Abstract
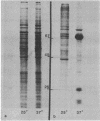
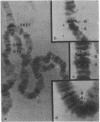
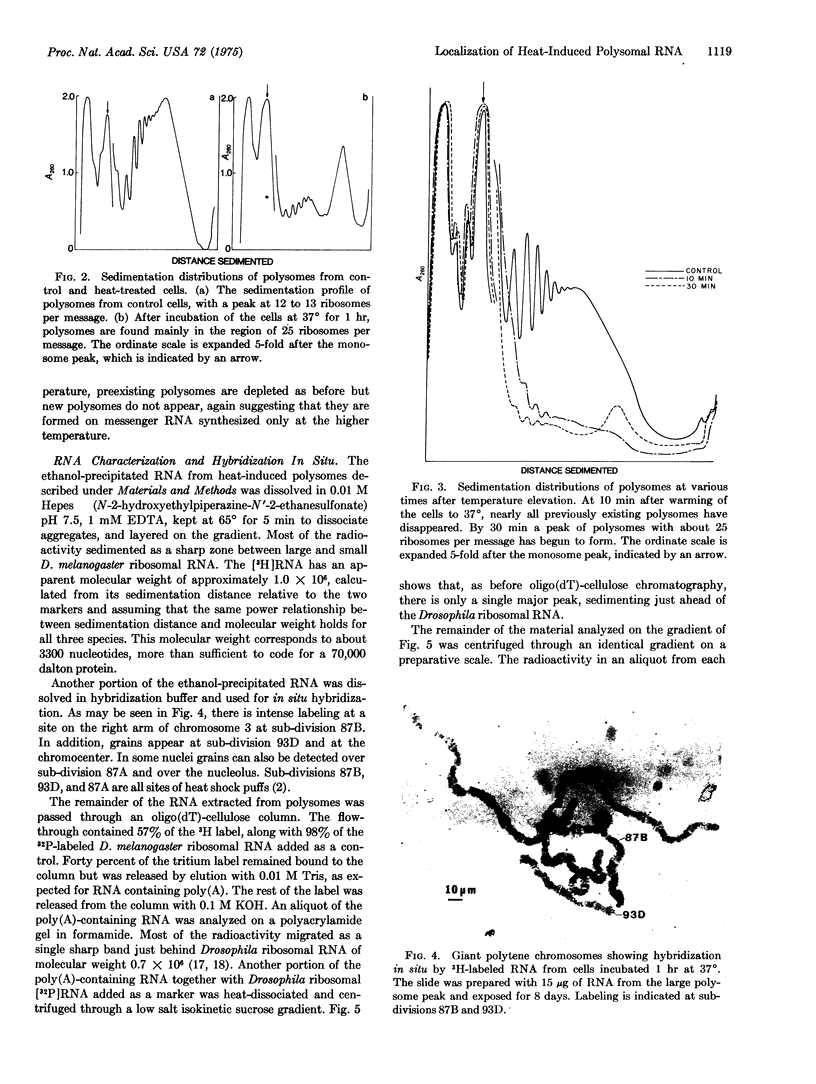
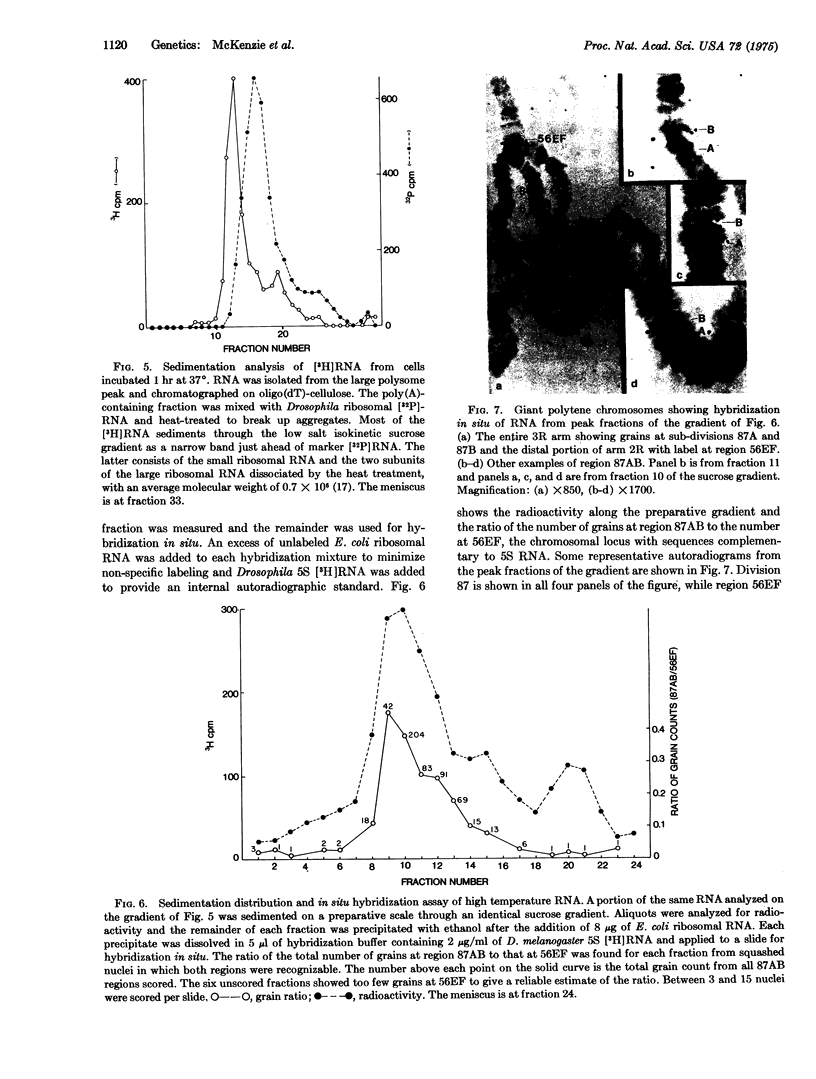
Heat treatment of D. melanogaster tissue culture cells causes drastic changes in the pattern of protein synthesis and the size distribution of polysomes. Like the heat shock puffs on polytene chromosomes which appear while preexisting puffs regress, heat shock proteins appear on gels while the synthesis of preexisting proteins is sharply reduced, and heat-induced polysomes appear on gradients after preexisting polysomes have disappeared. Most of the poly(adenylic acid)-containing RNA isolated from high-temperature polysomes sediments in sucrose gradients and migrates in gels as a rather narrow band. This RNA is of sufficient size to code for one particular protein that is found to account for more than half of the total synthesis at high temperature. The RNA hybridizes in situ mainly at chromosome sub-division 87B, the site of the major heat shock puff.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. V. Responses to environmental treatments. Chromosoma. 1970;31(3):356–376. doi: 10.1007/BF00321231. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., Ephrussi B. The Differentiation of Eye Pigments in Drosophila as Studied by Transplantation. Genetics. 1936 May;21(3):225–247. doi: 10.1093/genetics/21.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard E. G., Clever U. RNA metabolism during puff induction in Drosophila melanogaster. Chromosoma. 