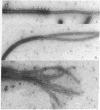
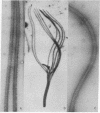
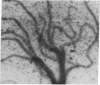
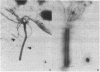
Abstract
Flagellar microtubules from Chlamydomonas and sea urchin sperm were used as in vitro assembly sites for chick brain tubulin. Brain microtubules assembled onto the A-tubules, central tubules, and, to a limited extent, onto the distal ends of the axonemes at low tubulin concentrations and onto distal and proximal ends at high tubulin concentrations; however, the rate of assembly onto the distal end was always greater. The rate of neurotubule assembly onto axonemes was shown to be dependent upon tubulin concentration and a forward rate constant for assembly was determined.
Full text
PDF
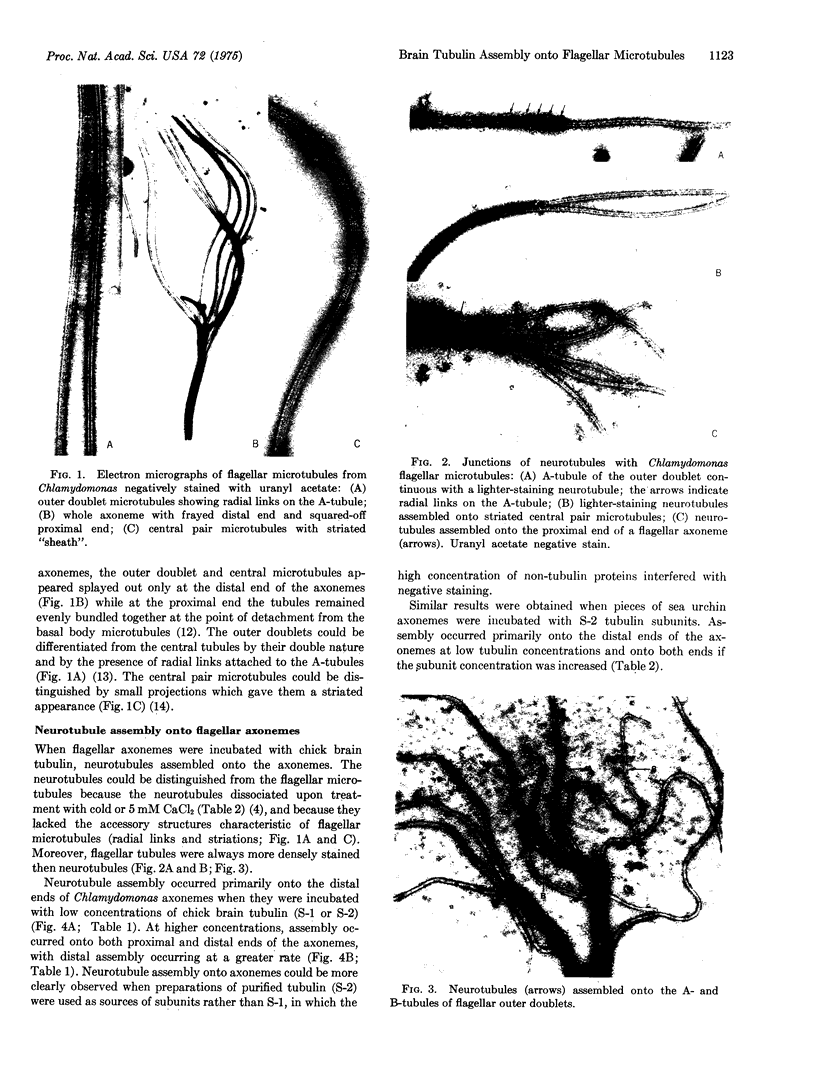
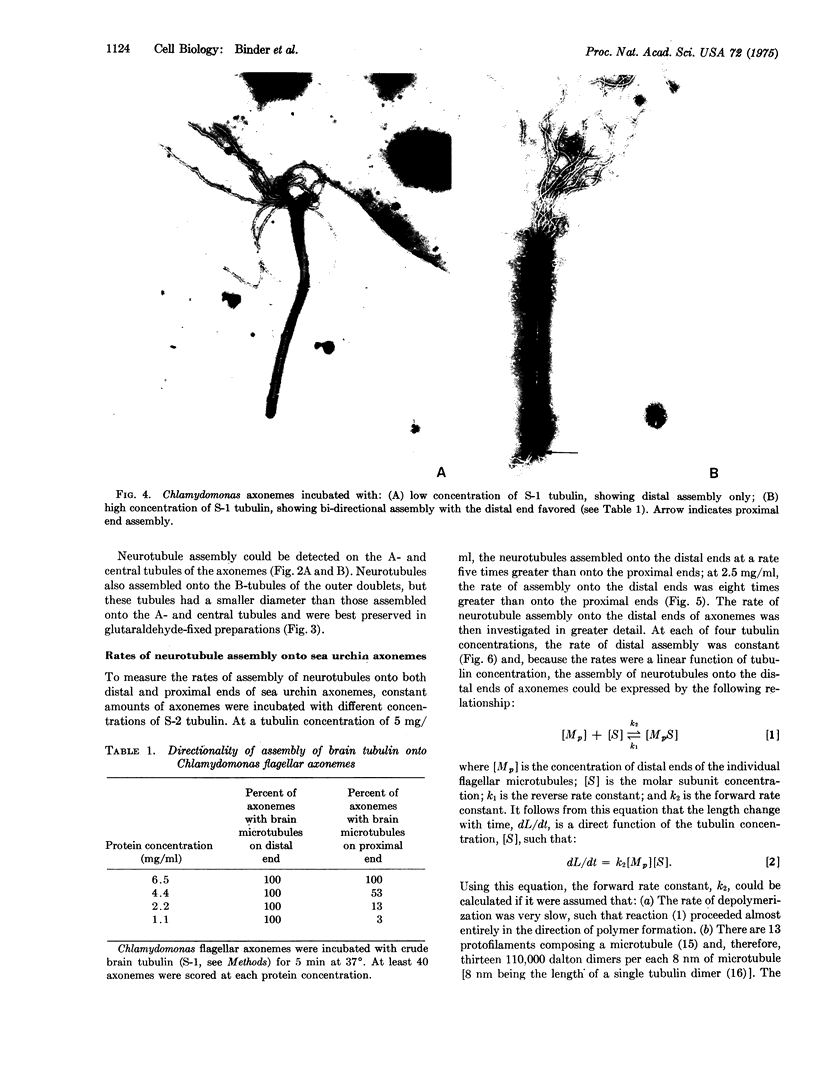
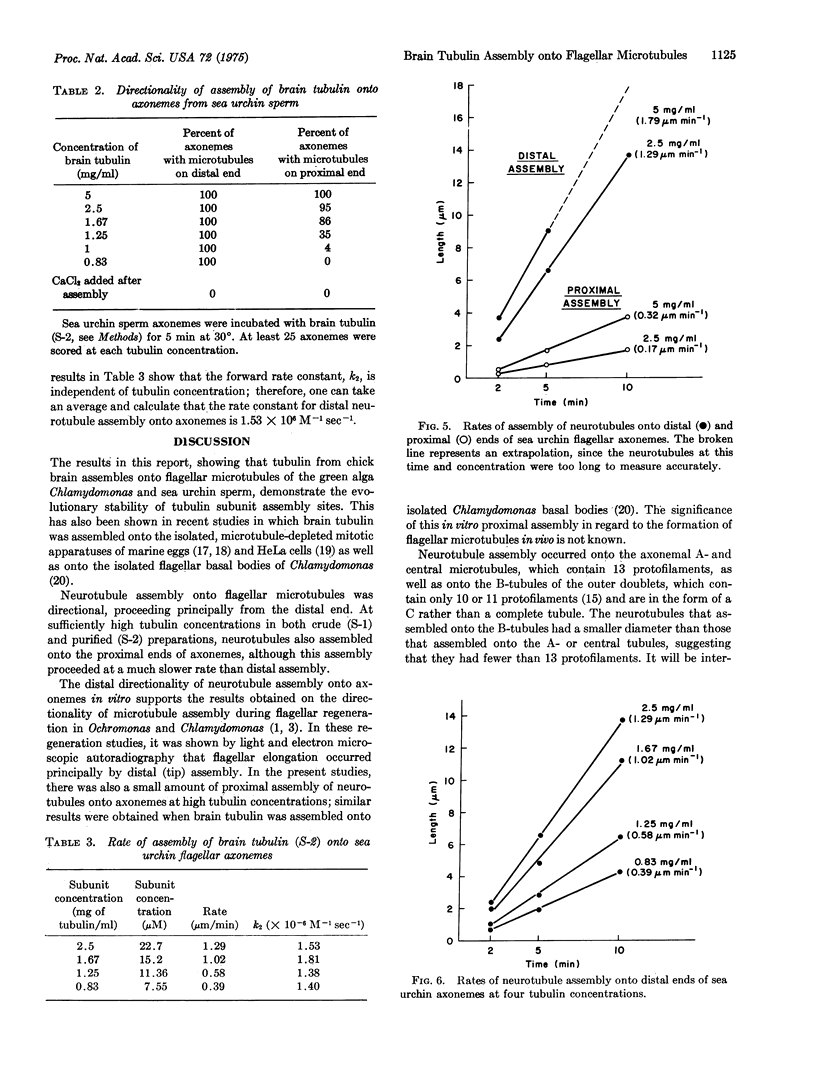

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L., Klug A. Arrangement of subunits in flagellar microtubules. J Cell Sci. 1974 May;14(3):523–549. doi: 10.1242/jcs.14.3.523. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B. Nucleated assembly of microtubules in porcine brain extracts. Science. 1972 Sep 29;177(4055):1196–1197. doi: 10.1126/science.177.4055.1196. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Fernandez H. L. Delineation by lanthanum staining of filamentous elements associated with the surfaces of axonal microtubules. J Cell Sci. 1973 Mar;12(2):567–583. doi: 10.1242/jcs.12.2.567. [DOI] [PubMed] [Google Scholar]
