Abstract
Background
Glycemic control in critically ill patients decreases infection and mortality. While capillary blood glucose values are accurate in normotensive patients and correlate with arterial samples, patients on vasopressors have altered peripheral perfusion that may affect accuracy of capillary blood glucose values tested using point of care devices.
Objectives
To compare capillary and arterial blood samples using point of care testing (POCT) with arterial blood samples using the clinical chemistry lab in patients following cardiothoracic surgery, and to determine if vasopressor medications or diminished peripheral perfusion influenced the accuracy of POCT values.
Methods
In a prospective, convenience sample (n=50) of adult post-operative cardiothoracic patients on insulin and vasopressors, samples (n=162) were obtained simultaneously from capillary and arterial sites during insulin infusion and tested on both POCT and clinical chemistry lab, respectively. Quality of peripheral perfusion was recorded using a standardized scale. Clarke error grid analysis and ISO 15197 were used to analyze the level of agreement between the three samples. Two-way ANOVA was used to analyze differences in blood glucose values with respect to vasopressor use and peripheral perfusion.
Results
An unacceptable level of agreement was found between the capillary POCT and arterial samples tested in the clinical chemistry lab (only 88.3% of values fell in Zone A, or within the ISO 15197 tolerance bands). Arterial POCT showed 94.4% agreement with the clinical chemistry lab. Vasopressor use demonstrated a statistically significant effect on the accuracy of arterial blood glucose values (F=15.01; p= .0001).
Conclusions
Capillary POCT is not within acceptable limits of agreement with the clinical chemistry lab. Even when using the more accurate arterial blood with POCT, patients with >2 vasopressors demonstrate significantly less accuracy as compared to patients on fewer vasopressors. Using the clinical chemistry lab may be safer for insulin titration in these patients.
Keywords: insulin, glucose, glycemic control, adrenergic beta-agonists, vasoconstrictor agents, catecholamine therapy, heart surgery, point-of-care testing, postoperative care, practice guidelines
Glycemic control is an essential part of caring for critically ill patients and has been shown to improve outcomes.(1) Hyperglycemia is associated with increased morbidity and mortality in diabetic and non-diabetics following cardiac surgery and maintaining glucose < 180 mg/dl has been shown to reduce mortality and morbidity, decrease the incidence of wound infections, reduce hospital length of stay and enhance long-term survival. The use of intravenous insulin infusion to maintain an early postoperative glucose < 180 mg/dl while avoiding hypoglycemia is a class 1b recommendation for patients following cardiac surgery in the 2011 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Guideline for Coronary Artery Bypass Surgery.(2, 3)
The accuracy of blood glucose measurements is paramount to nurses’ ability to manage and control blood glucose. Patients on insulin infusions require frequent blood glucose measurements for glycemic control. These measurements are performed every two hours minimum and generally every hour due to frequent changes in blood glucose values and to achieve the benefits of glycemic control. Blood glucose measurement using a finger-stick to obtain capillary samples is one way to minimize blood sampling from arterial lines in patients on insulin infusions. Additionally, the capillary glucose check is beneficial as it is a task that can be safely delegated to a nursing care assistant.
Studies have shown that capillary blood glucose measurements are accurate in normotensive patients and provide good correlation with samples tested in a clinical chemistry lab.(4) However, patients receiving vasopressor agents may be normotensive by arterial blood pressure measurement, but have altered peripheral perfusion due to the vasoconstrictor effects of the medication.(5) Altered peripheral perfusion may affect the accuracy of capillary blood glucose measurements, however actual best practice for this patient population is not known. One study of 50 postoperative cardiovascular surgery patients demonstrated high correlation in capillary glucose sampling,(6) while others have shown poor correlation in cardiopulmonary resuscitation (CPR),(7) shock states,(5, 8) presence of edema,(9) and following major surgery.(9, 10) The small sample sizes in these studies and the conflicting results support the need for further study in populations with compromised peripheral perfusion.
