Abstract
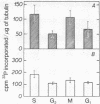
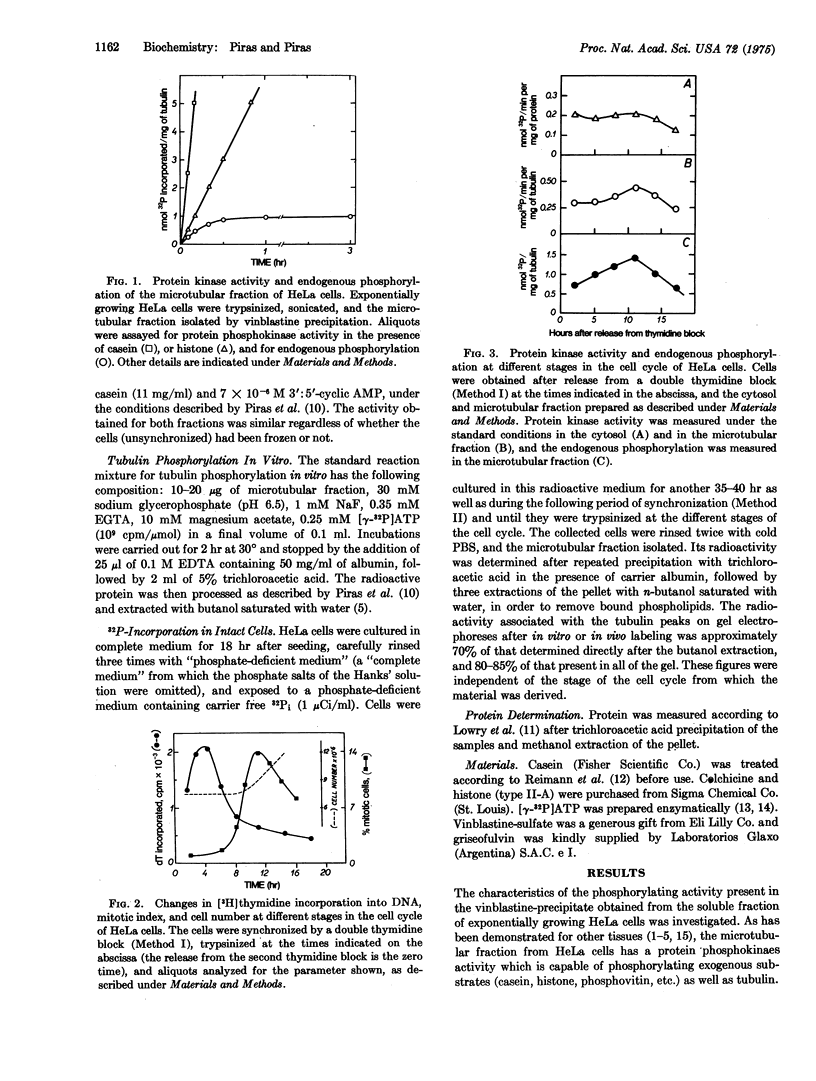
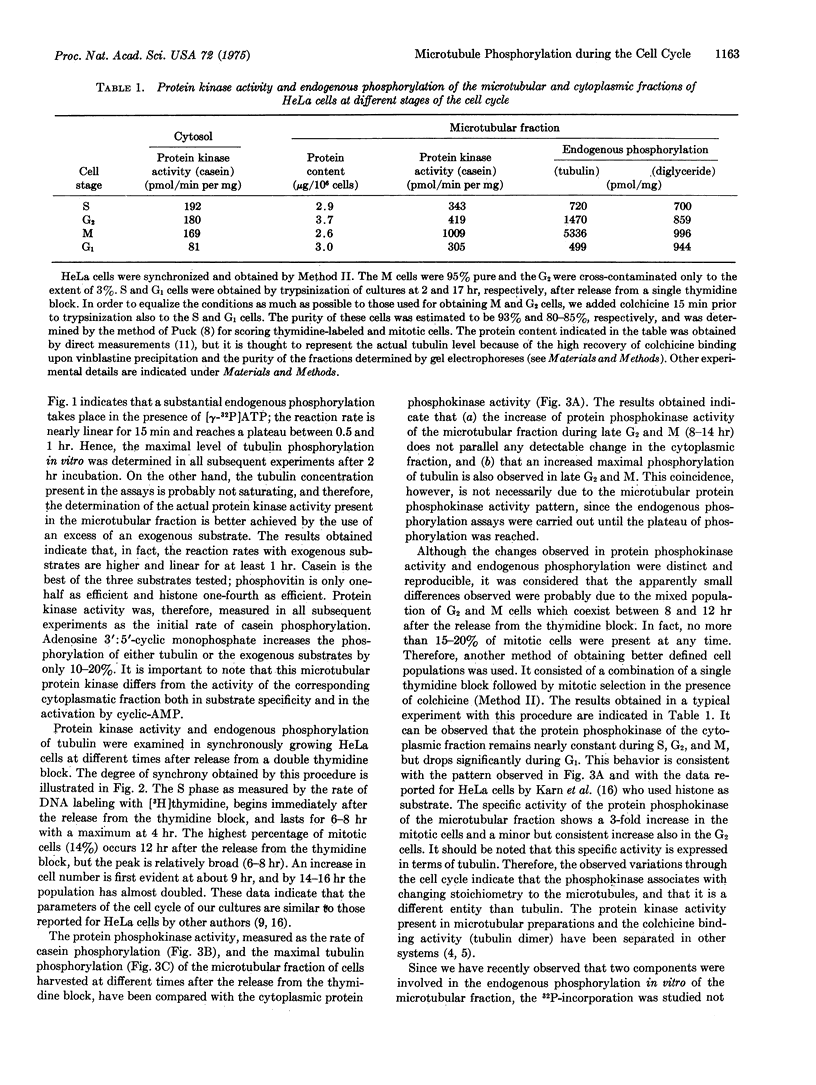
The phosphorylation in vitro and in vivo of tubulin isolated from HeLa cells has been examined during the cell cylce. The results obtained indicate that: (a) the protein kinase (ATP:protein phosphotransferase, EC 2.7.1.37) activity present in the microtubules, and measured in vitro with exogenous casein as substrate, is maximal in M cells, whereas that present in the cytosol is nearly constant during the S, G-2, and M stages, and decreases during G-1; (b) the patterns through the cell cycle of the maximal number of tubulin sites phosphorylated in vitro and of the microtubular protein kinase activity are similar; (c) the degree of tubulin phosphorylation in vivo is 2- to 3-fold higher in the microtubules isolated from the S and M stages of the cell cycle, than those from G-1 and G-2. This variable phosphate content of tubulin through the cell cycle suggests that such covalent modification might be important to enable tubulin to carry over some of its functions during the cell cycle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya B., Wolff J. Thyroid tubulin: purification and properties. Biochemistry. 1974 May 21;13(11):2364–2369. doi: 10.1021/bi00708a020. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B. Nucleated assembly of microtubules in porcine brain extracts. Science. 1972 Sep 29;177(4055):1196–1197. doi: 10.1126/science.177.4055.1196. [DOI] [PubMed] [Google Scholar]
- Daleo G. R., Piras M. M., Piras R. The presence of phospholipids and diglyceride kinase activity in microtubules from different tissues. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1043–1050. doi: 10.1016/0006-291x(74)90260-5. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2283–2287. doi: 10.1073/pnas.69.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain tubulin and protein kinase activity. J Biol Chem. 1974 Mar 10;249(5):1398–1406. [PubMed] [Google Scholar]
- Forrest G. L., Klevecz R. R. Synthesis and degradation of microtubule protein in synchronized Chinese hamster cells. J Biol Chem. 1972 May 25;247(10):3147–3152. [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. B., Rasmussen H., DiBella F., Guthrow C. E., Jr Cyclic adenosine 3':5'-monophosphate-stimulated phosphorylation of isolated neurotubule subunits. Proc Natl Acad Sci U S A. 1970 Oct;67(2):652–659. doi: 10.1073/pnas.67.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham L. M., Wilson L., Bensch K. G. Antimitotic action of griseofulvin does not involve disruption of microtubules. Nature. 1973 Aug 3;244(5414):294–296. doi: 10.1038/244294a0. [DOI] [PubMed] [Google Scholar]
