area project heavily to the ventrolateral periaqueductal gray (vlPAG) (Rizvi et al., 1992; Thompson and Swanson, 2003; Uschakov et al., 2007). The PAG has been implicated in pain modulation (Liebeskind et al., 1973), the control of vocalization (Jurgens and Pratt, 1979; Larson, 1991), motor behavior (Skultety, 1962), thermoregulation, sleep and cardiorespiratory function (Chen et al., 2002; Ni et al., 1990; Ward and Darlington, 1987).
Electrical stimulation of the AV3V area (which includes the MnPO, OVLT and medial preoptic area) has demonstrated a frequency dependent increase in hindquarter blood flow and decreases in mesenteric and renal blood flow, resulting overall in a depressor response. Similar changes occur with electrical stimulation of the rostral PAG and lesions of the PAG area reduce the hemodynamic responses to AV3V stimulation (Knuepfer et al., 1984). This shunting of blood peripherally can allow for better heat exchange/cooling. c-Fos expression has also been identified in the rostral PAG following preoptic warming (Yoshida et al., 2002) and direct connectivity of thermosensitive MnPO neurons projecting to the vlPAG has been demonstrated (Yoshida et al., 2005).
Neurons that are active during both nonREM and REM sleep have been localized to the MnPO (Gong et al., 2000; Suntsova et al., 2002). The majority of MnPO neurons activated during sleep are GABAergic (Gong et al., 2004). MnPO GABAergic neurons exhibit increased activity in response to sleep deprivation, indicating a possible role in homeostatic sleep regulation (Gvilia et al., 2006). A subset of MnPO sleep active neurons are a source of descending projections to the hypothalamic paraventricular nucleus and to the perifornical lateral hypothalamus (Uschakov et al., 2006).
The role of the PAG in the regulation of thirst and body fluid homeostasis is unknown although modulation of vigilance has been suggested (Denton et al., 1999).
Therefore, to examine if neurons in the LT that project to the PAG display similar or different activations, likely relating to function, we tested a number of stimuli known to activate the LT. We analyzed LT to vlPAG projection neurons by combining visualization of retrograde tracer with immunostaining for c-Fos protein in separate groups of rats subjected to hypertonic saline injection, water deprivation, heat exposure, systemic administration of isoproterenol (increases circulating AngII) or spontaneous sleep.
Methods
Prior to experiments, all investigations and procedures were approved by either the Animal Ethics Committee of the Howard Florey Institute Australia, or the Animal Care and Use Committee at the Veterans Administration of the Greater Los Angeles Health Care System. Experiments were performed on male Sprague-Dawley rats obtained from Harlan, Indiana, USA (sleep experiments) and the Animal Resource Centre in Willeton, Western Australia. Animals weighed between 275-300 grams at the time of tracer injection. Rats were maintained on a 12:12 light dark cycle, at an ambient temperature of 22±2°C, with food available ad libitum except as noted below. Initially they were prepared with microinjections of retrograde tracer and allowed one week for recovery. Experimental procedures were then performed on conscious animals.
Microinjection of tracer
For tracer injections, deep anesthesia was induced via an intraperitoneal administration of ketamine/xylazine (80mgkg-1/8mgkg-1). Animals were placed in a Kopf stereotaxic frame and prepared for aseptic surgery. Borosilicate glass capillaries (O.D. 1.2mm, I.D. 0.69mm) were pulled to give micropipettes with a desired tip outer diameter of ∼20μm. Those of high standard were filled with fluoro-gold (Fluorochrome, Colorado; 5% in filtered deionised water). A General Valve Corporation ‘Picospritzer II’ administered Fluoro-gold via pressure pulse (20ms pulse at 40psi) allowing approximately 100nl of tracer to be ejected into vlPAG. For the sleep and sleep control experiments, fluoro-gold (5% in filtered deionised water ∼300nl) was injected into the vlPAG using a kd-Scientific microdrive at a rate of 10nl/min. At the time of tracer injection, rats for the spontaneous sleep condition and the corresponding control rats received chronic implantation of bilateral stainless steel screw electrodes placed in the skull to record the EEG, and a pair of insulated stainless steel wires inserted into the dorsal neck muscle to record EMG, as per Bergmann et al (Bergmann et al., 1987). All injections were made with coordinates at 6.85mm caudal to bregma, lateral to the midline 0.6mm with a depth of 6.1mm from the top of the brain. One week post surgery, all animals n=47 were surveyed for health and general vitality; those that had recovered well were included for further study.
Physiological stimuli
Hypertonic saline administration
At 09.00h (∼2 hrs after lights on), male Sprague-Dawley rats, two per cage (n=6), were administered a warm (30-37°C) hypertonic salt load (0.5M NaCl, 1ml/100g) by intraperitoneal (i.p.) administration. Control animals, two per cage (n=6), were administered an equivalent volume of warm isotonic saline. Animals were handled briefly and gently to minimise stress. Ninety minutes later, they were killed by i.p. injection of pentobarbital (100 mg/kg), then brains were perfused via the left ventricle of the heart with saline (200 ml) followed by 4% paraformaldehyde/phosphate buffered saline (300 ml) and prepared as required for Fos immunohistochemistry (see below).
Water Deprivation
Male Sprague-Dawley rats, two per cage (n=5), had their water bottles removed at 09.00 one-week post surgery. Control animals, two per cage (n=4), were inspected and had their water bottles checked at the same time that experimental animals had their water bottles removed. At approximately 09.00-09.30h the following day (∼3hrs after lights on), animals were killed by i.p. administration of sodium pentobarbitone (100 mg/kg). They were then perfused through the left ventricle as described above and their brains prepared for Fos immunohistochemistry.
Isoproterenol treatment
At 09.00-09.30 hours (∼3hrs after lights on), animals, two per cage, were independently tagged by placing a small mark on their pelt with a felt tipped pen. One rat per cage was given isoproterenol (n=4; 50μg/kg in 500μl sterile isotonic saline) administered subcutaneously behind the neck. The other served as control and was given 500μl of sterile isotonic saline subcutaneously (n=4). After 90 minutes they were killed with i.p. sodium pentobarbitone. They were subsequently perfused with saline fixative as described above and their brains prepared for Fos immunohistochemistry.