1971;36(1):60–78. doi: 10.1007/BF00326422. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Synthesis and properties of ribosomal RNA in Drosophila. J Mol Biol. 1969 Nov 28;46(1):85–98. doi: 10.1016/0022-2836(69)90059-x. [DOI] [PubMed] [Google Scholar]
- Grigliatti T. A., White B. N., Tener G. M., Kaufman T. C., Holden J. J., Suzuki D. T. Studies on the transfer RNA genes of Drosophila. Cold Spring Harb Symp Quant Biol. 1974;38:461–474. doi: 10.1101/sqb.1974.038.01.050. [DOI] [PubMed] [Google Scholar]
- Haines M. E., Carey N. H., Palmiter R. S. Purification and properties of ovalbumin messenger RNA. Eur J Biochem. 1974 Apr 16;43(3):549–560. doi: 10.1111/j.1432-1033.1974.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Harrison P. R., Hell A., Birnie G. D., Paul J. Evidence for single copies of globin genes in the mouse genome. Nature. 1972 Sep 22;239(5369):219–221. doi: 10.1038/239219a0. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Hunt J. A. Rate of synthesis and half-life of globin messenger ribonucleic acid. Rate of synthesis of globin messenger ribonucleic acid calculated from data of cell haemoglobin content. Biochem J. 1974 Mar;138(3):499–510. doi: 10.1042/bj1380499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. W., Robertson F. W. Localisation of reiterated nucleotide sequences in Drosophila and mouse by in situ hybridisation of complementary RNA. Chromosoma. 1970;31(3):331–345. doi: 10.1007/BF00321229. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]