Abstract
Background
Studies report contrasting results regarding the efficacy and safety of pharmacological, psychological, and combined interventions in psychosis and schizophrenia in children, adolescents and young adults.
Methods
Systematic review and meta-analysis. Embase, Medline, PreMedline, PsycINFO, and CENTRAL were searched to July 2013 without restriction to publication status. Randomised trials comparing any pharmacological, psychological, or combined intervention for psychosis and schizophrenia in children, adolescents and young adults were included. Studies were assessed for bias, and GRADE criteria were used to describe the quality of the results.
Results
Twenty-seven trials including 3067 participants were identified. Meta-analyses were performed for 12 comparisons: symptoms, relapse, global state, psychosocial functioning, depression, weight and discontinuation. Low quality evidence demonstrated that antipsychotics have small beneficial effects on psychotic symptoms (SMD = -0.42, 95% CI -0.58 to -0.26), and a medium adverse effect on weight gain (WMD = 1.61, 95% CI 0.61 to 2.60) and discontinuation due to side effects (RR = 2.44, 95% CI, 1.12 to 5.31). There were no trials of psychological treatments in under-18 year olds. There was no evidence of an effect of psychological interventions on psychotic symptoms in an acute episode, or relapse rate, but low quality evidence of a large effect for family plus individual CBT on the number of days to relapse (WMD = 32.25, 95% CI -36.52 to -27.98).
Conclusions
For children, adolescents and young adults, the balance of risk and benefit of antipsychotics appears less favourable than in adults. Research is needed to establish the potential for psychological treatments, alone and in combination with antipsychotics, in this population.
Introduction
Early-onset schizophrenia, that is, schizophrenia occurring prior to 17 years [1], affects approximately 1.6 to 1.9 per 100,000 of the child and adolescent population [2–5]. It is a severe and debilitating disorder associated with considerable long-term impairments in psychological, social, educational and occupational functioning [6], poor physical health, reduced life expectancy [7,8], and substantial direct and indirect costs [9,10]).
Compared with adult-onset schizophrenia, early-onset schizophrenia may be a more severe disorder, negatively influencing social, cognitive and psychological development [6]. While antipsychotic medications play an integral role in the treatment and management of schizophrenia in children, adolescents and young adults, the nature of adverse effects that can follow first exposure occurs during a vulnerable phase of physical growth and brain development, and at a time when young people may be particularly vulnerable to rapid weight gain [11] and disturbances to the cardiometabolic system [12,13], bone growth [14] and sexual development [15]. Such health risks raise important public health concerns given the widespread use of these medications [16]. Furthermore, children, adolescents and young adults are more likely than adults to exhibit negative symptoms, and less likely to exhibit systematized delusions and hallucinations [17]. This has implications for the potential efficacy in children, adolescents and young adults of psychological interventions developed for adults with psychosis or schizophrenia. The increased recognition of the limitations associated with antipsychotic medication has stimulated greater interest in psychological interventions in this population [18]. A recent systematic review of interventions for people who do not have established psychosis, found that psychological interventions may have a positive impact if delivered before the onset of psychosis in individuals with attenuated or transient psychotic symptoms [19]. Additionally, demand for psychological therapies in general has also grown. In England, this has culminated in the Department of Health’s Improving Access to Psychological Therapies (IAPT) initiative, which is set to receive further funding to extend to children, adolescent and young adults and to those with major mental health problems, particularly schizophrenia, under the UK coalition government’s mental health strategy [20]. Finally, families may play an even greater role in providing care and support to children, adolescents and young adults with schizophrenia compared to adults. Given the robust evidence for the efficacy of family interventions in adult schizophrenia [21], these interventions may be particularly promising in children, adolescents and young adults.
A previous review of antipsychotic medications for childhood-onset schizophrenia found limited evidence regarding the effectiveness of antipsychotic medication in this population [22], but searches were conducted in 2007 and the review did not include participants over the age of 13 years. The evidence indicates there are few advantages of second-generation antipsychotics over first-generation antipsychotics in treating psychosis [22], suggesting they could be combined in a meta-analysis. Research in this field has advanced rapidly in recent years, and a current review is needed to determine the efficacy and safety of pharmacological, psychological and combination interventions in the treatment of children, adolescents and young adults with psychosis and schizophrenia.
Methods
This systematic review and meta-analysis was conducted as part of a clinical guideline for the management of psychosis and schizophrenia in children, adolescents and young adults [23], following a published protocol (see Appendix A in S1 File).
Eligibility criteria
We included all randomised controlled trials evaluating pharmacological, psychological or combination treatment for children, adolescents and young adults (18 years of age or younger) with a first episode psychosis (FEP) (a first experience of psychotic symptoms [24]) or a subsequent acute episode of psychosis or schizophrenia. Given the evidence from longitudinal neuroimaging studies demonstrating that brain growth continues into the twenties [25], and epidemiological studies which show the incidence of schizophrenia rising in late adolescence and early adulthood [26], we included studies in which the sample consisted of some participants under 18 years and some over 18 years, as long as the sample mean age did not exceed 25 years. We excluded studies of individuals with bipolar disorder only; studies of people who failed to respond to previous antipsychotic medication (i.e. treatment resistant); studies comparing a single treatment without a placebo arm; studies containing less than 10 participants per group; and studies not available in English. In addition, the current systematic review and meta-analysis was conducted as part of a clinical guideline for England and Wales and therefore studies of drugs not licensed in the UK were also excluded.
Types of outcome measures
Primary
We examined symptoms of psychosis (total, positive and negative) and relapse, at post-treatment and follow-up.
Secondary
We also analysed symptoms of depression, symptoms of anxiety, psychosocial functioning, global state, weight, and discontinuation due to side effects or for any reason.
Search Strategy
We searched Embase, MEDLINE, PreMedline, PsycINFO and CENTRAL from inception to July 2013 (see Appendix B in S1 File for the Medline population terms and the full list of search terms used across databases). In addition, we searched the reference lists of included studies, excluded studies, and previous reviews, and contacted study authors and experts.
Assessment of Bias
Studies were assessed independently by two authors (MRS, CEL) using the Cochrane Collaboration Risk of Bias Tool [27]. Disagreements were discussed with a third author (EMW) and resolved by consensus. Each study was rated for risk of bias due to: sequence generation; allocation concealment; blinding of participants, assessors, and providers; selective outcome reporting; and incomplete data. Risk of bias for each domain was rated as high (seriously weakens confidence in the results), low (unlikely to seriously alter the results) or unclear.
Due to the small number of trials, we were unable to assess publication bias formally (e.g. using a trim and fill analysis) [28]. However, previous reviews have demonstrated systematic under-reporting of such interventions in mental health for children, resulting in effects that have been systematically overstated and harms that have been systematically underestimated [29]. Many of these interventions were developed before the introduction of mandatory trial registration [30], rules with which manufacturers often fail to comply [31]. We believe there is a high risk of publication bias because the addition of one or two small unpublished studies could change our view of the relative benefits and harms of these interventions.