Heat exposure
Two hours after lights on, water bottles were removed from the animals cage (two animals per cage) and the cage was then placed into a thermal chamber maintained at 39°C for 60 minutes (n=5). Control animals (n=6) also had their water bottles removed and were placed in the thermal chamber for 60 minutes at standard laboratory temperature (22°C). The animals were then placed back in the animal storage facility for thirty minutes at 22°C. The animals were then sacrificed and prepared for Fos immunohistochemistry.
Sleep
One week after tracer injection and electrode implantation, animals were placed into the recording chamber and attached by the head plug to a cable with suitable counterbalance and via that cable to a Grass 15LT physiodata amplifier system for polysomnographic recording. The recording chamber was housed inside a sound attenuated, ventilated incubator maintained at 22±2°C. Animals were permitted 3-4 days to acclimatize to the recording chamber and cables prior to the experimental day. On the experimental day, one group of rats (n=3) was left undisturbed and permitted spontaneous sleep for a three hour period beginning at the time of lights-on. Sleep was confirmed by polysomnographic recording. The control/awake group of animals (n=4) underwent the same surgery, recovery and acclimatization protocols, but were kept awake by gentle handling for the corresponding three hours following lights-on. For the gentle handling procedure, animals were gently aroused by cue-tip introduction and gentle whisker brushing that was initiated at the earliest signs of synchronous EEG activity. After 180 minutes animals in both groups were given an anesthetic overdose via i.p. administration of sodium pentobarbitone, perfused and prepared for Fos immunohistochemistry.
Immunohistochemical processing for c-Fos
After sacrifice, animals were perfused through the left ventricle of the heart with 200ml isotonic saline followed by then 300ml of 4% paraformaldehyde in phosphate buffer (PB; 0.1M, pH 7.2). The brain was removed and placed in 20% sucrose/PB solution overnight. 40μm coronal sections were then cut through the LT and the injection site; this tissue was then transferred to a 10% solution of normal horse serum (NHS) for one hour at room temperature with gentle agitation. Subsequently the sections were transferred to a well containing the primary antibody for c-Fos (oncogene; Ab-5, Rabbit Polyclonal IgG, dilution 1:12,500) with 2% NHS and 0.3% Triton X-100 made up in PB. This was then incubated at ∼4°C overnight. Tissue sections were then given 3 × 5 minute washes in PB to remove unbound antibody and transferred to a well containing the secondary antibody, biotinylated anti-rabbit (Vectastain-elite; bio-α Rb) at a dilution of 1:200 in 2% NHS in PB. Sections were agitated for 45mins at room temperature and then washed 3 × 5 minutes in PB to remove unbound antibody. The tissue was transferred to wells containing the avidin-biotinylated horseradish peroxidase complex diluted at 1:100 in PB and was gently agitated for 45mins at room temperature. Sections were then washed 3 × 5 mins in 0.05M Tris buffer at pH 7.6 and subsequently agitated for 10mins in a nickel-diaminobenzidine solution (40mg ammonium nickel sulphate, 50mg diaminobenzidine and 100ml Tris buffer). 15μl of hydrogen peroxide (30% v/v) was then added and the tissue was left to agitate for a further 6min. The tissue was then washed in Tris buffer and mounted on gelatine coated slides. Mounted slides were then coverslipped using a xylene based media (Depex).
Sleep Analysis
Sleep or awake states were scored in 20-s epochs on the basis of the predominant state in each epoch. Non-paradoxical sleep was defined by synchronous high-amplitude EEG activity predominantly in the 2-4Hz range, with concurrent low EMG voltage. Paradoxical sleep was defined by moderate amplitude EEG activity with dominant theta frequency activity (6-8Hz) combined with a low EMG potential difference often interrupted by brief spiking events. Wake was defined by asynchronous low-voltage, high frequency EEG potential differences with concurrent elevated voltage between EMG electrodes from that of sleeping periods. Animals in the spontaneous sleep group attained 70% or more sleep in the three-hour period of the experiments; an average of 5% of this was paradoxical sleep. Animals in the awake group displayed 15% or less sleep in the three-hour period of the experiments. As previous work on the SFO identified negligible c-Fos-IR neurons during spontaneous sleep (Uschakov et al., 2006), cell counts were not performed in the SFO for the spontaneously sleeping and corresponding control groups.
Injection Sites
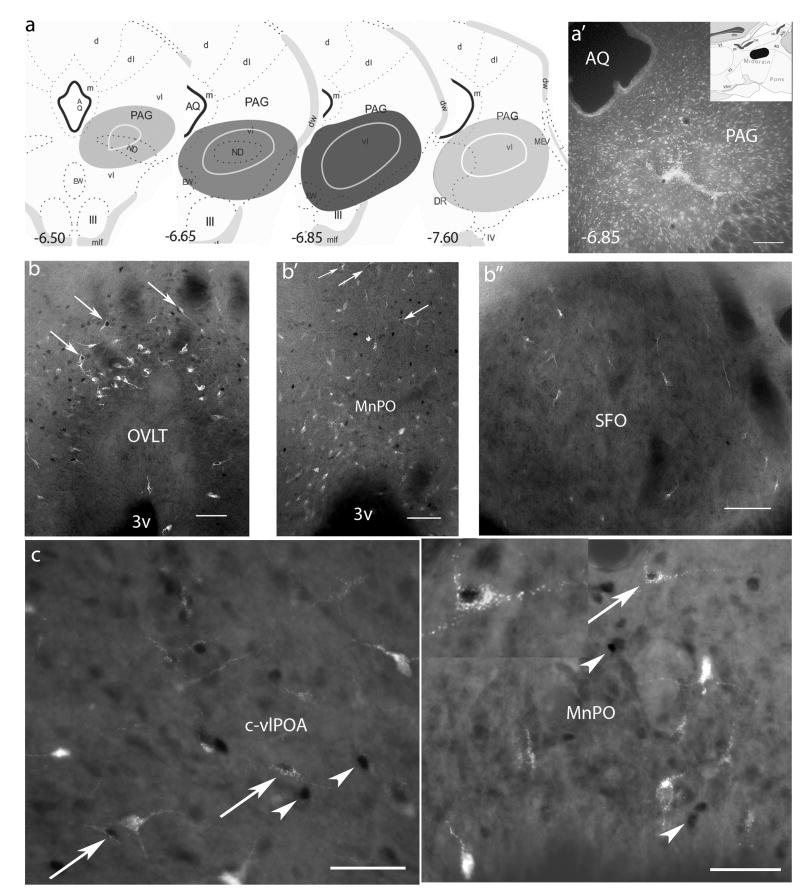
Injections of fluoro-gold into the PAG resulted in an intense yellow/gold staining that encompassed a sizable area, mostly within the vlPAG. A reproducibly defined area within the midbrain was stained likely due to tracer containment by surrounding fiber tracts (e.g. the medial longitudinal fascicle and/or the mesencephalic tract of the trigeminal nerve). The area consistently labeled throughout the experiments is represented in Figure 1a. Animals in the sleep and wake cohort encompassed the entire area demarcated in Figure 1a due to a greater quantity of tracer being injected, all the other injection sites were smaller and located within the central region. Animals with tracer injections that deviated from this area of staining were excluded from analysis.