The standard of practice for continuous insulin infusions in many intensive care unit settings is hourly blood glucose monitoring, particularly in the acute post-operative phase. Standard procedure however does not dictate method, and as a result either or both arterial and capillary blood samples are collected. Both finger sticks and arterial samples may be drawn concomitantly for purposes of validation; however, this is not required and is a nurse-dependent variation in care. Patients are typically admitted to the cardiothoracic intensive care unit from the operating room on insulin infusions and vasoactive medications. During early post-operative recovery frequent titration of both insulin and vasopressor drips occurs,(2) however, studies to date do not consistently support the accuracy of capillary blood glucose in this setting or the correlation of arterial versus capillary blood glucose values in this critically ill patient population. In order to answer the research question regarding accuracy and correlation in the setting of high doses of vasopressor drugs, early access to temporally related samples is required. The purpose of this study was to compare capillary and arterial blood samples using point of care testing (POCT) with arterial blood samples using the clinical chemistry lab in patients following cardiothoracic surgery, and to determine if vasopressor medications or peripheral perfusion influenced the POCT values.
METHODS
Design, Setting and Sample
This study was a prospective, case-controlled design, conducted in the 20-bed cardiothoracic intensive care unit (CTICU) at a large academic tertiary care hospital. The unit provides care for patients undergoing cardiac and thoracic surgery including bypass grafting, valve surgery, heart and lung transplant, ventricular assist devices, and thoracic surgeries. A convenience sample (n=50) of adult post-operative cardiothoracic patients on insulin and vasopressors were prospectively enrolled. All participants completed informed consent and the study was approved by the institutional review board.
Measures
Prospective data collection of all study variables occurred for participants on admission to the CTICU and each time thereafter that a basic metabolic panel was ordered while the patient was on a continuous insulin infusion drip. Variables collected included: pharmacotherapy, vital signs and blood samples obtained simultaneously from arterial and capillary sites. Samples were tested using point of care testing (POCT) and the clinical chemistry laboratory testing (CLT) as the “gold standard”. The samples sent to the clinical lab were run on a Beckman Coulter Unicel DxC 600/800 utilizing the oxygen consumption rate method.
Vasopressor medications included in the evaluation for this study were: dopamine, epinephrine, norepinephrine, phenylephrine or vasopressin. The quality of peripheral tissue perfusion was assessed by standard scales for pulse (0-4), capillary refill (< 3 sec, > 3 sec), color (pink-mottled) and temperature (hot-cold) of the extremity used for study samples. The scales used for these assessments in this study are a part of the usual care procedures for all nurses certified to care for patients in the CTICU. The competency-based orientation process accounts for reliability, inter-observer, and intra-observer variation for these scales. The temperature of the extremities was categorized into two groups (cool or warm) based on the distribution of the data and the lack of subject variability across groups. The documented temperature of the extremities fell into one of these two categories for all participants. The categories were coded as 1 = cold or cool and 2 = warm or hot. These categories were used as the measure of peripheral tissue perfusion for comparison in the study.
Study Procedures
Demographics and medical history were abstracted from the medical record. Fifty adult (age 18 years and above) post-operative cardiothoracic subjects on insulin and vasopressor infusions were enrolled and consented to participate in the study. Since samples were needed early in the post-operative recovery process and patients were sedated and therefore incapable of giving consent, we obtained ethical review and approval for a conditional waiver of consent and authorization. We collected the early matched samples and these data were used only if the participant or legally authorized representative later gave consent for use of the samples.
Patients with a hematocrit of < 20% or > 70% were excluded from the study due to reported inaccuracy of the glucometer (Abbott Precision PCx). All participants were required to have undergone coronary artery bypass grafting, valve repair or replacement, lung transplant, or heart transplant, and were receiving insulin as a part of standard care for blood glucose management following cardiothoracic surgery. The first blood draw was from the immediate post-operative admission as per standard post-operative care orders. Results of blood glucose checks from capillary and arterial sources were recorded. The type and number of intravenous vasopressor medications and intravenous insulin were also recorded. Additional assessment data included the presence of edema, quality of capillary refill, and quality of pulse from the arm from which the blood glucose samples were drawn. The test nurse was not blinded to the results; however bias was limited by the objective nature of the data and the assessment strategy. The majority of variables were objective data (blood glucose values and the number of vasopressive medication used) with the exception of the perfusion assessment. The possibility for introduction of bias in the perfusion assessment was limited by the fact that nurses used a standardized assessment scale and evaluated all patients in the same manner. There was no ‘treatment group’ per se, all patients had exactly the same assessment. In addition, nurses documented the same information in the patient record as was used for the research study.
Analysis
A descriptive, quantitative evaluation of all demographic variables was conducted to estimate the frequency and distribution of patient characteristics. Each patient had blood samples obtained simultaneously from arterial and capillary sites for the duration of the insulin infusion drip. This procedure resulted in all patients having 2-5 sets of blood samples. Total 162 sets of blood glucose values were used in the analysis after removing missing values and outliers. The “outliers” consist of three samples with blood glucose values of 1002, 14, and 18. These values are inconsistent with the correlating capillary POCT value taken from these same patients at the same time points. We therefore consider these values to be errors and we have eliminated these samples from the analysis.
