Abstract
Biological therapy revolutionized the treatment of inflammatory bowel disease (IBD) during the last decade. These monoclonal antibodies, which target tumor necrosis factor (TNF), integrins or IL12/23, have been approved—or are in development for—both Crohn’s disease (CD) and ulcerative colitis (UC). Early use of these agents taught clinicians that induction and maintenance therapy, coupled with immunomodulator agents, reduced the immunogenicity of these agents, and led to sustained remission in many patients. More recent data has demonstrated that, through dose adjustments, optimizing serum drug levels may also provide more durable maintenance of remission, and improved mucosal healing. This review examines clinical practices that may enhance clinical outcomes from biological therapy in IBD.
Keywords: inflammatory bowel disease, biological therapy
Introduction
Biological therapy currently refers to monoclonal antibodies directed against specific targets implicated in the pathogenesis of chronic inflammatory conditions. For inflammatory bowel disease (IBD), this primarily encompasses the approved anti-tumor necrosis factor (TNF) therapies (infliximab, adalimumab, certolizumab, golimumab), but also agents approved, or under development, that target integrins (natalizumab, vedolizumab) and interleukin (IL)-12/23 (ustekinumab), amongst others. Table 1 summarizes some of the biological therapies that have been studied in clinical trials in IBD.
Table 1.
Examples of biologics for inflammatory bowel disease, approved and under development
| Name | Primary target | FDA-approved indication |
|---|---|---|
| Infliximab (Remicade®) | TNF | CD, UC |
| Adalimumab (Humira®) | TNF | CD, UC |
| Certolizumab (Cimzia®) | TNF | CD |
| Golimumab (Simponi®) | TNF | UC |
| Natalizumab (Tysabri®) | alpha-4 integrin | CD |
| Vedolizumab (Entyvio®) | alpha-4-beta-7 integrin | CD, UC |
| Ustekinumab (Stelara®) | IL-12/23 | N/A |
| Etrolizumab | beta-7 integrin | N/A |
| Anrukinzumab | IL-13 | N/A |
FDA = Food and Drug Administration; TNF = tumor necrosis factor; IL = interleukin; CD = Crohn’s disease; UC = ulcerative colitis; N/A = not available
The introduction of anti-TNF agents in the United States and Europe in the late 1990s gradually led to a paradigm shift in our approach to IBD therapy [1]. The deluge of studies that followed their approval provided evidence that this class of drug could induce sustained clinical remission in a cohort of patients, avoid the chronic need for steroids, reduce hospitalizations, and potentially prevent surgical interventions for complications [2]. The goal of treatment shifted from simply improving patients’ symptoms, to aiming for objective reversal of mucosal inflammation and prevention of long-term complications. On the down side, the use of anti-TNFs led in practice to reports of hypersensitivity reactions, reactivation of TB, and lymphomas in patients receiving these agents. The unprecedented enrolment of larger cohorts of IBD patients in registry studies to track adverse events led to a characterization of many disease- and drug- related safety signals [3]. For the first time, extensive capture of health outcomes, pharmaco-economic analyses, quality-of-life measures and work productivity were undertaken to assess the impact of these diseases—and their treatment—on the population of patients with IBD.
One of the conclusions of long-term follow-up studies, in practice and clinical trials, was that many patients who initially obtained ‘remission' with anti-TNF agents subsequently experienced reduced response over time: for example, in clinical trials, only ∼40% of patients were in remission at 1 year after induction, and in clinical practice less than 50% of initial responders were in remission at this time-point [4]. A review of these long-term outcomes suggests that there is clearly a population of patients who clinically respond to induction therapy and then maintain remission over time, with little need for dose adjustments; however, there is also a sizeable population of patients whose symptoms recur despite continued biological therapy. Strategies to address this problem and improve the rates of retention for this drug class have emerged in recent years [5].
Mechanisms for loss of response to Biologics
The recurrence of clinical symptoms after induced remission with anti-TNFs is a common one; up to 60% of patients experience recurrence of symptoms in clinical practice over time [6]. There are many reasons for this event, and these are summarized in Table 2. The relative contribution of each cause is difficult to determine in practice, but cohort studies suggest that immunogenicity and overlap functional symptoms account for most cases of loss of response [7].
Table 2.