Figure 1.
Retrograde labeling in the preoptic area following injections of fluoro-gold into the vlPAG. a. rostrocaudal maps through the PAG indicating the location of injection sites, animals in the sleep and wake cohort encompassed a larger area as more tracer was injected, all other injection sites were located within the central shaded area; a′ photomicrograph of a typical injection site with inset of midsagittal cartoon indicating the rostrocaudal boundaries of the injection sites, values denote millimeters posterior to bregma. b. retrograde labeling and Fos-IR in the dorsal cap of the OVLT; b′. the MnPO and b″. the SFO following hypertonic saline administration (0.5M NaCl, 1ml/100g); c. retrograde labeling and Fos-IR in the c-vlPOA and c′. the MnPO following 3hrs sleep; inset, higher magnfication of a double labeled neuron, note the darkly labeled nucleus and the fluorescent retrograde label within the soma. Arrows and arrowheads indicate double labeled neurons or single Fos label respectively. Scale bars = 100μm
Cell Counts
Total retrogradely labeled cells and double labeled cells were counted and grouped according to the area in which they were located (i.e. OVLT, MnPO or SFO). The ventrolateral preoptic nucleus (vlPOA), extended and core regions, were also counted for the sleep cohort as these areas are known to contain sleep active neurons. Counts included all neurons in a particular subdivision of the lamina terminalis, i.e. all sections were counted throughout the area examined. The OVLT was identified rostrally as the area connecting the rostral part of the optic chiasm to the ventral part of the medial forebrain. Caudally, the OVLT was determined as the highly vascularized region subjacent to the rostral part of the MnPO. The dorsal cap of the OVLT was also included in all cell counts of the OVLT. The dorsal cap of the OVLT was considered as that part of the OVLT located atop the vascularized region with high cellular density, uniform orientation of cells, and its relative distance from the vascularised zone, approximately 50-80μm dorsal (Oldfield et al., 1994; Bisley et al., 1996; McKinley et al., 1998). The MnPO was determined rostrally as that area directly dorsal to the OVLT, its rostral most/dorsal most boundaries were determined by a greater density of neurons to that of the surrounding nuclei/areas. The lateral boundaries were also determined on cellular packing density, being greater than the surrounding neural parenchyma. A standard guide utilized the lateral walls of the third ventricle as the lateral limits to the MnPO. The SFO was determined as the area dorsal/caudal to the MnPO at the level where the fornical bundle separates and the third ventricle opens dorsal to the anterior commissure. The SFO was most easily recognized at its most rostral extent as the area that displayed clustered arrangements of cells distinct from the ventrodorsally orientated cells within the dorsal part of the MnPO.
The vlPOA core was counted throughout the preoptic area from −0.1mm A/P from bregma to −0.46mm A/P from bregma ipsilateral to the injection site. Cells within the vlPOA core were counted within an equilateral triangle grid with a side length of 300μm. The vlPOA core was readily recognized without galanin/GAD staining by the location of clustered c-Fos cells during sleep or by clustering of retrogradely labeled neurons from the vlPAG. The extended vlPOA was considered as that region that extends medially and dorsally from the vlPOA core. The area that was counted was an extension of the grid along the lateral vertically oriented side of the triangle by 600μm and an extension of the horizontal side of the triangular grid by 600μm. c-Fos-IR was detected in retrogradely-labeled neurons following Fluoro-gold injections into the vlPAG. The presence of the tracer within the soma of any particular neuron did not seem to affect its basal c-Fos production as in no cases were all retrogradely labeled neurons double labeled or unlabeled. Only neurons that displayed significant morphology of the cell soma as determined by the intensity of retrograde labeling which included the recognition of somatic appendages (dendrites) and significant (>70%) portions of the cell nucleus were counted. Counts were conducted at 200× magnification on a Nikon Eclipse E600 microscope using standard bright field parameters and a fluorescent attachment utilizing a 100-watt high-pressure mercury vapour short arc photo-optic lamp (Osram) and a UV-2A filter block for the fluorescent label. Counts were aided with the use of a Neurolucida (Microbrightfield) computer aided plotting program incorporating a Microfire camera with 2M pixel resolution at a pixel size of 7.4μm × 7.4μm.
Statistical analysis
Statistics were performed on these cell counts with the analytical program, SigmaStat-version 3.0.1. Excluding the sleep study, these data were analyzed against all the control animals as there was no statistical difference between any of the four variables in the control groups. The animals in the sleep cohort were analyzed against the wake group only (control group; n=4) due to different quantities of tracer being administered into the vlPAG compared to the previous studies. The independent-samples t-test was used to analyze these data sets. Data was plotted as percentages with respect to the numbers of retrogradely labeled neurons that were also c-Fos-IR found throughout the lamina terminalis or the vlPOA. Error values (Table 1) indicate the standard error of the mean, significance P < 0.050 is indicated by an asterisk and all statistical values are included in the text in the following format; t(degrees freedom) = x.xx, P = x.xxx.