Clarke error grid analysis (EGA) (11) was performed to assess the level of agreement between both capillary and arterial blood glucose using the POCT and arterial blood glucose using the clinical chemistry laboratory test. The error grid analysis has 5 zones of relative accuracy of blood glucose estimation. Zone A indicates a difference of less than 20% in the blood glucose values being compared. This zone represents acceptably equivalent values and no difference would be expected with regard to clinical decision-making in response to values that fall in this zone. Zone A is the only acceptable zone which has the same criteria as the ISO 15197 gold standard guideline. Zone B represents a difference of greater than 20% in glucose values but has minimal impact on the clinical treatment. Zone C indicates that the difference in glucose values overcorrects the acceptable blood glucose and causes a change in the clinical action. Zone D indicates that the difference in glucose values has the risk of failure to detect or treat an error. Zone E indicates that the difference in glucose values causes erroneous treatment.
The ISO 15197 guideline states that measurements should be within 15 mg/dl of reference for glucose <75 mg/dl and within 20% for glucose ≥75 mg/dl. A blood glucose device is considered accuracy if 95% of pairs satisfy these criteria. Results fell outside the ISO 15197 tolerance bands are considered inaccurate.
Analysis of variance (ANOVA) was used to evaluate the association between the differences in sets of blood glucose values within each time-point, peripheral perfusion and vasopressor use. The patients were classified into two groups according to medication use at the time of the blood sampling: 1-2 medications and 3-5 medications. The patients’ peripheral perfusion was classified as one of two categories: cool or warm. The analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
Of 50 patients who agreed to participate in the study, one patient had incomplete data and was not able to be included in the final analysis. Among the 49 remaining participants, a total of 162 sets of blood glucose values were available for the final analysis. The mean age of participants was 61.3 years (standard deviation 13.9) and 81.6% (n=40) were Caucasian race. The mean body mass index (BMI) for the sample was 28.8 kg/m2 (standard deviation, 7.0 kg/m2), and 26.5% (n=13) of the participants were smokers. The average core temperature was 37.2 °C (standard deviation, 0.9), and the average number of vasopressor medications (dopamine, epinephrine, vasopressin, norepinephrine, and phenylephrine) that patients were receiving was 3.2 (standard deviation, 1.0). Table 1 shows demographic characteristics of the study population.
Table 1.
Demographic Characteristics (n=50)
| Participant Characteristics | Value | |
|---|---|---|
| Age in years | mean (SD) | 61.3 (13.9) |
| White (vs. Black) | number (%) | 40.0 (81.6) |
| Tobacco use, yes | number (%) | 13.0 (26.5) |
| BMI, kg/m2 | mean (SD) | 28.8 (7.0) |
| Inotropic drugs (#) | mean (SD) | 3.2 (1.0) |
| Core temperature, °C | mean (SD) | 37.2 (0.9) |
SD = Standard deviation; BMI = body mass index
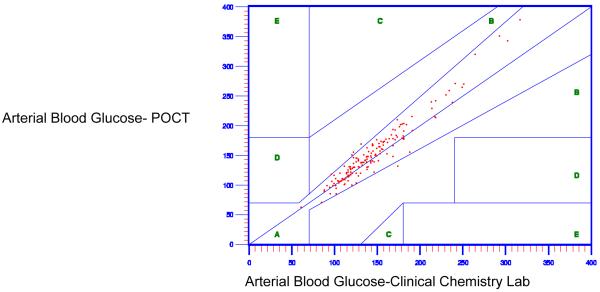
First, the level of agreement between the capillary blood glucose using the POCT and the arterial blood glucose using the clinical chemistry lab was evaluated using Clarke EGA and ISO 15197 guideline. Only eighty-eight percent (88.3%) of the capillary POCT values fell in Zone A (Figure 1), with 88.3% within the ISO 15197 tolerance bands. The capillary blood glucose using the POCT was therefore considered inaccurate as less than 95% of the values satisfy the ISO clinical standards guideline criteria. A systematic bias between the POCT and clinical chemistry lab test results was present, as was expected based on previously reported research.
Figure 1.