Data Management
For continuous outcomes, the magnitude of treatment effects were calculated as a standardised mean difference (SMD), Hedges g [32]. A small effect was considered to be a SMD of between 0.00 and 0.49, a medium effect between 0.50 and 0.79 and a large effect >0.80. We also calculated the weighted mean difference (WMD) for weight and time to relapse when studies reported the same measure. Sensitivity analysis was conducted for continuous outcomes when endpoint and change data were included in the same analysis. For dichotomous outcomes, an overall risk ratio (RR) was calculated. We analysed individually randomised units. When data were extracted in several formats that could not be combined directly in RevMan, we used the generic inverse variance option. All outcomes are reported with 95% confidence intervals (CI). Overall effects were calculated using random-effects. Continuous effects were weighted by the inverse of variance; dichotomous effects were weighted using the Mantel-Haenszel method [33,34].
Missing data were noted for each outcome. When dropout was not reported, we contacted the authors. For both primary and secondary outcomes reporting data for completers as well as controlling for dropout (for example, imputed using regression methods), we used the latter.
Subgroup analyses were conducted for different doses of antipsychotic medication, where more than one dose was compared with placebo. We used the lower and upper dose ranges identified by the Prescribing Observatory for Mental Health, United Kingdom Topic 10 benchmarking exercise of antipsychotic prescribing in children and young people in practice [35], to categorise doses administered in the included trials as either ‘lower’ or ‘higher’ doses of medication. Therefore, ‘higher’ doses are those exceeding the maximum dose stated in the manufacturers’ summary of product characteristics for that drug, and ‘lower’ doses are those under the minimum dose stated in the manufacturers’ summary of product characteristics for that drug. Because children, adolescents and young adults previously unexposed to antipsychotics may be particularly vulnerable to weight gain associated with antipsychotic use [36], we also conducted subgroup analyses for FEP and subsequent acute episode groups. FEP and subsequent acute episode groups were defined as reported by the trial authors.
Statistical heterogeneity was assessed by visual inspection of forest plots, by performing the Chi2 test (assessing the p value), and by calculating the I2 statistic [37,38], which describes the percentage of observed heterogeneity that would not be expected by chance. If the p value was less than 0.10 and I2 exceeded 50%, we considered heterogeneity to be substantial. Meta-analyses were conducted using RevMan [39]. Confidence in the results was assessed using the GRADE method [40], which is a structured assessment of the quality of evidence attending to the following factors: (1) risk of bias; (2) inconsistency; (3) indirectness; (4) imprecision; and (5) publication bias.
Results
Trial flow
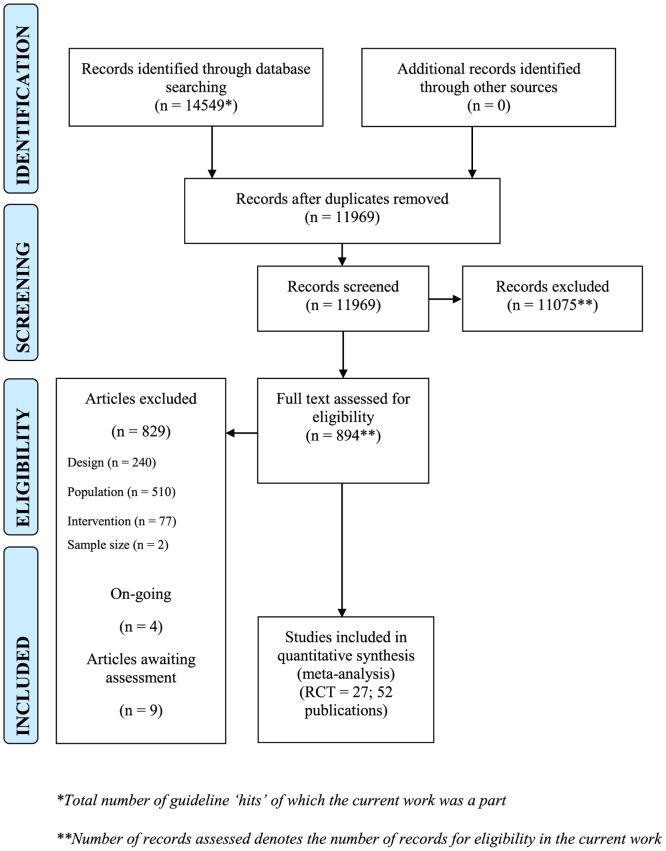
This review was conducted as part of a clinical guideline for the management of psychosis and schizophrenia in children, adolescents and young adults which identified 11969 potentially relevant citations, for which 894 papers were retrieved. Of these, 829 were not relevant and were excluded. The most common reason for exclusion was that the study was conducted in an adult population and included no-one under the age of 18 years. Thirty studies were excluded from this review with reasons (Appendix C in S1 File). Two studies [41,42] were not published in English and were identified via an included systematic review of antipsychotic medication for childhood-onset schizophrenia [22]. Nine trials await assessment (six trials were not published in English, and three trials reported insufficient information in a conference abstract to make an assessment, see Appendix D in S1 File) and four ongoing trials were identified (Appendix E in S1 File). Therefore, 27 randomised controlled trials reported in 52 published papers were included in this systematic review and meta-analysis (Fig. 1).
Fig 1. PRISMA flowchart.
Study characteristics
Pharmacological study characteristics
Nineteen included pharmacological trials assigned 2338 participants. The median sample size was 75 (range 22 to 400) and 1552 (66%) randomised participants were male. Table 1 contains the characteristics of included pharmacological trials. Comparisons included seven placebo controlled trials and 12 head-to-head trials. The median length of treatment was 8 weeks (range 4 to 52) with only two trials reporting long-term follow-up assessments at 104 [43] and 156 weeks [44].
Table 1. Study characteristics for pharmacological interventions.