Table 1.
Mean numbers of neurons within particular regions of the lamina terminalis that project to the vlPAG and express Fos in response to particular stimuli
| Stimulus | Region of the lamina terminalis | No. of animals | Mean no. of retrogradely labelled neurons/animal | Mean no. of retrogradely labelled neurons expressing Fos/animal | Percentage of retrogradely labelled neurons expressing Fos |
|---|---|---|---|---|---|
| i.p. hypertonic NaCl | |||||
| OVLT | 6 | 158 ± 48 | 11 ± 4 | 7* | |
| MnPO | 337 ± 105 | 13 ± 6 | 4 | ||
| SFO | 40 ± 19 | 1.5 ± 1 | 4 | ||
| 24 hrs dehydration | |||||
| OVLT | 5 | 77 ± 25 | 12 ± 2 | 16* | |
| MnPO | 208 ± 79 | 22 ± 9 | 11* | ||
| SFO | 49 ± 11 | 5 ± 1 | 10* | ||
| s.c. isoproterenol | |||||
| OVLT | 4 | 67 ± 20 | 6 ± 1 | 9* | |
| MnPO | 152 ± 41 | 3 ± 2 | 2 | ||
| SFO | 32 ± 5 | 5 ± 2 | 16* | ||
| heat exposure | |||||
| OVLT | 5 | 89 ± 10 | 4 ± 1 | 5* | |
| MnPO | 172 ± 53 | 14 ± 5 | 8* | ||
| SFO | 36 ± 15 | 0.4 ± 0.2 | 1 | ||
| Control | |||||
| OVLT | 20 | 83 ± 12 | 0.2 ± 0.09 | 0.2 | |
| MnPO | 205 ± 38 | 1 ± 0.23 | 0.5 | ||
| SFO | 28 ± 5 | 0.2 ± 0.15 | 0.7 | ||
| sleep | |||||
| OVLT | 3 | 106 ± 39 | 4 ± 1 | 4 | |
| MnPO | 973 ± 51 | 78 ± 13 | 8* | ||
| c-vlPOA | 161 ± 33 | 32 ± 15 | 20* | ||
| ex-vlPOA | 545 ± 96 | 33 ± 20 | 6 | ||
| sleep control | |||||
| OVLT | 4 | 92 ± 19 | 2 ± 0.5 | 2 | |
| MnPO | 788 ± 69 | 39 ± 9 | 5 | ||
| c-vlPOA | 138 ± 17 | 3 ± 1.5 | 2 | ||
| ex-vlPOA | 601 ± 62 | 33 ± 3 | 6 | ||
Results
Injection Sites
Injections of Fluoro-gold into the vlPAG consistently resulted in retrogradely labeled cells within the LT and within the surrounding preoptic area ipsilateral to the injection site. Injections into the vlPAG were large enough to encompass the vlPAG area and in some cases included the lateral division of the dorsal raphé nucleus and/or part of the dorsolateral part of the PAG. Injections that were included for analysis were located in the rostral/middle part of the vlPAG (Fig. 1a, see methods). Animals included in the study all displayed high numbers of retrogradely labeled neurons within the MnPO (57% ± 3.2) and proportionately less within the OVLT (30% ± 2.4) and the SFO (13% ± 1.7).
Hypertonicity
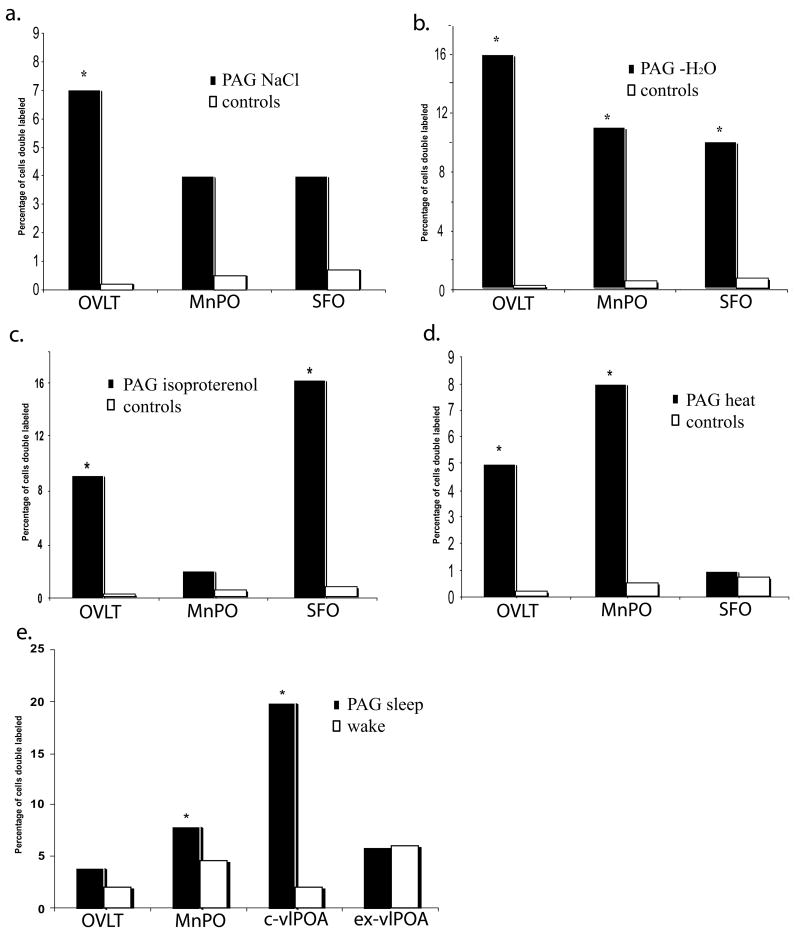
Following i.p. administration of hypertonic saline, Fos immunoreactive (IR) cells were found mainly within the SON, the dorsal cap of the OVLT, the periphery of the SFO and within the MnPO (Fig. 1b). A relatively large proportion of vlPAG projecting neurons within the LT were Fos-IR following i.p.-hypertonic NaCl administration (∼15%; Table 1, Figs. 1b & 2a). The OVLT displayed the greatest percentage of double labeled cells with respect to total retrogradely labeled cells (∼7%, t(24) = 6.19; P < 0.001). The MnPO and the SFO had lower percentages at ∼4%, (t(24) = 1.79; P = 0.462, t(24) = 1.98; P = 0.137 respectively). Only the OVLT reached significance over control values.