Capillary Blood Glucose Values Using Point of Care Testing (POCT) as Compared to Arterial Blood Glucose Values Using Clinical Chemistry Lab
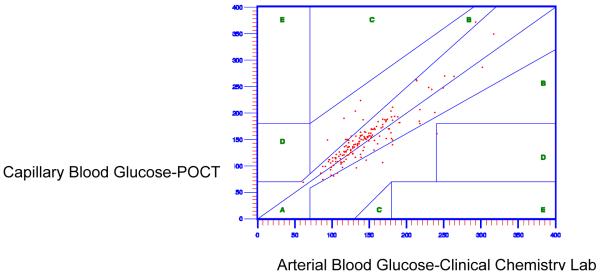
Next, Clarke EGA was used to evaluate the level of agreement in the arterial blood glucose using POCT and the arterial blood glucose using the clinical chemistry lab. As shown in Figure 2, acceptable agreement between arterial blood glucose at the POCT and the arterial blood glucose at the clinical chemistry laboratory test was indicated, with 94.4% of values falling in Zone A. The comparison performed by ISO 15197 guideline has the same results, 94.4% pairs of arterial blood glucose using POCT and the arterial blood glucose using the clinical chemistry lab within the tolerance bands.
Figure 2.
Accuracy of Arterial Point of Care Testing Versus Clinical Chemistry Lab
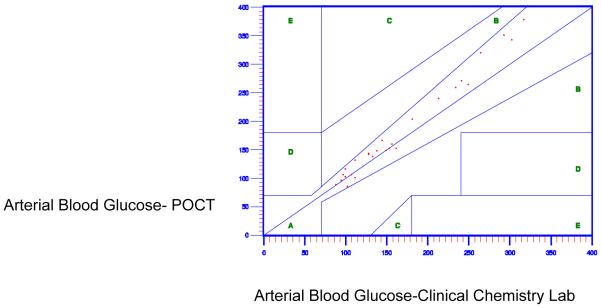
Finally, ANOVA was used to examine the relationship among vasopressor medication use, the peripheral perfusion scores, and the accuracy and reliability of arterial POCT blood glucose values. Patients with more vasopressors (greater than two concomitant vasopressor medications) demonstrated a statistically significant difference in arterial blood glucose POCT accuracy as compared to patients with fewer vasopressors (1-2 vasopressor medications) (F= 15.01; p = .0001). Patients with decreased peripheral perfusion, categorized as cool, demonstrated no significant difference in arterial POCT blood glucose accuracy as compared to patients with better perfusion, categorized as warm (F=0; p=0.98).
In summary, higher vasopressor use demonstrated significant differences in accuracy when using arterial POCT blood glucose testing.
Discussion
This study evaluated the accuracy of both capillary and arterial blood samples using POCT in patients on insulin after cardiothoracic surgery. Accuracy was determined by the level of agreement between capillary as well as arterial blood glucose samples when tested using POCT and compared with the gold standard measure, arterial blood glucose from the clinical chemistry lab. Two statistical approaches were used to verify level of agreement, the Clarke error grid analysis and the ISO 15197, the standard analysis used in the clinical chemistry lab upon which guidelines for clinical acceptability in levels of agreement are based.
In addition and most importantly, this study evaluated the relationship between both vasopressor medications and altered peripheral perfusion on the accuracy of arterial POCT blood glucose values in patients on insulin after cardiothoracic surgery. Results showed that patients on more than two vasopressor infusions had significantly lower accuracy of arterial blood glucose values. Peripheral perfusion did not affected the accuracy of arterial POCT blood glucose values
These findings play an important role in ICU care, particularly for patients undergoing cardiothoracic surgery. The importance of glycemic control for all patients undergoing cardiac surgery has been demonstrated in numerous studies.(1, 2, 12) Higher glucose (> 180 mg/dl ) during the perioperative period is an independent predictor of mortality in diabetics and nondiabetics.(3) Although glycemic control in critically ill patients decreases infection and mortality, tight glycemic control (< 150 mg/dl) is associated with increased incidence of hypoglycemic events and increased mortality.(13) These results led to the early discontinuation of tight glycemic control research protocols.(13-15) Glycemic control (glucose < 180 mg/dl) is a Class I recommendation in the 2011 ACCF/AHA Guidelines.(3) Despite this recommendation, the evidence for titration of insulin infusions following cardiothoracic surgery in patients who are subsequently receiving high doses of vasopressor medications is scant.