- Cande W. Z., Snyder J., Smith D., Summers K., McIntosh J. R. A functional mitotic spindle prepared from mammalian cells in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1559–1563. doi: 10.1073/pnas.71.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Witman G. B., Rosenbaum J. L. Directionality of brain microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1974 May;71(5):1710–1714. doi: 10.1073/pnas.71.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P. Microtubule surface lattice and subunit structure and observations on reassembly. J Cell Biol. 1974 Jan;60(1):153–167. doi: 10.1083/jcb.60.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Hopkins J. M. Subsidiary components of the flagella of Chlamydomonas reinhardii. J Cell Sci. 1970 Nov;7(3):823–839. doi: 10.1242/jcs.7.3.823. [DOI] [PubMed] [Google Scholar]
- Inoué S., Borisy G. G., Kiehart D. P. Growth and lability of Chaetopterus oocyte mitotic spindles isolated in the presence of porcine brain tubulin. J Cell Biol. 1974 Jul;62(1):175–184. doi: 10.1083/jcb.62.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane N. J., Treherne J. E. Lanthanum staining of neurotubules in axons from cockroach ganglia. J Cell Sci. 1970 Jul;7(1):217–231. doi: 10.1242/jcs.7.1.217. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Witman G. B., Carlson K., Rosenbaum J. L. Comparison of the microtubule proteins of neuroblastoma cells, brain, and Chlamydomonas flagella. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2273–2277. doi: 10.1073/pnas.68.9.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun L. I., Rosenbaum J., Lefebvre P., Smith G. Reversible restoration of the birefringence of cold-treated, isolated mitotic apparatus of surf clam eggs with chick brain tubulin. Nature. 1974 May 10;249(453):113–115. doi: 10.1038/249113a0. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. L., Child F. M. Flagellar regeneration in protozoan flagellates. J Cell Biol. 1967 Jul;34(1):345–364. doi: 10.1083/jcb.34.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E., Ringo D. L. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969 May;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional studies with Chlamydomonas reinhardi. Ann N Y Acad Sci. 1953 Oct 14;56(5):831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Snell W. J., Dentler W. L., Haimo L. T., Binder L. I., Rosenbaum J. L. Assembly of chick brain tubulin onto isolated basal bodies of Chlamydomonas reinhardi. Science. 1974 Jul 26;185(4148):357–360. doi: 10.1126/science.185.4148.357. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Thermal fractionation of outer fiber doublet microtubules into A- and B-subfiber components. A- and B-tubulin. J Mol Biol. 1970 Feb 14;47(3):353–363. doi: 10.1016/0022-2836(70)90307-4. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Bryan J., Bush D. J., Fujiwara K., Mooseker M. S., Murphy D. B., Snyder D. H. Microtubules: evidence for 13 protofilaments. J Cell Biol. 1973 Nov;59(2 Pt 1):267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner F. D. New observations on flagellar fine structure. The relationship between matrix structure and the microtubule component of the axoneme. J Cell Biol. 1970 Oct;47(1):159–182. doi: 10.1083/jcb.47.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Rosenbaum J. L. Chlamydomonas flagella. II. The distribution of tubulins 1 and 2 in the outer doublet microtubules. J Cell Biol. 1972 Sep;54(3):540–555. doi: 10.1083/jcb.54.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971 Jun;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]