Figure 2.
Histograms of the percentage of vlPAG projecting neurons within the lamina terminalis/preoptic area that were double-labeled (Fos-IR & fluoro-gold) following a. i.p.-hypertonic saline (0.5M NaCl, 1ml/100g), b. 24hrs water deprivation, c. subcutaneous isoproterenol (50μg/kg), d. high ambient heat (39°C for 60mins) and e. 3hrs sleep or wake. * p < 0.05
Water Deprivation
Fos-IR cells were found throughout the LT, in the dorsal cap and lateral margins of the OVLT, throughout the MnPO and within the center and periphery of the SFO. Numerous Fos-IR cells were also observed within the SON. Water deprivation resulted in the greatest percentage of double labeled neurons found within the LT with respect to total retrogradely labeled neurons (∼37%). The majority of double labeled neurons, were located within the OVLT (∼16%, t(23) = 6.93; P < 0.001) the MnPO (∼11%, t(23) = 5.35; P = 0.022) and the SFO (∼10%, t(23) = 6.01; P = 0.009). All results were significantly greater than control values (Table 1, Fig. 2b).
Isoproterenol (endogenously generated angiotensin II)
Following the s.c. administration of isoproterenol, Fos-IR cells were observed within the MnPO, the SFO, the lateral margins of the OVLT and the SON in significantly greater numbers than in control animals. Isoproterenol administration resulted in a high percentage of vlPAG projecting-neurons that were Fos-IR (∼27%). The SFO displayed the highest percentage with ∼16% (t(22) = 6.57; P < 0.001) of PAG projecting neurons being double labeled, the OVLT ∼9% (t(22) = 7.29; P < 0.001) and the MnPO ∼2% (t(22) = 1.74; P = 0.258; Table 1, Fig. 2c). Values in the SFO and OVLT were significantly different from the control group.
Heat Exposure
The distribution of Fos-IR within the LT following exposure to elevated ambient temperature (39°C) resulted in high Fos-IR cells within the lateral margins of the ventral part of the MnPO and within the dorsal division of the MnPO. Fos-IR cells were rarely observed in the SFO while a few were located within the dorsal aspect of the dorsal cap of the OVLT. Following heat exposure, ∼14% of retrogradely labeled neurons within the LT were Fos-IR. The MnPO displayed the greatest percentage (8%, t(23) = 6.62; P < 0.001) compared to the OVLT (∼5%, t(23) = 8.39; P < 0.001) and the SFO (∼1%, t(23) = 2.54; P = 0.524). All values were significant except those of the SFO (Table 1, Fig. 2d).
Sleep vs Waking
Within the preoptic area, Fos immunoreactivity following sleep was readily identified within the core of the ventrolateral preoptic area (c-vlPOA), the dorsal cap of the OVLT and throughout the MnPO and the extended-vlPOA (ex-vlPOA). Negligible Fos-IR cells were observed within the SFO. PAG projecting neurons that were Fos positive were identified within the c-vlPOA and the MnPO, with fewer observed within the OVLT and the ex-vlPOA. Significantly greater double-labeled neurons were located within the MnPO (8%, t(5) = 3.78; P = 0.036) and the c-vlPOA (20%, t(5) = 4.03; P = 0.008) during spontaneous sleep than that during a comparable period of enforced wakefulness. No significant difference in Fos label was observed between sleep or wake in vlPAG projecting neurons located within the OVLT (4%, t(5) = 1.60; P = 0.13) or the ex-vlPOA (6%, t(5) = 0.64; P = 0.857; Table 1, Figs. 1c & 2e).
Discussion
Injections of retrograde tracer into the vlPAG resulted in significant numbers of retrogradely labeled neurons within the preoptic area. Many of these retrogradely labeled neurons were Fos-IR following water deprivation, hypertonic salt loading, isoproterenol administration, heat exposure or sleep.
Our results indicate that PAG projecting neurons within the LT activated by these stimuli display three distinct patterns of activity (Fig. 2). 1-where the OVLT has a predominant double labeled population and the MnPO and the SFO display a smaller double labeled population, i.e. following water deprivation and hypertonic salt loading. 2-where the OVLT and the SFO have a predominant double labeled population significantly greater than the MnPO, i.e. following isoproterenol administration and 3-where the MnPO displays the most activity of vlPAG projecting neurons followed by lesser but substantial activity in OVLT-vlPAG projecting neurons and insignificant activity in the SFO, i.e. following heat exposure or sleep.
We hypothesize that these differing patterns of activation result in varied functional modulations of vlPAG activity.
Water deprivation and hypertonic salt loading
The LT is recognized as an activated region of the anterior hypothalamus in many experimental thirst and drinking paradigms. Its role in fluid homeostasis is best underscored by lesion studies in which animals markedly cease drinking water subsequent to the lesion (Andersson et al., 1975; Johnson and Buggy, 1978). The homeostatic activity of the LT during body fluid perturbations was evidenced here during water deprivation and hypertonic salt loading where we noted significant excitation of ventral LT neurons, the highest activity being observed within the OVLT. Within the LT, of all the experimental stimuli tested, we found that the highest proportion of vlPAG projection neurons expressing Fos-IR was observed following water deprivation (∼37%). This is interesting as previous findings indicate that the vlPAG is significantly activated by hypovolemic stimuli and suggests that this activation may be mediated, in part, via projections from the LT (Dampney et al., 1984).
The heightened activity in LT-vlPAG projecting neurons in water deprived as opposed to salt loaded animals likely reflects the multiple influences during dehydration including volume, osmolality and hormonal variations. The heightened activation of the LT may also reflect modulation of the sensitivity of the LT from baroreceptors or other peripheral relays through the parabrachial nucleus and/or the nucleus of the solitary tract (Miura et al., 1994; Saper and Levisohn, 1983).
