Abstract
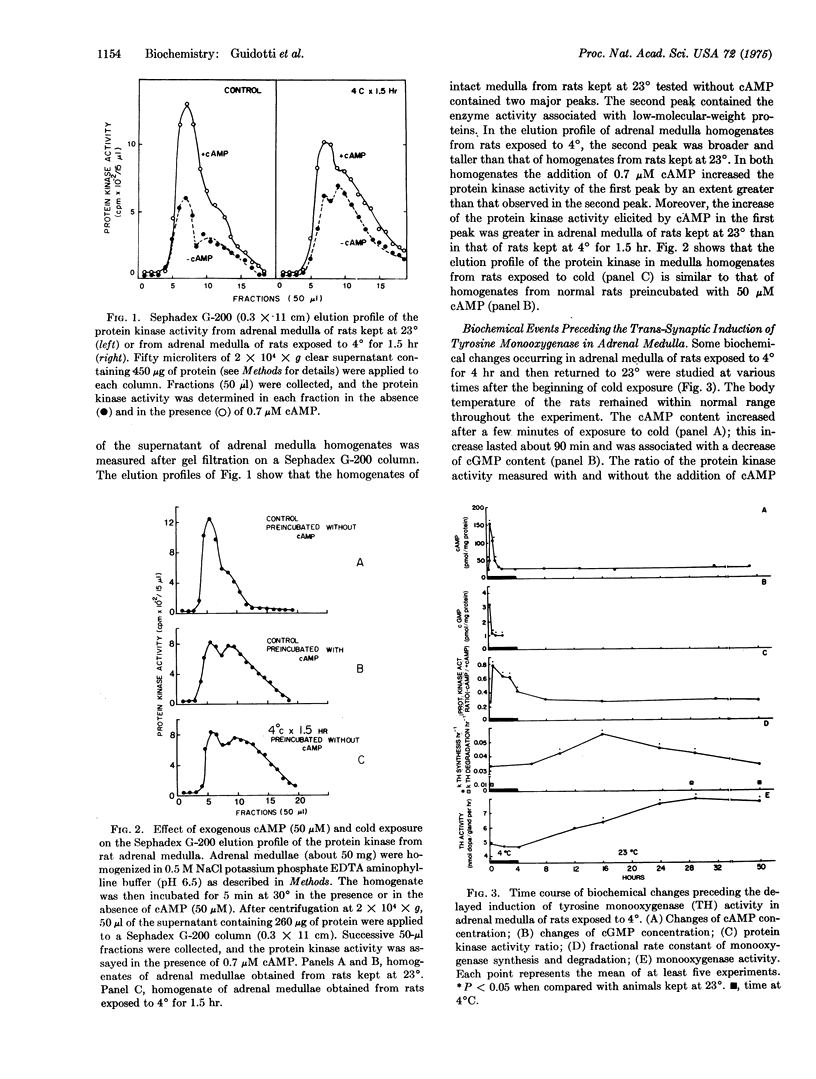
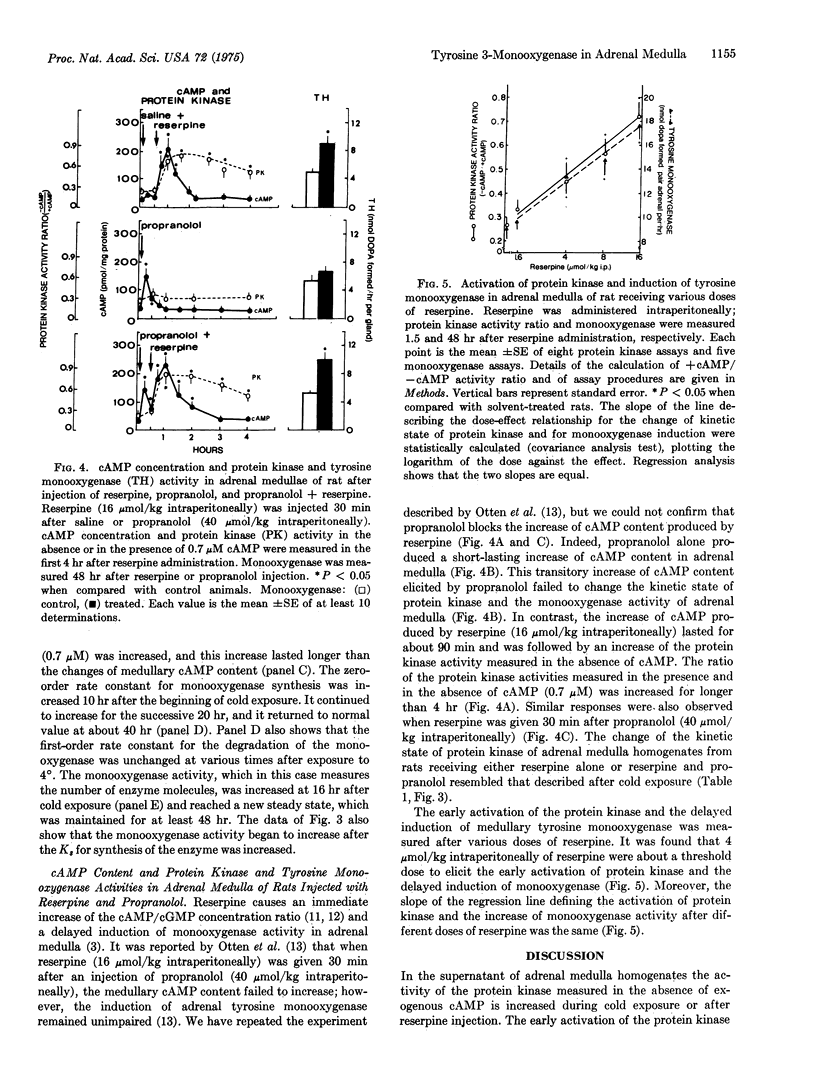
An increase of cAMP/cGMP concentration ratio is the earliest stimulus-coupled biochemical change that has been measured in the adrenal medulla during the trans-synaptic induction of tyrosine 3-monooxygenase [EC 1.14.16.2; L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating)]. In adrenal medulla of rats receiving reserpine alone (16 mumol/kg intraperitoneally) or reserpine and propranolol (40 mumol/kg intraperitoneally 30 min before reserpine), or exposed to 4 degrees for 4 hr, the extent and duration of the increase of the cAMP/cGMP concentration ratio exceeds the critical value that is required to activate the protein kinases (EC 2.7.1.37; ATP:protein phosphotransferase). Gel filtration experiments indicate that during this activation, the catalytic subunit of the protein kinase (low-molecular-weight enzyme) is released from the holoenzyme. The activation of protein kinase lasts longer than the increase in the cAMP/cGMP concentration ratio and appears to be an obligatory early event that mediates the increase of tyrosine monooxygenase synthesis. The trans-synaptic induction of the monooxygenase in adrenal medulla appears to be due to an increased synthesis of the enzyme;the rate for monooxygenase degradation is proportional to the number of enzyme molecules that are present at various stages of the induction process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuang D. M., Costa E. Biosynthesis of tyrosine hydroxylase in rat adrenal medulla after exposure to cold. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4570–4574. doi: 10.1073/pnas.71.11.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Soderling T. R., Park C. R. Regulation of adenosine 3',5'-monophosphate-dependent protein kinase. I. Preliminary characterization of the adipose tissue enzyme in crude extracts. J Biol Chem. 1973 Mar 10;248(5):1813–1821. [PubMed] [Google Scholar]
- Costa E., Guidotti A., Hanbauer I. Do cyclic nucleotides promote the trans-synaptic induction of tyrosine hydroxylase? Life Sci. 1974 Apr 1;14(7):1169–1188. doi: 10.1016/0024-3205(74)90425-1. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Costa E. A role for nicotinic receptors in the regulation of the adenylate cyclase of adrenal medulla. J Pharmacol Exp Ther. 1974 Jun;189(3):665–675. [PubMed] [Google Scholar]