| Study ID | N | Country | Mean age yrs (SD) | Intervention (mg/day) | PT (FU) weeks |
|---|---|---|---|---|---|
| First episode psychosis | |||||
| ARANGO2009 | 50 | ESP | 15.9 (1.3) | Quetiapine (438.8) vs olanzapine (12.1) | 26 (None) |
| LIEBERMAN2003 | 263 | Multiple | 23.8 (4.8) | Olanzapine (10.2) vs haloperidol(4.82) | 12 (104) |
| MCEVOY2007 | 400 | Multiple | 24.5 (5.8) | Olanzapine(11.7) vs quetiapine(506) vs risperidone (2.4) | 52 (None) |
| ROBINSON2006 | 120 | USA | 23.3 (5.1) | Olanzapine (11.8) vs risperidone (3.9) | 16 (156) |
| SIKICH2008b 1 | 119 | USA | 13.8 (2.4) | Olanzapine (11.4) vs risperidone (2.8) | 52 2 (None) |
| SWADI2010 | 22 | N Z | 16.7 (nr) | Quetiapine (607.0) vs risperidone (2.9) | 6 (None) |
| VANBRUGGEN2003 | 44 | NL | 20.8 (2.9) | Olanzapine (15.6) vs risperidone(4.4) | 6–10 (None) |
| Subsequent acute episode | |||||
| FINDLING2012 | 222 | Multiple | 15.4 (1.3) | Quetiapine (400.0) vs quetiapine (800.0) vs placebo (na) | 26 (None) |
| FINDLING2008A | 302 | Multiple | 15.5 (1.4) | Aripiprazole (10.0) vs aripiprazole (30.0) vs placebo (na) | 6 (None) |
| HAAS2009B | 160 | Multiple | 15.6 (1.3) | Risperidone (1.0–3.0) vs risperidone (4.0–6.0) vs placebo (na) | 6 (None) |
| JENSEN2008 | 30 | USA | 15.2 (2.1) | Olanzapine (14.0) vs quetiapine (611.0) vs risperidone (3.4) | 12 (None) |
| KRYZHANOVSKAYA2009B | 107 | Multiple | 16.7 (1.4) | Olanzapine (11.1) vs placebo (na) | 6 (None) |
| MOZES2006 | 25 | IL | 11.1 (1.6) | Olanzapine (8.2) vs risperidone (1.6) | 12 (None) |
| PAILLERE-MARTINOT1995 | 27 | FR | 20 (4.0) | Amisulpride (50.0–100.0) vs placebo (na) | 6 (None) |
| POOL1976* | 75 3 | USA | 15.5 (nr) | Haloperidol (11.9) vs placebo (na) | 4 (None) |
| SIKICH2004 | 51 | USA | 14.8 (2.8) | Olanzapine (12.3) vs risperidone (4.0) vs haloperidol (5.0) | 8 (None) |
| SINGH2011 4 | 201 | Multiple | 15.4 (1.5) | Paliperidone (1.5) vs paliperidone (3–6) vs placebo (na) | 6 (None) |
| KENNEDY2012/ XIONG2003 | 60 | CN | 13.0 (nr) | Risperidone (0.5–5.0) vs chlorpromazine (50.0–400.0) | 8 (None) |
| KENNEDY2012/ YAO2004 | 60 | CN | 11.0 (nr) | Risperidone (0.25–3.0) vs haloperidol (0.5–12.0) | 6 (None) |
Note.
* Data not reported in sufficient detail to include in analysis
1 Molindone was the third arm of this trial (n = 40), however as it was discontinued by its sole supplier, Endo Pharmaceuticals, on January 13, 2010, only data for risperidone and olanzapine are used in this review
2 The study design consisted of an 8 week ‘acute phase’ and a blind ‘maintenance phase’ up to 52 weeks post randomization. During the maintenance phase participants continued to be administered treatment within their randomised groups and at same dose range.
3 Loxapine was the third arm of this trial (n = 26), however it was not included in this guideline as it was discontinued in the UK in 2003.
4This trial included a fourth arm of paliperidone 6–12mg/day. The 3–6mg/day arm was selected as the ‘higher dose’ antipsychotic medication in accordance with POMH-UK Topic 10 benchmarking exercise and therefore the 6–12mg/day arm was not included in the current work.
N = number randomised; nr = not reported; na = not applicable; mg = milligrams; DU = duration of treatment; PT = post-treatment data collection; FU = follow-up data collection
We conducted meta-analysis for seven pharmacological comparisons. Antipsychotic drugs that were compared with a placebo included quetiapine [45], aripiprazole [46], risperidone [47], paliperidone [48], amisulpride [49], olanzapine [50] and haloperidol [51]. The median of the mean ages was 15.5 years (range 15.4 to 20.0 years). None of these trials were conducted in FEP. These trials were included in a meta-analysis of antipsychotic medications compared to placebo and subgroup analyses were conducted for ‘lower’ and ‘higher’ doses of antipsychotic medication (see ‘Data management‘). Total, positive and negative symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) and global state was measured using the Clinical Global Impressions Scale (CGI). A variety of measures were used to measure depression including the Montgomery-Åsberg Depression Rating Scale (MADRS), the Hamilton Depression Rating Scale (HAM-D) and the PANSS-Depression; and psychosocial functioning including the Children’s Global Assessment Scale (CGAS) and the Global Assessment of Functioning (GAF). Relapse and anxiety were not measured in any trials of antipsychotic medication compared with placebo.
Additionally, head-to-head comparisons included risperidone compared with olanzapine [44,52–56], haloperidol [22,53], quetiapine [55,57,58], and chlorpromazine [22]; and olanzapine compared with quetiapine [55,57,59] and haloperidol [43,53]. The median of the mean ages was 15.5 years (range 11.0 to 24.5 years). Subgroup analyses were conducted for FEP and subsequent acute episode groups. Total, positive and negative symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) and the Brief Psychiatric Rating Scale (BPRS). Global state and psychosocial functioning were measured using the CGI and the CAS respectively. Relapse and anxiety were not measured in any of the included head-to-head trials.
Psychological study characteristics
Eight included psychological trials assigned 729 participants with a median sample size of 64 (range 30 to 309). The median of the mean ages was 22.3 years (range 15.0 to 24.0 years), with no samples of exclusively under 18 year olds identified. Four hundred and fifty seven (63%) randomised participants were male. The median length of treatment across trials was 22 weeks (range 10 to 65) and all trials except one [60] conducted follow-up assessments, with a median follow-up of 78 weeks (range 26 to 260). All participants were either currently experiencing FEP or a subsequent acute episode of psychosis or schizophrenia, apart from two trials that were specifically designed to test a relapse prevention strategy following remission from first psychotic episode [61,62]. Table 2 contains the characteristics of included psychological trials.
Table 2. Study characteristics for psychological interventions.
| Study ID | N | Country | Mean age yrs (SD) | Intervention (mg/day) | PT (FU) weeks |
|---|---|---|---|---|---|
| APTER1978* | 30 | NR | nr | Individual movement therapy vs group movement therapy vs non-specific dance therapy | 12 (None) |
| JACKSON2008 | 62 | AU | 22.3 (3.6) | Individual CBT + EPPIC TAU vs befriending + EPPIC TAU | 14 (52) |
| JACKSON2009 | 66 | AU | 23.3 (4.6) | Individual CBT vs TAU | 26 (52) |
| HADDOCK2006* | 309 | GBR | nr | Individual CBT+TAU (UK) vs supportive counselling+TAU (UK) vs TAU | 18 (78) |
| MAK2007* | 48 | CN | 24 (4) | Individual CBT vs waitlist | CBT:36 (60); waitlist:26 (84) |
| POWER2003 | 56 | AU | nr (range 15–29) | Individual CBT + EPPIC TAU in acutely suicidal patients vs EPPIC TAU in acutely suicidal patients | 10 (26) |
| GLEESON2009 | 82 | AU | 20.1 (3.1) | Family CBT + individual CBT vs EPPIC TAU | 28 (130) |
| LINSZEN1996 | 76 | NL | 20.6 (2.5) | Family CBT vs individual CBT | 65 (260) |
Note.