The SFO not only displayed few neurons that projected to the vlPAG, it also displayed low activity with respect to osmotic pressure in vlPAG projecting neurons. These findings are consistent with earlier studies that propose osmoreceptive cells are located principally within the dorsal cap of the OVLT (McKinley et al., 1978; McKinley et al., 2004; Oldfield et al., 1994).
Isoproterenol
Isoproterenol, a beta-adrenergic agonist, was chosen for this study as it is a strong dipsogen, increases circulating AngII (Johnson et al., 1981) and has vasodilator properties which counteract the hypertensive effects of AngII acting on the vasculature (Moosavi and Johns, 2003; Robinson and Evered, 1987). The dipsogenic response to isoproterenol is principally mediated by increased AngII within the bloodstream (Houpt and Epstein, 1971). Given that increased c-Fos immunoreactivity in the lamina terminalis following isoproterenol administration is abolished by the AT1 receptor blocker losartan or the ACE inhibitor captopril (Oldfield and McKinley, 1994) it is recognized that circulating AngII is responsible for activation of the lamina terminalis after drug administration as opposed to vascular responses.
Circulating AngII acting centrally via the circumventricular organs is implicated in regulating thirst (Buggy et al., 1979; Phillips et al., 1978), evoking a pressor response (Johnson et al., 1978; Mangiapane and Simpson, 1980) and initiating a salt appetite (Bryant et al., 1980; Denton et al., 1984; Henry, 1988; Nicolaidis and Jeulin, 1984; Rowland et al., 1996; Schulkin and Fluharty, 1985; Thunhorst, 1996; Weisinger et al., 1987). To the extent that vlPAG is involved in one or more of these regulatory responses, our findings suggest that circulating AngII effects on the vlPAG are mediated by projections from the OVLT and SFO but not the MnPO. Our results also indicate that elevations in circulating AngII seems an unlikely cause of significant MnPO activation during water deprivation, as Fos label in this nucleus was not elevated in response to systemic isoproterenol (Table 1; Figure 2c).
Heat Exposure
Following acute heat exposure (39°C for one hour), the distribution of vlPAG projecting LT neurons that were Fos-IR were primarily located within the OVLT and MnPO (Table 1, Fig. 2). The SFO displayed few cells projecting to the vlPAG that were Fos-IR. This is consistent with studies that describe high proportions of Fos positive cells within the MnPO/preoptic area subsequent to increased ambient temperature (Gong et al., 2000; Patronas et al., 1998; Scammell et al., 1993; Yoshida et al., 2005). Our data correspond well with earlier investigations of vlPAG-projecting neurons and heat exposure. Differences between the studies reflect differences in experimental design.
Heat exposure leads to increased peripheral vasodilatation, and reduces splanchnic, renal and muscular blood flow (Kregel et al., 1991; Rowell, 1974). The activation of cells projecting to the rostral-middle vlPAG from the LT particularly the MnPO may in part mediate these responses as preoptic warming increases Fos-IR in the vlPAG (Yoshida et al., 2002). Additionally, the activation of neurons within the MnPO, often within the lateral lamina (i.e. the sides of the MnPO) indicates that local circuits within the preoptic area, possibly the medial preoptic area (MPO) are being influenced by MnPO activity. Whether local preoptic circuits are directly influenced or part of an en passant circuit is yet to be defined. Possibly, disinhibition or activation of inhibitory MPO neurons projecting to caudal brain areas involved in thermogenesis are involved (Morrison et al., 2008).
Sleep
Compared to wake animals, Fos-IR was readily found in the core-vlPOA and the ventral lamina terminalis of spontaneously sleeping animals, as previously described (Gong et al., 2000; Sherin et al., 1996; Uschakov et al., 2006). The location of Fos-IR in the MnPO tended to be distributed throughout the nucleus and within the dorsal cap of the subjacent OVLT. The proportion of vlPAG projecting neurons that were also Fos-IR was lower in the OVLT (4%) than in the MnPO (8%), with double-labeled cell counts in the MnPO being significantly higher than in awake animals.
Levels of Fos expression in the OVLT and MnPO during sleep most closely resembled that observed in response to heat exposure. This is consistent with the finding that many warm-sensing neurons in the preoptic hypothalamus also exhibit spontaneous increases in activity during sleep compared to waking (Alam et al., 1995; Alam et al., 1997). MnPO neurons that exhibit sleep-related c-Fos expression are GABAergic (Gong et al., 2004) and these neurons have been hypothesized to promote sleep via descending inhibition of arousal regulatory neurons in the posterior and lateral hypothalamus and the rostral brainstem (Szymusiak and McGinty, 2008; Uschakov et al., 2006).
Sleep active MnPO-vlPAG projection neurons may be involved in coupling the occurrence of nonREM and REM sleep, as evidence indicates that the vlPAG contains a population of neurons that exert inhibitory control over REM sleep-generating circuits in the paramedian pontine reticular formation (Luppi et al., 2006). We observed a tendency of increased Fos expression in MnPO-vlPAG projecting neurons in animals with greater REM sleep duration (data not shown). Alternatively or in addition, the sleep-active component of the MnPO-vlPAG projection could function to promote peripheral vasodilation during sleep, in the same way that this pathway is implicated in promoting vasodilation during heat exposure (Yoshida et al., 2005).
Acknowledgments
This work was supported by NHMRC project grant 454601 and NHMRC fellowship 454369 and by the Medical Research Service of the Department of Veterans Affairs and NIH grant MH63323.