- Guidotti A., Costa E. Association between increase in cyclic AMP and subsequent induction of tyrosine hydroxylase in rat adrenal medulla. Experiments with swimming stress. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(2):217–221. doi: 10.1007/BF00499036. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Costa E. Involvement of adenosine 3',5'-monophosphate in the activation of tyrosine hydroxylase elicited by drugs. Science. 1973 Mar 2;179(4076):902–904. doi: 10.1126/science.179.4076.902. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Hiestand P. C., Schweppe J. S. Mechanism of action of gonadotropin. IV. Cyclic adenosine monophosphate-dependent translocation of ovarian cytoplasmic cyclic adenosine monophosphate-binding protein and protein kinase to nuclear acceptor sites. Endocrinology. 1974 Jan;94(1):168–183. doi: 10.1210/endo-94-1-168. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. A cell-free system with ribosomal protein kinase activity. Biochemistry. 1971 Jan 19;10(2):197–203. doi: 10.1021/bi00778a001. [DOI] [PubMed] [Google Scholar]
- Korenman S. G., Bhalla R. C., Sanborn B. M., Stevens R. H. Protein kinase translocation as an early event in the hormonal control of uterine contraction. Science. 1974 Feb 1;183(4123):430–432. doi: 10.1126/science.183.4123.430. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. VI. Isolation and partial purification of a protein kinase activated by guanosine 3',5'-monophosphate. J Biol Chem. 1970 May 25;245(10):2493–2498. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maeno H., Greengard P. Phosphoprotein phosphatases from rat cerebral cortex. Subcellular distribution and characterization. J Biol Chem. 1972 May 25;247(10):3269–3277. [PubMed] [Google Scholar]
- Mao C. C., Guidotti A. Simultaneous isolation of adenosine 3',5'-cyclic monophosphate (cAMP) and guanosine 3',5'-cyclic monophosphate (cGMP) in small tissue samples. Anal Biochem. 1974 May;59(1):63–68. doi: 10.1016/0003-2697(74)90009-8. [DOI] [PubMed] [Google Scholar]
- Molinoff P. B., Axelrod J. Biochemistry of catecholamines. Annu Rev Biochem. 1971;40:465–500. doi: 10.1146/annurev.bi.40.070171.002341. [DOI] [PubMed] [Google Scholar]