* Data not reported in sufficient detail to include in analysis
CBT = cognitive behavioural therapy; EPPIC = Early Psychosis Prevention and Intervention Centre; TAU = treatment as usual; UK = United Kingdom; N = number randomised; nr = not reported; na = not applicable; PT = post-treatment data collection; FU = follow-up data collection
We conducted meta-analysis for five psychological comparisons. Psychological interventions included arts therapy, CBT and family interventions. Comparisons included individual body movement therapy compared with group body movement therapy and a non-specific dance therapy control [60]; CBT compared with waitlist [63]; CBT compared with treatment as usual (TAU) [64,65]; CBT compared with supportive counselling [64]; CBT compared with befriending [66]; CBT for acutely suicidal patients compared with TAU [67]; family CBT compared with individual CBT [61]; and family plus individual CBT compared with TAU [62]. Three of eight included trials were conducted in a specialist Early Psychosis Prevention and Intervention Centre (EPPIC) in Australia, which offers a highly comprehensive service to people aged 15 to 25 years with emerging psychotic disorders [62,66,67]. All participants in these studies received TAU by the EPPIC centre. Appendix F in S1 File provides a detailed description of the psychological interventions used in the included trials. Total, positive and negative symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) and the Brief Psychiatric Rating Scale (BPRS). The Scale for the Assessment of Negative Symptoms (SANS) was also used to measure negative symptoms. Relapse was measured using the BPRS. Depression was measured using the Beck Depression Inventory (BDI), Montgomery-Åsberg Depression Rating Scale (MADRS), and the Calgary Depression Scale for Schizophrenia (CDSS). Global state was measured using the Clinical Global Impressions scale (CGI). Psychosocial functioning and anxiety were not measured in any of the psychological trials.
Risk of bias
We rated risk of bias for each trial (Appendix G in S1 File) using the Cochrane risk of bias tool [27].
Pharmacological trials
Nine out of 19 included pharmacological trials employed adequate methods of sequence generation, however the risk of bias due to inadequate allocation concealment was unclear in 17 trials. Lack of blinding of assessors created a high risk of bias for some outcomes in two studies and for 12 studies this was unclear. Five studies were at high risk of bias because participants or staff were not blind and for 11 studies this was unclear. There was a high risk of bias due to incomplete outcome data for 15 included trials and only two trials were clearly free of attrition bias. Only nine studies were clearly free of selective outcome reporting; seven trials did not report all outcomes and for two trials data were not reported in sufficient detail to be included in meta-analysis. It was unclear whether three trials reported all outcomes.
Psychological trials
Four out of eight included psychological trials were considered to have employed adequate methods of sequence generation, however the risk of bias owing to poor allocation concealment was unclear for all trials. There was a high risk of bias due to incomplete outcome data for four trials, and for one trial this was unclear. Four trials were at a high risk of selective outcome reporting, and for three of these trials, no data could be extracted for any outcomes.
Quantitative data synthesis
We analysed psychotic symptoms, relapse, global state, psychosocial functioning, depression, weight and discontinuation of treatment. Summary of effects for all comparisons and all outcomes can be found in Appendix H in S1 File.
Pharmacological quantitative data synthesis
A meta-analysis of antipsychotic medications compared to placebo was conducted because of the small number of trials and participants, and therefore lack of statistical power, for any single antipsychotic compared to placebo. Six of seven placebo-controlled trials reported data for at least one outcome in sufficient detail to be included in this meta-analysis [45–50]. Subgroup analyses were conducted for ‘lower’ and ‘higher’ doses of antipsychotic medication.
Eleven of twelve head-to-head trials reported data for at least one outcome to be included in meta-analysis [22,43,52–59]. We analysed nine trials comparing two antipsychotics and three trials comparing three antipsychotics. Subgroup analyses were conducted for FEP and subsequent acute episodes of schizophrenia.
Efficacy of antipsychotic medication versus placebo
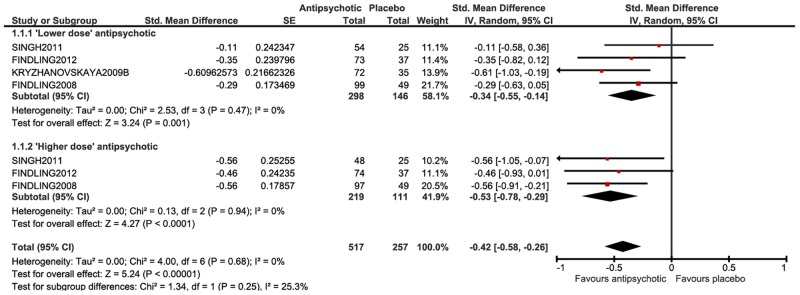
At post-treatment, low quality evidence suggested small effects for antipsychotic medication on total symptoms (SMD = -0.42, 95% CI-0.58 to-0.26) (Fig. 2), positive symptoms (SMD = -0.42, 95% CI-0.56 to-0.28), negative symptoms (SMD = -0.32, 95% CI-0.46 to-0.18), depression (SMD = -0.24, 95% CI-0.45 to-0.03), psychosocial functioning (SMD = -0.37, 95% CI-0.52 to-0.23) and global state severity (SMD = -0.41, 95% CI-0.58 to-0.25), and a large effect for global state improvement (RR = 1.89, CI 1.26 to 2.83). These effects remained small but statistically significant in subgroup analyses, with marginal decreases and increases in the size of the effects for ‘lower dose’ and ‘higher dose’ groups respectively. However, the effect of treatment was relatively small compared with change over time, even in the absence of intervention. In one trial, PANSS scores fell approximately 20 points in the placebo group [45], constituting a minimum clinically important difference of greater than 15 PANSS points as estimated by Hermes et al. [68]. The difference between post-treatment mean scores between groups was approximately eight points, and in another trial, the difference between placebo and antipsychotic treated groups was approximately nine points [48]. These differences are small and do not meet this threshold.
Fig 2. Antipsychotic medication compared with placebo at post-treatment—total symptoms.
Side effects of antipsychotic medication versus placebo
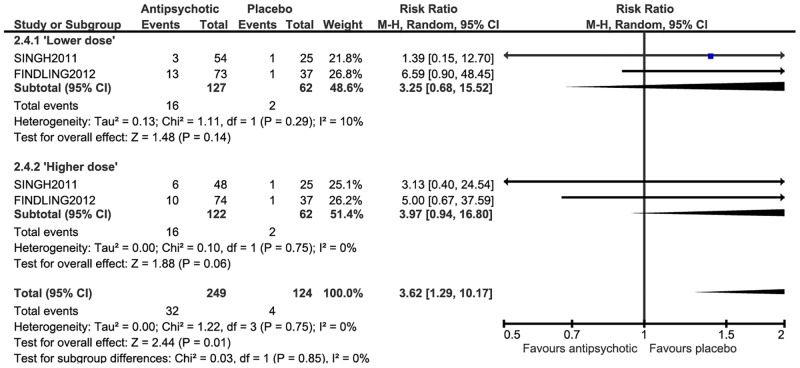
There was very low quality evidence for a medium effect on weight (kg), with antipsychotic treated participants gaining significantly more weight than the placebo group at post-treatment (SMD = 0.63, 95% CI 0.32 to 0.93) (WMD = 1.61, 95% CI 0.61 to 2.60), however there was significant heterogeneity across studies (p = 0.0007, I2 = 68% and p = 0.00001, I2 = 84% respectively). A large effect was observed for the number of participants gaining >7% of their baseline body weight (RR = 3.62, 95% CI 1.29 to 10.17) (Fig. 3), however, there was no significant effect for either of the ‘lower’ and ‘higher’ dose subgroups (‘lower dose’: RR = 3.25, 95% CI 0.68 to 15.52; ‘higher dose’: RR = 3.97, 95% CI 0.94 to 16.80). A large effect favouring placebo was found for leaving the study early due to side effects at post-treatment (RR = 2.44, 95% CI, 1.12 to 5.31), however, there was no significant effect for either of the ‘lower’ and ‘higher’ dose subgroups (‘lower dose’: RR = 2.53, 95% CI 0.87 to 7.34; ‘higher dose’: RR = 2.33, 95% CI 0.74 to 7.30).