References
- Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol. 1995;269(5 Pt 2):R1240–1249. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Szymusiak R. Thermosensitive neurons of the diagonal band in rats: relation to wakefulness and non-rapid eye movement sleep. Brain Res. 1997;752(1-2):81–89. doi: 10.1016/s0006-8993(96)01452-7. [DOI] [PubMed] [Google Scholar]
- Andersson B, Leksell LG, Lishajko F. Perturbations in fluid balance induced by medially placed forebrain lesions. Brain Res. 1975;99(2):261–275. doi: 10.1016/0006-8993(75)90028-1. [DOI] [PubMed] [Google Scholar]
- Aradachi H, Honda K, Negoro H, Kubota T. Median preoptic neurones projecting to the supraoptic nucleus are sensitive to haemodynamic changes as well as to rise in plasma osmolality in rats. J Neuroendocrinol. 1996;8(1):35–43. doi: 10.1111/j.1365-2826.1996.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Bergmann BM, Winter JB, Rosenberg RS, Rechtschaffen A. NREM sleep with low-voltage EEG in the rat. Sleep. 1987;10(1):1–11. doi: 10.1093/sleep/10.1.1. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis 31 Suppl. 2000;5:S157–161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Bryant RW, Epstein AN, Fitzsimons JT, Fluharty SJ. Arousal of a specific and persistent sodium appetite in the rat with continuous intracerebroventricular infusion of angiotensin II. J Physiol. 1980;301:365–382. doi: 10.1113/jphysiol.1980.sp013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggy J, Hoffman WE, Phillips MI, Fisher AE, Johnson AK. Osmosensitivity of rat third ventricle and interactions with angiotensin. Am J Physiol. 1979;236(1):R75–82. doi: 10.1152/ajpregu.1979.236.1.R75. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Hardy JD. [Single-unit and thermoregulatory responses during local heating and cooling of the preoptical region and mesencephalon in rabbits] J Physiol (Paris) 1969;61(4):331–347. [PubMed] [Google Scholar]
- Chen XM, Nishi M, Taniguchi A, Nagashima K, Shibata M, Kanosue K. The caudal periaqueductal gray participates in the activation of brown adipose tissue in rats. Neurosci Lett. 2002;331(1):17–20. doi: 10.1016/s0304-3940(02)00757-7. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Goodchild AK, Tan E. Identification of cardiovascular cell groups in the brain stem. Clin Exp Hypertens A. 1984;6(1-2):205–220. doi: 10.3109/10641968409062561. [DOI] [PubMed] [Google Scholar]
- Denton D, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc Natl Acad Sci U S A. 1999;96(5):2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton DA, Coghlan JP, Fei DT, McKinley M, Nelson J, Scoggins B, Tarjan E, Tregear GW, Tresham JJ, Weisinger R. Stress, ACTH, salt intake and high blood pressure. Clin Exp Hypertens A. 1984;6(1-2):403–415. doi: 10.3109/10641968409062573. [DOI] [PubMed] [Google Scholar]
- Fuller CA, Horwitz BA, Horowitz JM. Shivering and nonshivering thermogenic responses of cold-exposed rats to hypothalamic warming. Am J Physiol. 1975;228(5):1519–1524. doi: 10.1152/ajplegacy.1975.228.5.1519. [DOI] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556(Pt 3):935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279(6):R2079–2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26(37):9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP. Stress, salt and hypertension. Soc Sci Med. 1988;26(3):293–302. doi: 10.1016/0277-9536(88)90393-0. [DOI] [PubMed] [Google Scholar]
- Houpt KA, Epstein AN. The complete dependence of beta-adrenergic drinking on the renal dipsogen. Physiol Behav. 1971;7(6):897–902. doi: 10.1016/0031-9384(71)90061-8. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Buggy J. Periventricular preoptic-hypothalamus is vital for thirst and normal water economy. Am J Physiol. 1978;234(3):R122–129. doi: 10.1152/ajpregu.1978.234.3.R122. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Hoffman WE, Buggy J. Attenuated pressor responses to intracranially injected stimuli and altered antidiuretic activity following preoptic-hypothalamic periventricular ablation. Brain Res. 1978;157(1):161–166. doi: 10.1016/0006-8993(78)91007-7. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Mann JF, Rascher W, Johnson JK, Ganten D. Plasma angiotensin II concentrations and experimentally induced thirst. Am J Physiol. 1981;240(3):R229–234. doi: 10.1152/ajpregu.1981.240.3.R229. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Pratt R. Role of the periaqueductal gray in vocal expression of emotion. Brain Res. 1979;167:367–378. doi: 10.1016/0006-8993(79)90830-8. [DOI] [PubMed] [Google Scholar]
- Knuepfer MM, Johnson AK, Brody MJ. Vasomotor projections from the anteroventral third ventricle (AV3V) region. Am J Physiol. 1984;247(1 Pt 2):H139–145. doi: 10.1152/ajpheart.1984.247.1.H139. [DOI] [PubMed] [Google Scholar]
- Kobayashi S. Warm- and cold-sensitive neurons inactive at normal core temperature in rat hypothalamic slices. Brain Res. 1986;362(1):132–139. doi: 10.1016/0006-8993(86)91406-x. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Overton JM, Johnson DG, Tipton CM, Seals DR. Mechanism for pressor response to nonexertional heating in the conscious rat. J Appl Physiol. 1991;71(1):192–196. doi: 10.1152/jappl.1991.71.1.192. [DOI] [PubMed] [Google Scholar]
- Larson CR. On the relation of PAG neurons to laryngeal and respiratory muscles during vocalization in the monkey. Brain Res. 1991;552(1):77–86. doi: 10.1016/0006-8993(91)90662-f. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10(9):1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Liebeskind JC, Guilbaud G, Besson JM, Oliveras JL. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973;50(2):441–446. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100(5-6):271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Mangiapane ML, Simpson JB. Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol. 1980;239(5):R382–389. doi: 10.1152/ajpregu.1980.239.5.R382. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Denton DA, Leksell LG, Mouw DR, Scoggins BA, Smith MH, Weisinger RS, Wright RD. Osmoregulatory thirst in sheep is disrupted by ablation of the anterior wall of the optic recess. Brain Res. 1982;236(1):210–215. doi: 10.1016/0006-8993(82)90048-8. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Denton DA, Weisinger RS. Sensors for antidiuresis and thirst--osmoreceptors or CSF sodium detectors? Brain Res. 1978;141(1):89–103. doi: 10.1016/0006-8993(78)90619-4. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Hards DK, Oldfield BJ. Identification of neural pathways activated in dehydrated rats by means of Fos-immunohistochemistry and neural tracing. Brain Res. 1994;653(1-2):305–314. doi: 10.1016/0006-8993(94)90405-7. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol. 2004;16(4):340–347. doi: 10.1111/j.0953-8194.2004.01184.x. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Oldfield BJ. The brain as an endocrine target for peptide hormones. Trends Endocrin Met. 1998;9(9):349–354. doi: 10.1016/s1043-2760(98)00092-7. [DOI] [PubMed] [Google Scholar]