- Otten U., Mueller R. A., Oesch F., Thoenen H. Location of an isoproterenol-responsive cyclic AMP pool in adrenergic nerve cell bodies and its relationship to tyrosine 3-monooxygenase induction. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2217–2221. doi: 10.1073/pnas.71.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick R. L., Kirshner N. Acetylcholine-induced stimulation of catecholamine recovery in denervated rat adrenals after reserpine-induced depletion. Mol Pharmacol. 1971 Jul;7(4):389–396. [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970 Nov 28;228(5274):861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Kettler R., Burkard W., Saner A. Neurally mediated control of enzymes involved in the synthesis of norepinephrine; are they regulated as an operational unit? Naunyn Schmiedebergs Arch Pharmakol. 1971;270(2):146–160. doi: 10.1007/BF00997085. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Mueller R. A., Axelrod J. Increased tyrosine hydroxylase activity after drug-induced alteration of sympathetic transmission. Nature. 1969 Mar 29;221(5187):1264–1264. doi: 10.1038/2211264a0. [DOI] [PubMed] [Google Scholar]
- Udenfriend S. Tyrosine hydroxylase. Pharmacol Rev. 1966 Mar;18(1):43–51. [PubMed] [Google Scholar]
- Walaas O., Walaas E., Gronnerod O. Hormonal regulation of cyclic-AMP-dependent protein kinase of rat diaphragm by epinephrine and insulin. Eur J Biochem. 1973 Dec 17;40(2):465–477. doi: 10.1111/j.1432-1033.1973.tb03215.x. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Krebs E. G., Reimann E. M., Brostrom M. A., Corbin J. D., Hickenbottom J. P., Soderling T. R., Perkins J. P. The receptor protein for cyclic AMP in the control of glycogenolysis. Adv Biochem Psychopharmacol. 1970;3:265–285. [PubMed] [Google Scholar]
- Wastila W. B., Stull J. T., Mayer S. E., Walsh D. A. Measurement of cyclic 3',5'-denosine monophosphate by the activation of skeletal muscle protein kinase. J Biol Chem. 1971 Apr 10;246(7):1996–2003. [PubMed] [Google Scholar]
- Waymire J. C., Bjur R., Weiner N. Assay of tyrosine hydroxylase by coupled decarboxylation of DOPA formed from 1- 14 C-L-tyrosine. Anal Biochem. 1971 Oct;43(2):588–600. doi: 10.1016/0003-2697(71)90291-0. [DOI] [PubMed] [Google Scholar]
- Zivkovic B., Guidotti A. Changes of kinetic constant of striatal tyrosine hydroxylase elicited by neuroleptics that impair the function of dopamine receptors. Brain Res. 1974 Oct 25;79(3):505–509. doi: 10.1016/0006-8993(74)90448-x. [DOI] [PubMed] [Google Scholar]