Fig 3. Antipsychotic medication compared with placebo at post-treatment—weight.
Efficacy of antipsychotic medication in head-to-head trials
In very low quality evidence there were no significant effects between antipsychotics in head-to-head trials for any of our included measures of efficacy, except a small effect for olanzapine on negative symptoms compared with haloperidol in FEP participants (SMD = -0.25, 95% CI-0.50 to-0.00), and a small effect for risperidone compared with quetiapine on positive symptoms in FEP participants (SMD = -0.43, 95% CI-0.82 to-0.03). Relapse and anxiety were not measured in any of the included head-to-head trials. Psychosocial functioning was not measured in trials of olanzapine compared with haloperidol, and depression was not measured in trials of risperidone compared with haloperidol.
Side effects of antipsychotic medication in head-to-head trials
For weight gain in FEP participants, very low quality evidence indicated a large differential effect favouring quetiapine to olanzapine (RR = 1.86, 95% CI 1.33 to 2.61), and moderate differential effects, favouring risperidone to olanzapine (RR = 0.68, 95% CI 0.47 to 0.98), and on haloperidol to olanzapine (SMD = 0.70, 95% CI 0.45 to 0.95) (WMD = 6.08, 95% CI 3.97 to 8.20). A large effect was observed in the FEP subgroup, favouring olanzapine to haloperidol, for leaving the study early due to side effects at post-treatment (RR = 0.37, 95% CI 0.16 to 0.85). In very low quality evidence there were no further significant effects on weight or leaving the study early due to side effects, between antipsychotics in head-to-head trials.
Psychological quantitative data synthesis
Five of eight included psychological trials reported data for at least one outcome in sufficient detail to be included in an analysis [61,62,65–67], however the comparators used in these trials were considered to be too different to combine in a meta-analysis and so single pairwise comparisons were conducted.
Family and individual CBT compared to TAU at the Early Psychosis Prevention and Intervention Centre
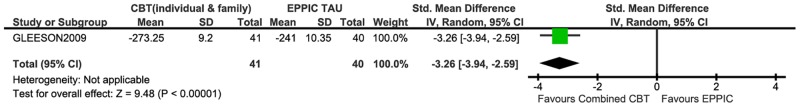
At 33 weeks post-treatment, there was low quality evidence that time to relapse was significantly extended by 32.25 days in family plus individual CBT compared to TAU at EPPIC (SMD = -3.26, 95% CI-3.94 to-2.59) (WMD = 32.25, 95% CI-36.52 to-27.98) (Fig. 4) [62]. Time to relapse was not reported at 130 weeks follow-up. However, the number of participants relapsing was not significantly different between groups at 33 weeks post-treatment (RR = 0.24, 95% CI 0.06 to 1.08) or at 130 weeks follow-up (RR = 0.98, 95% CI 0.31 to 3.11). No differential effects between groups were found for total or positive symptoms at either time point, or for negative symptoms at 33 weeks post-treatment, however a medium effect favouring TAU at EPPIC was found for negative symptoms at 130 weeks follow-up (SMD = 0.60, 95% CI 0.15 to 1.05). No significant difference was found between groups on psychosocial functioning at 33 weeks post-treatment, however a small effect for psychosocial functioning was observed at 130 weeks follow-up (SMD = -0.45, 95% CI-0.89 to-0.01). No differential effects between groups were found for depression or leaving the study early for any reason. Anxiety and global state were not measured in this trial.
Fig 4. Family plus individual CBT compared with TAU at EPPIC—Time to relapse at post-treatment.
CBT compared with TAU in the UK
No significant differences were found for depression between CBT and TAU in the UK at post treatment (SMD = -0.29, 95%CI-0.87 to 0.30) or follow-up (SMD = -0.05, 95% CI-0.63 to 0.52); or leaving the study early for any reason at post-treatment (RR = 1.94, 95% CI 0.85 to 4.43) or follow-up (RR = 1.77, 95% CI 0.89 to 3.52) [65]. Symptoms, relapse, global state, psychosocial functioning and anxiety were not measured in this trial.
CBT plus TAU compared with befriending at EPPIC
No significant differences were found between CBT plus TAU at EPPIC and befriending at post treatment for positive symptoms (SMD = -0.05, 95% CI-0.55 to 0.45), negative symptoms (SMD = -0.44, 95% CI-.095 to 0.06), and psychosocial functioning (SMD = -0.40, 95% CI-0.90 to 0.11) [64]. These effects remained non-significant at follow-up: SMD = -0.08, 95% CI-0.58 to 0.42 (positive symptoms), SMD = -0.37, 95% CI-0.87 to 0.13 (negative symptoms) and SMD = -0.08, 95% CI-0.58 to 0.41 (psychosocial functioning). No significant differences were found between CBT plus TAU at EPPIC and befriending on leaving the study early for any reason (RR = 0.57, 95% CI 0.19 to 1.76). Relapse, depression, global state and anxiety were not measured by this trial.
CBT compared with TAU for acutely suicidal patients at EPPIC
In a trial conducted in acutely suicidal participants, no significant differences were found between CBT and TAU at EPPIC for leaving the study early at post-treatment (RR = 2.02, 95% CI 0.72 to 5.66) [67]. The primary outcome in this trial was suicidality; however symptom and global state data were also collected, but not reported in sufficient detail to be included in an analysis. Relapse, depression, anxiety and psychosocial functioning were not measured in this trial.
Family CBT compared with individual CBT
No significant differences were found between family CBT and individual CBT at post-treatment for rates of relapse (RR = 0.95, 95% CI 0.34 to 2.68), and symptom data was collected but not reported in sufficient detail to be used in an analysis [61]. Global state, psychosocial functioning, depression, anxiety and numbers of participants leaving the study early for any reason were not measured by this trial.
Discussion
Findings
This is the first systematic review and meta-analysis of pharmacological and psychological treatments for children, adolescents and young adults with psychosis and schizophrenia. The data set on antipsychotics includes 19 trials, with 2338 participants with a median mean age of 15.5 years (range 11.0 to 24.5 years). Low quality evidence suggests small effects for antipsychotic medication on positive and negative symptoms, depression and psychosocial functioning and a large effect on global state, but also a medium effect on weight gain which increased quickly, especially over the first six weeks of treatment, with a median of the mean change in weight across antipsychotic treated participants of 1.25kg (range 0 to 4.3kg); and greater discontinuation due to side effects where this was reported. We note that in these trials the placebo groups improved substantially, with antipsychotics adding relatively small additional benefit. Head-to-head trials of antipsychotics showed medium to large differential effects on weight gain with olanzapine. However, most head-to-head trials of antipsychotics were underpowered and the evidence was very low quality, making any comparisons between individual antipsychotics unreliable.