- Hepler P. K., McIntosh J. R., Cleland S. Intermicrotubule bridges in mitotic spindle apparatus. J Cell Biol. 1970 May;45(2):438–444. doi: 10.1083/jcb.45.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane R. E. The mitotic apparatus. Identification of the major soluble component of the glycol-isolated mitotic apparatus. J Cell Biol. 1967 Feb;32(2):243–253. doi: 10.1083/jcb.32.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Johnson E. M., Vidali G., Allfrey V. G. Differential phosphorylation and turnover of nuclear acidic proteins during the cell cycle of synchronized HeLa cells. J Biol Chem. 1974 Feb 10;249(3):667–677. [PubMed] [Google Scholar]
- Kirschner M. W., Williams R. C., Weingarten M., Gerhart J. C. Microtubules from mammalian brain: some properties of their depolymerization products and a proposed mechanism of assembly and disassembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1159–1163. doi: 10.1073/pnas.71.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevecz R. R. Rapid protein catabolism in mammalian cells is obscured by reutilization of amino acids. Biochem Biophys Res Commun. 1971 Apr 2;43(1):76–81. doi: 10.1016/s0006-291x(71)80088-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McIntosh J. R. Bridges between microtubules. J Cell Biol. 1974 Apr;61(1):166–187. doi: 10.1083/jcb.61.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilas M. M., Staneloni R., Leiderman B., Piras R. Protein phosphokinases of chick muscle: changes during embryonic development. FEBS Lett. 1972 Jun 15;23(2):199–202. doi: 10.1016/0014-5793(72)80340-5. [DOI] [PubMed] [Google Scholar]
- Piras M. M., Piras R. Phosphorylation of vinblastine-isolated microtubules from chick-embryonic muscles. Eur J Biochem. 1974 Sep 16;47(3):443–452. doi: 10.1111/j.1432-1033.1974.tb03711.x. [DOI] [PubMed] [Google Scholar]
- Puck T. T. Phasing, Mitotic Delay, and Chromosomal Aberrations in Mammalian Cells. Science. 1964 May 1;144(3618):565–566. doi: 10.1126/science.144.3618.565-c. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Robbins E., Jentzsch G., Micali A. The centriole cycle in synchronized HeLa cells. J Cell Biol. 1968 Feb;36(2):329–339. doi: 10.1083/jcb.36.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Shelanski M. Synthesis of a colchicine-binding protein during the HeLa cell life cycle. J Cell Biol. 1969 Nov;43(2):371–373. doi: 10.1083/jcb.43.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Brosom C. O., Ho E. S., Kreb E. G. Catlysis of the phosphrylaseinase actition reaction. J Biol Chem. 1971 Apr 10;246(7):1968–1976. [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Wilson H. J. Arms and bridges on microtubules in the mitotic apparatus. J Cell Biol. 1969 Mar;40(3):854–859. doi: 10.1083/jcb.40.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]