- Miura M, Takayama K, Okada J. Neuronal expression of Fos protein in the rat brain after baroreceptor stimulation. J Auton Nerv Syst. 1994;50(1):31–43. doi: 10.1016/0165-1838(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Moosavi SM, Johns EJ. The effect of isoprenaline infusion on renal renin and angiotensinogen gene expression in the anaesthetised rat. Exp Physiol. 2003;88(Pt 2):221–227. doi: 10.1113/eph8802490. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93(7):773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol. 2008;586(10):2611–2620. doi: 10.1113/jphysiol.2008.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HF, Zhang JX, Harper RM. Respiratory-related discharge of periaqueductal gray neurons during sleep-waking states. Brain Res. 1990;511(2):319–325. doi: 10.1016/0006-8993(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Nicolaidis S, Jeulin AC. Converging projections of hydromineral imbalances and hormonal co-action upon neurons surrounding the anterior wall of the third ventricle. J Physiol (Paris) 1984;79(6):406–415. [PubMed] [Google Scholar]
- Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience. 1994;60(1):255–262. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Bicknell RJ, McAllen RM, Weisinger RS, McKinley MJ. Intravenous hypertonic saline induces Fos immunoreactivity in neurons throughout the lamina terminalis. Brain Res. 1991;561(1):151–156. doi: 10.1016/0006-8993(91)90760-s. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, McKinley MJ. Distribution of Fos in rat brain resulting from endogenously-generated angiotensin II. Kidney Int. 1994;46(6):1567–1569. doi: 10.1038/ki.1994.448. [DOI] [PubMed] [Google Scholar]
- Patronas P, Horowitz M, Simon E, Gerstberger R. Differential stimulation of c-fos expression in hypothalamic nuclei of the rat brain during short-term heat acclimation and mild dehydration. Brain Res. 1998;798(1-2):127–139. doi: 10.1016/s0006-8993(98)00405-3. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Quinlin J, Keyser C, Phipps J. Organum vasculosum of the lamina terminalis (OV) as a receptor site for ADH release, drinking, and blood pressure responses to angiotensin II (AII) Fed Proc. 1978;38:438. [Google Scholar]
- Richard D, Bourque CW. Synaptic activation of rat supraoptic neurons by osmotic stimulation of the organum vasculosum lamina terminalis. Neuroendocrinology. 1992;55(5):609–611. doi: 10.1159/000126174. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J Comp Neurol. 1992;315(1):1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- Robinson MM, Evered MD. Pressor action of intravenous angiotensin II reduces drinking response in rats. Am J Physiol. 1987;252(4 Pt 2):R754–759. doi: 10.1152/ajpregu.1987.252.4.R754. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Fregly MJ, Han L, Smith G. Expression of Fos in rat brain in relation to sodium appetite: furosemide and cerebroventricular renin. Brain Res. 1996;728(1):90–96. [PubMed] [Google Scholar]
- Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res. 1983;288(1-2):21–31. doi: 10.1016/0006-8993(83)90078-1. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Price KJ, Sagar SM. Hyperthermia induces c-fos expression in the preoptic area. Brain Res. 1993;618(2):303–307. doi: 10.1016/0006-8993(93)91280-6. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Fluharty SJ. Further studies on salt appetite following lateral hypothalamic lesions: effects of preoperative alimentary experiences. Behav Neurosci. 1985;99(5):929–935. doi: 10.1037//0735-7044.99.5.929. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271(5246):216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science. 1973;181(105):1172–1175. doi: 10.1126/science.181.4105.1172. [DOI] [PubMed] [Google Scholar]
- Skultety FM. Circus movements in cats following mid-brain stimulation through chronically implanted electrodes. J Neurophysiol. 1962;25:211–225. doi: 10.1152/jn.1962.25.2.152. [DOI] [PubMed] [Google Scholar]
- Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543(Pt 2):665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41(2-3):153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Thrasher TN, Keil LC, Ramsay DJ. Lesions of the organum vasculosum of the lamina terminalis (OVLT) attenuate osmotically-induced drinking and vasopressin secretion in the dog. Endocrinology. 1982;110(5):1837–1839. doi: 10.1210/endo-110-5-1837. [DOI] [PubMed] [Google Scholar]
- Thunhorst RL. Role of peripheral angiotensin in salt appetite of the sodium-deplete rat. Neurosci Biobehav Rev. 1996;20(1):101–106. doi: 10.1016/0149-7634(95)00050-o. [DOI] [PubMed] [Google Scholar]
- Travis KA, Johnson AK. In vitro sensitivity of median preoptic neurons to angiotensin II, osmotic pressure, and temperature. Am J Physiol. 1993;264(6 Pt 2):R1200–1205. doi: 10.1152/ajpregu.1993.264.6.R1200. [DOI] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur J Neurosci. 2006;23(12):3284–3296. doi: 10.1111/j.1460-9568.2006.04860.x. [DOI] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150(1):104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DG, Darlington DN. A blood pressure lowering effect of lesions of the caudal periaqueductal gray: relationship to basal pressure. Brain Res. 1987;423(1-2):373–377. doi: 10.1016/0006-8993(87)90866-3. [DOI] [PubMed] [Google Scholar]
- Weisinger RS, Denton DA, Di Nicolantonio R, McKinley MJ, Muller AF, Tarjan E. Role of angiotensin in sodium appetite of sodium-deplete sheep. Am J Physiol. 1987;253(3 Pt 2):R482–488. doi: 10.1152/ajpregu.1987.253.3.R482. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133(4):1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Maruyama M, Hosono T, Nagashima K, Fukuda Y, Gerstberger R, Kanosue K. Fos expression induced by warming the preoptic area in rats. Brain Res. 2002;933(2):109–117. doi: 10.1016/s0006-8993(02)02287-4. [DOI] [PubMed] [Google Scholar]