The data set for psychological treatments amounted to eight trials, including 729 participants. Disappointingly, there were no psychological treatment trials for children or young people under 18 years of age reporting data in a sufficient format to be included in an analysis; but for young people under 25 years old, we found some low quality evidence that combining family interventions with individual CBT had a strong, statistically significant effect on extending time to relapse, although there were no differences in relapse rate. No significant beneficial effects were found for psychological interventions for psychotic symptoms or for depression. It was not possible to meta-analyse data from any of the psychological treatment trials, largely because of variation in controls, and we found no other statistically significant effects.
It is important to note, however, that in several psychological treatment trials, the control conditions included active interventions which mirror the effect of the psychological interventions in practice, where they are always administered in addition to treatment as usual. For example, three studies [62,66,67] were conducted in EPPIC, a very intensive and comprehensive treatment centre which includes an inpatient unit, an outpatient case management system, family work, accommodation, prolonged recovery programmes, tailored group programmes and clear, low dose medication protocols.
Limitations
The two most important limitations we identified for this review were the age range of participants in studies included and the quality of the evidence. The dataset for children, adolescents and young adults under 18 years old is very much smaller than in adults (k = 10; n = 1217), with no studies in under 18 year olds identified for psychological interventions. Furthermore, most studies used outcomes measures that have been validated in adults. In addition, the quality of the evidence across the whole dataset was poor.
The quality of the evidence for antipsychotics was low or very low. Most trials were at high or unclear risk of selection bias and some trials were rated as having high or unclear performance and detection bias. However, we considered it unlikely that blinding of participants or providers would introduce any important bias, and we did not downgrade for this reason. Only two trials were rated as having low risk of attrition bias and less than half of the trials were completely free of selective outcome reporting, with many studies not reporting all outcomes. Two trials could not be included in any analyses because data reporting was inadequate. Another important reason for downgrading the quality of the evidence is the high risk of selective publication bias. The small number of trials meant it was not possible to assess publication bias formally (e.g. using a trim and fill analysis [28]).
Another limitation is the range of available outcomes, in particular relating to side effects. Weight was the most consistently reported outcome across trials, while other potentially relevant outcomes of antipsychotics, such as extrapyramidal side effects and other metabolic changes, are reported far less frequently. Poor reporting of side effects also raises the possibility of selective publication of outcomes and of whole studies, a practice that is common and leads to overestimating the benefits and underestimating the harm of drug treatments [29]. Unfortunately, it is not possible to be sure if all negative trials have been published.
The quality of the evidence for the psychological treatment trials was also low or very low. Several studies were at high risk of selection bias, attrition bias and selective outcome reporting. There was also a high risk of performance bias, but a low risk of detection bias across trials. As with pharmacological trials, we did not downgrade outcomes due to the risk of performance bias. Reassuringly, we found no evidence of psychological treatment trials being registered but not published.
Conclusion
Compared to the substantially larger data-set for both pharmacological and psychological treatment trials for adults with psychosis and schizophrenia [69], this review suggests that while the efficacy of antipsychotics is similar in children, adolescents and young adults, side effects, in particular weight gain, are greater. The discontinuation rates due to side effects also suggests that these drugs are not well tolerated and that the balance of risks and benefits for antipsychotics may be less favourable in children, adolescents and young adults. This is in line with previous reviews of antipsychotics in children that conclude the benefits of antipsychotic medication are offset by the risks of serious side effects [22,70] and a cohort study demonstrating substantial weight gain following 12 weeks of treatment with antipsychotics [36]. The improvements observed for placebo treated groups in the current work points to a possible role of support and the passage of time, in recovery. A recent study shows that the difference between drugs and placebo becomes smaller as the length of the studies increase [71] and it remains to be tested whether the endpoint level of recovery remains different after a longer follow-up. The weight gain we have observed here, suggests that long, comprehensive investigations are needed for a complete and balanced cost-benefit analysis of long-term use of antipsychotic drugs, beginning in adolescence. In addition, the value of psychological treatments remains uncertain and largely untested in the young. Trials of psychological treatments in adults, strongly suggest that family interventions clearly and reliably reduce relapse rates and CBT reduces symptoms and length of hospitalisation [69]; with similar effects for people with first episode psychosis across all ages, both within and without early interventions services [21]. This could lead us to infer that these treatments are likely to be effective in younger age groups. However, this cannot be assumed for reasons such as cognitive immaturity. The lack of effect of psychological interventions on psychotic symptoms in our analyses suggests that similar benefits cannot be assumed when using interventions developed for adults with children, adolescents and young adults.
Psychosis and schizophrenia in children, adolescents and young adults are very serious and debilitating illnesses, which in clinical practice usually leads to the use of antipsychotics. However, in the absence of high quality evidence for the effectiveness of antipsychotic medication in children, adolescents and young adults, their routine use in the treatment of psychosis and schizophrenia should be undertaken cautiously. Furthermore, given the growing evidence that antipsychotics are associated with often severe metabolic, neurological and other side effects associated with significant premature mortality [72], these drugs should only be used under specialist psychiatric supervision and with careful monitoring [23]. Moreover, the complete absence of research on the effectiveness of psychological interventions in individuals under 18 years old needs to be urgently addressed. This is practically important because young people should not be denied potentially effective interventions because of an absence of research evidence. Although treatments developed for adults cannot be assumed to have the same benefits in children, adolescents and young adults, their benefits in first episode psychosis [21] suggests that family interventions and CBT would be good candidates for further research in younger age groups.
This review, we hope, can be used as a platform from which we can develop new clinical and research strategies, investigating pragmatic questions such as the benefits of combining family and individual psychological interventions, how psychological interventions should be adapted for children, adolescents and young adults, the benefits of psychological interventions alone and with limited and targeted use of antipsychotics, the predictors of response for different treatment approaches, and the most effective timing for interventions during the course of illness.
Supporting Information
(DOCX)
Acknowledgments
The full review protocol is available from the authors. The authors would like to acknowledge the support of Sarah Stockton of the National Collaborating Centre for Mental Health and Hannah Jackson, who worked at the National Collaborating Centre for Mental Health during guideline development.