As has been shown by other investigators in critically ill patient populations, altered blood pressure affects the accuracy of POCT blood glucose measurements.(5, 8) Studies have shown that POCT blood glucose measurements are accurate in normotensive patients and provide good correlation with arterial samples. However, following cardiothoracic surgery, patients are likely to be hypotensive and typically receive two or more vasopressor medications for short term hemodynamic support. These patients may be normotensive by arterial blood pressure measurement, but due to the alpha and beta adrenergic receptor agonist effects of the medication, may have resultant peripheral vasoconstriction. Our study supports that greater than two vasopressor medications affects the accuracy of POCT values in this patient population.
This study evaluated the relationship between peripheral perfusion in postoperative cardiac surgery patients and the accuracy of POCT blood glucose analysis and found that there was no statistically significant difference in accuracy in blood glucose values in patients with decreased perfusion.
Limitations
A limitation to the study was the short duration of ICU stay for patients following cardiothoracic surgery in this study. We were likely to lose data capture opportunities for data collection after the first 4-8 hours due to the rapid discontinuation of vasopressor drugs. Because all patients did not have data beyond the second time period, we were not able to conduct a multiple repeated measures analysis (MANCOVA), and therefore lacked the ability to have increased sensitivity in the statistical output beyond the second (Time 2) data collection point for each patient. For example, we could not evaluate the trends in warming and decreasing drug use on blood glucose values over time using repeated measures since all patients did not have more than two time points of evaluation. In addition, patients were warmer than anticipated (higher core body temperature) in the immediate post-operative period. This may have minimized the effect of peripheral vasoconstriction. Lastly, the care nurse served as the investigator as well as the care nurse, therefore patients with high dose, multiple inotropes may have required care that precluded regularly scheduled data collection.
Implications for Clinical Practice
Clinical practice recommendations based on these findings were presented to the Clinical Practice Council (CPC) and included the following:
POCT using arterial blood sampling was recommended to improve the efficiency and reduce the cost of obtaining frequent blood glucose results.
POCT was only within acceptable limits of accuracy in patients on no more than two concomitant vasopressors.
Use of a consistent blood source for testing was recommended to reduce unnecessary variation and improve safety in insulin dosing .
This study validated previous work showing a systematic bias in the use of capillary POCT as compared to clinical chemistry lab for management of insulin drips in patients. In addition, the arterial POCT was within the 95% acceptable level of agreement with clinical chemistry lab. Those patients on three or more vasopressor medications had significant and unacceptable differences in the blood glucose values as compared to the clinical chemistry lab. As a result the POCT device cannot be recommended for patients with three or more vasopressors.
Our research work group was interested in conducting this study because nurses had observed incidences when simultaneous testing in patients produced large variances in results. These differences were confirmed in the initial analysis of the accuracy of capillary blood glucose values. Due to the overall inaccuracy of capillary values, it was irrelevant to further analyze the effect of vasoconstrictors or peripheral perfusion. The recommendation to clinical practice council (CPC) supported the use of POCT using arterial blood to improve accuracy at the highest rate of precision, and avoid using capillary samples in this patient population. In addition, our study shows that arterial POCT in patients with > 2 vasopressors is not reliable in cardiac surgery patients. Study results were disseminated at the health-system wide patient safety conference and the study team received a first place award for research to improve patient safety.
Current practice includes use of POCT testing from arterial blood samples for titration of insulin drips in cardiac surgery patients. Our study results demonstrate inaccuracy in these values in patients with > 2 vasopressors. These results need to be verified in a larger sample of patients to recommend practice change. A quality improvement project to verify these results is planned.
Conclusions
In conclusion, our findings show that capillary POCT resulted in unacceptable, low levels of agreement with the gold standard clinical chemistry lab. Arterial POCT was within the 95% acceptable level of agreement with clinical chemistry lab, and is recommended for safe titration of insulin infusions in this patient population. In the context of three or more vasopressor infusions, arterial POCT yielded significantly different results from the clinical chemistry lab and cannot be recommended for safe insulin titration in this patient population. Lastly, arterial POCT was not significantly different from the clinical chemistry lab in patients with poor peripheral perfusion (cold) as compared to those with normal peripheral perfusion (warm).
Summary of Key Points.
Use of vasopressor medications significantly affected the accuracy of arterial POCT blood glucose values.
Patients on more than two vasopressor medications are more likely to have variances in POCT blood glucose testing.
Figure 3.
Accuracy of Arterial Point of Care Testing Versus Clinical Chemistry Lab With 3-5 Vasopressive Medications
Table 2.