We adhere to PLOS ONE policies on sharing data and materials.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Collaborating Centre for Mental Health and conducted as part of a guideline about psychosis in children, adolescents and young adults. The full review protocol is available from the authors. TK receives £1.4 million per year for the National Collaborating Centre for Mental Health from the National Institute for Health and Clinical Excellence to develop guidelines for the treatment of mental health problems (https://www.nice.org.uk/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mattai AK, Hill JL, Lenroot RK (2010) Treatment of early-onset schizophrenia. Curr Opin Psychiatry 23: 304–310. 10.1097/YCO.0b013e32833b027e [DOI] [PubMed] [Google Scholar]
- 2. Gillberg C (1984) Infantile autism and other childhood psychoses in a Swedish urban region. Epidemiological aspects. J Child Psychol Psychiatry 25: 35–43. [DOI] [PubMed] [Google Scholar]
- 3. Burd L, Kerbeshian J (1987) A North Dakota prevalence study of schizophrenia presenting in childhood. J Am Acad Child Adolesc Psychiatry 26: 347–350. [DOI] [PubMed] [Google Scholar]
- 4. Hellgren L, Gilberg C, Enerskog I (1987) Antecedents of adolescent psychoses: a population based study of school health problems in children who develop psychosis in adolescence. J Am Acad Child Adolesc Psychiatry 26: 351–355. [DOI] [PubMed] [Google Scholar]
- 5. Gillberg C (2001) Epidemiology of early onset schizophrenia. Cambridge: Cambridge University Press. [Google Scholar]
- 6. Hollis C (2000) Adult outcomes of child- and adolescent-onset schizophrenia: diagnostic stability and predictive validity. Am J Psychiatry 157: 1652–1659. [DOI] [PubMed] [Google Scholar]
- 7. Parks J, Svendsen D, Singer P, Foti ME (2006) Morbidity and Mortality in People with Serious Mental Illness, 13th Technical Report. Alexandria, Virginia: National Association of State Mental Health Program Directors. [Google Scholar]
- 8. Brown S, Kim M, Mitchell C, Inskip H (2010) Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry 196: 116–121. 10.1192/bjp.bp.109.067512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu EQ, Birnbaum HG, Shi L, Ball DE, Kessler RC, et al. (2005) The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 66: 1122–1129. [DOI] [PubMed] [Google Scholar]
- 10. Mangalore R, Knapp M (2007) Cost of schizophrenia in England . J Ment Health Policy Econ 10: 23–41. [PubMed] [Google Scholar]
- 11. Alvarez-Jiménez M, González-Blanch C, Crespo-Facorro B, Hetrick S, Rodríguez-Sánchez JM, et al. (2008) Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs 22: 547–562. [DOI] [PubMed] [Google Scholar]
- 12. Correll CU (2010) Symptomatic presentation and initial treatment for schizophrenia in children and adolescents. J Clin Psychiatry 71: e29 10.4088/JCP.9101tx4c [DOI] [PubMed] [Google Scholar]
- 13. Foley DL, Morley KI (2011) Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 68: 609–616. 10.1001/archgenpsychiatry.2011.2 [DOI] [PubMed] [Google Scholar]
- 14. Calarge CA, Ivins SD, Motyle KJ, Shibli-Rahhal AA, Bliziotes MM, et al. (2013) Possible mechanisms for the skeletal effects of antipsychotics in children and adolescents. Ther Adv in Psychopharmacol. 3(5): 278–293. 10.1177/2045125313487548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meunch J, Hamer A (2010) Adverse effects of anitpsychotic medication. American Family Physician 81(5): 617–622 [PubMed] [Google Scholar]
- 16. Sikich L (2008) Efficacy of atypical antipsychotics in early-onset schizophrenia and other psychotic disorders. J Clin Psychiatry 69 Suppl 4: 21–25. [PubMed] [Google Scholar]
- 17. Hollis C, Rapoport J (2011) Child and adolescent schizophrenia In: Weinberger D, Harrison P, editors. Schizophrenia 3rd ed. London: Wiley; pp. 22–46. [Google Scholar]
- 18. Perkins DO, Gu H, Weiden PJ, McEvoy JP, Hamer RM, et al. (2008) Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry 69: 106–113. 18312044 [Google Scholar]
- 19. Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T (2013) Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ 346: f185 10.1136/bmj.f185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NHS (30 July 2013). Children and Young People’s Programme: Key Facts Briefing Children and Young People’s IAPT Programme. Available: http://www.iapt.nhs.uk/silo/files/cyp-iapt-key-facts-july-2013-.pdf Accessed 11 August 2014.
- 21. Bird V, Premkumar P, Kendall T, Whittington C, Mitchell J, et al. (2010) Early intervention services, cognitive-behavioural therapy and family intervention in early psychosis: systematic review. Br J Psychiatry 197: 350–356. 10.1192/bjp.bp.109.074526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kennedy E, Kumar A, Datta SS (2012). Antipsychotic medication for childhood-onset schizophrenia. Cochrane Database of Systematic Reviews 2007, Issue 3 10.1002/14651858.CD004027.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. NICE (2013) Psychosis and schizophrenia in children and young people: Recognition and Management. London: National Institute of Health and Care Excellence; 10.1136/bmj.f150 [DOI] [PubMed] [Google Scholar]
- 24. Martens L, Baker S (2009) Promoting recovery from first episode psychosis: A guide for families. Canada: Centre for addiction and mental health. [Google Scholar]
- 25. Johnson SB, Blum RW, Giedd JN (2009) Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health 45: 216–221. 10.1016/j.jadohealth.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C (2011) Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull 37: 504–513. 10.1093/schbul/sbr030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins J, Green S (2008) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available: http://handbook.cochrane.org/ Accessed 11 August 2014.