Error Grid Analysis Of Accuracy Of Arterial Point Of Care Versus Clinical Chemistry Lab Testing By Medication Usage
| Medications(0-2) | Medications(3-5) | |||
|---|---|---|---|---|
| Zone | Frequency | Percentage (%) | Frequency | Percentage (%) |
| A | 120 | 95.24 | 27 | 90% |
| B | 6 | 4.76 | 3 | 10% |
| C | 0 | 0 | 0 | 0 |
| D | 0 | 0 | 0 | 0 |
| E | 0 | 0 | 0 | 0 |
Acknowledgments
This publication was made possible by Grant Number 1 UL1 RR024128-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
All work for this study was performed at Duke University Hospital and School of Nursing
References
- 1.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. The New England journal of medicine. 2001;345(19):1359–67. doi: 10.1056/NEJMoa011300. Epub 2002/01/17. [DOI] [PubMed] [Google Scholar]
- 2.Lazar HL, MM, Chipkin SR, et al. The Society of Thoracic Surgeons Practice Guideline Series: Blood Glucose Management During Adult Cardiac Surgery. Annals of Thoracic Surgery. 2009:663–9. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2011;58(24):e123–210. doi: 10.1016/j.jacc.2011.08.009. Epub 2011/11/11. [DOI] [PubMed] [Google Scholar]
- 4.Lonjaret L, Claverie V, Berard E, Riu-Poulenc B, Geeraerts T, Genestal M, et al. Relative accuracy of arterial and capillary glucose meter measurements in critically ill patients. Diabetes & metabolism. 2012 doi: 10.1016/j.diabet.2011.12.003. Epub 2012/02/22. [DOI] [PubMed] [Google Scholar]
- 5.Dubose JJ, Inaba K, Branco BC, Barmparas G, Lam L, Teixeira PG, et al. Discrepancies between capillary glucose measurements and traditional laboratory assessments in both shock and non-shock states after trauma. The Journal of surgical research. 2012 doi: 10.1016/j.jss.2012.04.003. Epub 2012/05/26. [DOI] [PubMed] [Google Scholar]
- 6.Maser RE, Butler MA, DeCherney GS. Use of arterial blood with bedside glucose reflectance meters in an intensive care unit: are they accurate? Critical care medicine. 1994;22(4):595–9. doi: 10.1097/00003246-199404000-00014. Epub 1994/04/01. [DOI] [PubMed] [Google Scholar]
- 7.Thomas SH, Gough JE, Benson N, Austin PE, Stone CK. Accuracy of fingerstick glucose determination in patients receiving CPR. Southern medical journal. 1994;87(11):1072–5. doi: 10.1097/00007611-199411000-00003. Epub 1994/11/01. [DOI] [PubMed] [Google Scholar]
- 8.Sylvain HF, Pokorny ME, English SM, Benson NH, Whitley TW, Ferenczy CJ, et al. Accuracy of fingerstick glucose values in shock patients. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 1995;4(1):44–8. Epub 1995/01/01. [PubMed] [Google Scholar]
- 9.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, et al. Reliability of point-of-care testing for glucose measurement in critically ill adults. Critical care medicine. 2005;33(12):2778–85. doi: 10.1097/01.ccm.0000189939.10881.60. Epub 2005/12/15. [DOI] [PubMed] [Google Scholar]
- 10.Akinbami F, Segal S, Schnipper JL, Stopfkuchen-Evans M, Mills J, Rogers SO., Jr. Tale of two sites: capillary versus arterial blood glucose testing in the operating room. American journal of surgery. 2012;203(4):423–7. doi: 10.1016/j.amjsurg.2011.10.013. Epub 2012/03/01. [DOI] [PubMed] [Google Scholar]
- 11.Clarke WL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622–8. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 12.Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2004;10(Suppl 2):21–33. doi: 10.4158/EP.10.S2.21. Epub 2004/07/15. [DOI] [PubMed] [Google Scholar]
- 13.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. The New England journal of medicine. 2009;360(13):1283–97. doi: 10.1056/NEJMoa0810625. Epub 2009/03/26. [DOI] [PubMed] [Google Scholar]
- 14.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. The New England journal of medicine. 2008;358(2):125–39. doi: 10.1056/NEJMoa070716. Epub 2008/01/11. [DOI] [PubMed] [Google Scholar]
- 15.Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive care medicine. 2009;35(10):1738–48. doi: 10.1007/s00134-009-1585-2. Epub 2009/07/29. [DOI] [PubMed] [Google Scholar]