- 28. Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR (2000) Empirical assessment of effect of publication bias on meta-analyses. BMJ 320: 1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, et al. (2004) Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 363: 1341–1345. [DOI] [PubMed] [Google Scholar]
- 30. Abbasi K (2004) Compulsory registration of clinical trials. BMJ 329: 637–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zarin DA, Ide NC, Tse T, Harlan WR, West JC, et al. (2007) Issues in the registration of clinical trials. JAMA 297: 2112–2120. [DOI] [PubMed] [Google Scholar]
- 32. Hedges LV (1994) Statistical considerations In: Cooper HM, and Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage; pp. 29–39. [Google Scholar]
- 33. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 34. Greenland S, Robins J (1985) Estimation of a common effect parameter from sparse follow-up data. Biometrics 41: 55–68. [PubMed] [Google Scholar]
- 35. POMH- UK (2012) POMH-UK Topic 10b re-audit report: Prescribing antipsychotics for children and adolescents. London: The Royal College of Psychiatrists. [Google Scholar]
- 36. Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, et al. (2009) Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302: 1765–1773. 10.1001/jama.2009.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 38. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- 40. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, et al. (2004) Grading quality of evidence and strength of recommendations. BMJ 328: 1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yao H (2003) A study of risperidone in the treatment of child schizophrenia. Journal of Clinical Psychological Medicine 7468: 801. [Google Scholar]
- 42. Xiong Y (2004) Comparison study of childhood schizophrenia treated with risperidone and chlorpromazine. Guizhou Medical Journal 28: 697–698. [Google Scholar]
- 43. Lieberman JA, Tollefson G, Tohen M, Green AI, Gur RE, et al. (2003) Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry 160: 1396–1404. [DOI] [PubMed] [Google Scholar]
- 44. Robinson DG, Woerner MG, Napolitano B, Patel RC, Sevy SM, et al. (2006) Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry 163: 2096–2102. [DOI] [PubMed] [Google Scholar]
- 45. Findling RL, McKenna K, Earley WR, Stankowski J, Pathak S (2012) Efficacy and safety of quetiapine in adolescents with schizophrenia investigated in a 6-week, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol 22: 327–342. 10.1089/cap.2011.0092 [DOI] [PubMed] [Google Scholar]
- 46. Findling RL, Robb A, Nyilas M, Forbes RA, Jin N, et al. (2008) A multiple-center, randomized, double-blind, placebo-controlled study or oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry 165: 1432–1441. 10.1176/appi.ajp.2008.07061035 [DOI] [PubMed] [Google Scholar]
- 47. Haas M, Unis AS, Armenteros J, Copenhaver MD, Quiroz JA, et al. (2009) A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol 19: 611–621. 10.1089/cap.2008.0144 [DOI] [PubMed] [Google Scholar]
- 48. Singh J, Robb A, Vijapurkar U, Nuamah I, Hough D (2011) A randomized, double-blind study of paliperidone extended-release in treatment of acute schizophrenia in adolescents. Biol Psychiatry 70: 1179–1187. 10.1016/j.biopsych.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 49. Paillère-Martinot ML, Lecrubier Y, Martinot JL, Aubin F (1995) Improvement of some schizophrenic deficit symptoms with low dose amisulpride. Am J Psychiatry 152: 130–133. [DOI] [PubMed] [Google Scholar]
- 50. Kryzhanovskaya L, Schulz S, McDouble C, Frazier J, Dittman R, et al. (2009) Olanzapine versus placebo in adolescents with schizophrenia: a 6-week, randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 48: 60–70. 10.1097/CHI.0b013e3181900404 [DOI] [PubMed] [Google Scholar]
- 51. Pool D, Bloom W, Mielke DH, Roniger JJ Jr, Gallant DM (1976) A controlled evaluation of loxitane in seventy-five adolescent schizophrenic patients. Curr Ther Res Clin Exp 19: 99–104. [PubMed] [Google Scholar]
- 52. van Bruggen J, Tijssen J, Dingemans P, Gersons B, Linszen D (2003) Symptom response and side-effects of olanzapine and risperidone in young adults with recent onset schizophrenia. Int Clin Psychopharmacol 18: 341–346. [DOI] [PubMed] [Google Scholar]
- 53. Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA (2004) A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology 29: 133–145. [DOI] [PubMed] [Google Scholar]
- 54. Mozes T, Ebert T, Michal SE, Spivak B, Weizman A (2006) An open-label randomized comparison of olanzapine versus risperidone in the treatment of childhood-onset schizophrenia. J Child Adolesc Psychopharmacol 16: 393–403. [DOI] [PubMed] [Google Scholar]
- 55. McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Gu H, et al. (2007) Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 164: 1050–1060. [DOI] [PubMed] [Google Scholar]
- 56. Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, et al. (2008) Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry 165: 1420–1431. 10.1176/appi.ajp.2008.08050756 [DOI] [PubMed] [Google Scholar]
- 57. Jensen JB, Kumra S, Leitten W, Oberstar JV, Anjum A, et al. (2008) A comparative pilot study of second-generation antipsychotics in children and adolescents with schizophrenia-spectrum disorders. J Child Adolesc Psychopharmacol 18: 317–326. 10.1089/cap.2007.0123 [DOI] [PubMed] [Google Scholar]
- 58. Swadi HS, Craig BJ, Pirwani NZ, Black VC, Buchan JC, et al. (2010) A trial of quetiapine compared with risperidone in the treatment of first onset psychosis among 15- to 18-year-old adolescents. Int Clin Psychopharmacol 25: 1–6. 10.1097/YIC.0b013e3283320511 [DOI] [PubMed] [Google Scholar]
- 59. Arango C, Robles O, Parellada M, Fraguas D, Ruiz-Sancho A, et al. (2009) Olanzapine compared to quetiapine in adolescents with a first psychotic episode. Eur Child Adolesc Psychiatry 18: 418–428. 10.1007/s00787-009-0749-5 [DOI] [PubMed] [Google Scholar]
- 60. Apter A, Sharir I, Tyano S, Wijsenbeek H (1978) Movement therapy with psychotic adolescents. Br J Med Psychol 51: 155–159. [DOI] [PubMed] [Google Scholar]
- 61. Linszen D, Dingemans P, Van der Does JW, Nugter A, Scholte P, et al. (1996) Treatment, expressed emotion and relapse in recent onset schizophrenic disorders. Psychol Med 26: 333–342. [DOI] [PubMed] [Google Scholar]
- 62. Gleeson JF, Cotton SM, Alvarez-Jiménez M, Wade D, Gee D, et al. (2009) A randomized controlled trial of relapse prevention therapy for first-episode psychosis patients. J Clin Psychiatry 70: 477–486. [DOI] [PubMed] [Google Scholar]
- 63. Mak GKL, Li FWS, Lee PWH (2007) A pilot study on psychological interventions with Chinese young adults with schizophrenia. Hong Kong J Psychiatry 17: 17–23. [Google Scholar]
- 64. Haddock G, Lewis S, Bentall R, Dunn G, Drake R, et al. (2006) Influence of age on outcome of psychological treatments in first-episode psychosis. Br J Psychiatry 188: 250–254. [DOI] [PubMed] [Google Scholar]
- 65. Jackson C, Trower P, Reid I, Smith J, Hall M, et al. (2009) Improving psychological adjustment following a first episode of psychosis: a randomised controlled trial of cognitive therapy to reduce post psychotic trauma symptoms. Behav Res Ther 47: 454–462. 10.1016/j.brat.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 66. Jackson HJ, McGorry PD, Killackey E, Bendall S, Allott K, et al. (2008) Acute-phase and 1-year follow-up results of a randomized controlled trial of CBT versus Befriending for first-episode psychosis: the ACE project. Psychol Med 38: 725–735. [DOI] [PubMed] [Google Scholar]
- 67. Power PJ, Bell RJ, Mills R, Herrman-Doig T, Davern M, et al. (2003) Suicide prevention in first episode psychosis: the development of a randomised controlled trial of cognitive therapy for acutely suicidal patients with early psychosis. Aust N Z J Psychiatry 37: 414–420. [DOI] [PubMed] [Google Scholar]
- 68. Hermes ED, Sokoloff D, Stroup TS, Rosenheck RA (2012) Minimum clinically important difference in the Positive and Negative Syndrome Scale with data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). J Clin Psychiatry 73: 526–532. 10.4088/JCP.11m07162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. NICE (2009) Core Interventions in the Treatment and Management of Schizophrenia in Adults in Primary and Secondary Care. London: National Institute of Health and Care Excellence. [Google Scholar]
- 70. Fraguas D, Correll CU, Merchán-Naranjo J, Rapado-Castro M, Parellada M, et al. (2011) Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol 21: 621–645. 10.1016/j.euroneuro.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 71. Leucht S, Tardy M, Komossa K, Heres S, Kissling W, et al. (2012) Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379: 2063–2071. 10.1016/S0140-6736(12)60239-6 [DOI] [PubMed] [Google Scholar]
- 72. Wildgust HJ, Hodgson R, Beary M (2010) The paradox of premature mortality in schizophrenia: new research questions. J Psychopharmacol 24: 9–15. 10.1177/1359786810382149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.