Reasons for loss of clinical response amongst patients receiving biologics for inflammatory bowel disease
| Mechanism |
|---|
| Immunogenicity (anti-drug antibodies) |
| Enhanced drug clearance (non-immunogenic) |
| Alternate inflammatory pathways |
| Non-inflammatory complications (e.g. strictures) |
| Overlap functional symptoms |
| Concurrent infections (e.g. C. difficile or Cytomegalovirus) |
Immunogenicity
Immunogenicity—the propensity for patients to develop anti-drug antibodies (ADAs) against the monoclonal agents—develops in a proportion of patients through both thyroid-dependent (high affinity, immunological memory), and thyroid independent (low affinity, occasional memory) mechanisms [8]. These ADAs are typically IgG antibodies that can impair binding of the biological agent to the target cytokine, or accelerate drug clearance by the reticulo-endothelial system (RES) [9]. When the anti-TNF antibody infliximab was initially given only intermittently in practice, up to 60% of patients developed anti-drug antibodies, and these patients were twice as likely as antibody-negative recipients to develop acute infusion reactions [10]. Since then, the use of induction and regular maintenance infusions has lowered the prevalence of anti-infliximab antibodies to 10–20% in randomized, controlled trials (RCTs) and observational cohorts [11]; in this patient population however, once they develop, ADAs are associated with a higher risk of loss of clinical response, and risk of infusions reactions [12, 13]. It has also recently been recognized that patients can develop transient ADAs, or persistent ADAs, and that drug-free periods lead to gradual disappearance of ADAs from circulation [14, 15]. While the factors that lead some patients to develop ADAs is only partially understood, persistently low drug serum levels have been associated with development of ADAs in some studies [16].
Overlap functional symptoms
More frequent—but certainly less well-characterized—is the phenomenon of patients with IBD experiencing non-inflammatory symptoms. These are usually described as intestinal symptoms of pain, diarrhea or rectal bleeding in the absence of objective evidence of active inflammation on endoscopy. For example, in one study, 26% of patients with inactive Crohn’s disease met criteria for a diagnosis of irritable bowel syndrome (IBS) while, in another study, 10% of patients with quiescent ulcerative colitis (UC) had significant on-going abdominal pain [17, 18]. In some individuals, physiological processes such as delayed gastric emptying or bile salt malabsorption may play a role even in the absence of macroscopic inflammation [19, 20]. These factors need to be considered when evaluating recurrence of symptoms, as biological therapy for is not an appropriate intervention for IBS.
Other processes
Whilst IBD manifests itself as a purely inflammatory process early on, later complications, such as strictures, fistulae, and malignancy, may lead to the development of symptoms that are unresponsive to any biological anti-cytokine therapy [9]. Small bowel strictures and their associated bacterial overgrowth can contribute to abdominal pain, bloating and diarrhea, and require mechanical correction [18]. Enteric fistulae to other abdominal compartments and organs may also lead to symptoms and a perceived loss of response to biological therapy.
Strategies for optimizing the use of Biological therapy
The observations noted above illustrate that maintaining a sustained clinical remission with biological therapies requires attention to the many factors that could lead to relapse of symptoms. In the scenario where active inflammation has been objectively confirmed, and concurrent infections have been excluded, there are a number of therapeutic decisions that can be made to pro-actively, or reactively, enhance the efficacy of biological agents (Table 3). The evidence base for this is primarily derived from studies of infliximab, particularly with regard to serum drug levels and ADAs.
Table 3.
Proposed strategies to optimize the efficacy of biological therapies in inflammatory bowel disease
| Strategy | Evidencea |
|---|---|
| Patient selection | |
| Early in disease course | B |
| Administration schedule | |
| Induction & maintenance | B |
| Concomitant therapy | |
| Thiopurines | B |
| Methotrexate | C |
| Therapeutic drug monitoring | |
| Reactive testing | B |
| Proactive testing | C |
| Biomarker monitoring | |
| Proactive CRP measurements | C |
aGrading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group 2007.
A = several high-quality studies; B = moderate quality: several studies with limitations; C = low quality: studies with many limitations; CRP = C-reactive protein
Patient selection
Biological agents were initially given only to patients with severe disease, which limited the potential long-term efficacy of these drugs. In RCTs of infliximab in Crohn’s disease, for example, enrolled subjects had the disease for an average of 8 years, 51% already required surgery for complications, and a third had failed to respond to thiopurines [21]. Since Crohn’s disease is a progressive condition, selecting only patients with long-standing disease for a given therapy reduces the proportion of recipients whose symptoms are caused primarily by active inflammation [22]. In contrast, post-hoc analysis of these RCTs has concluded that overall remission rates with biologics are numerically greater when administered to patients within 2 years of diagnosis [23]. Therefore, the preferred candidate for biological therapy is one with confirmed active intestinal inflammation, prior to the development of complications such as strictures. Using biologics in patients with overlap IBS or mostly fibrotic disease yields lower clinical remission rates, at considerable expense to those paying for treatment [24]. Of course, not every patient with IBD will require a biological therapy early after diagnosis. Selecting patients early in the course of their disease, who are at higher risk of complications, can be used to ‘risk stratify' individuals for early use of biological therapy. Clinical (age, phenotype, steroid use) and serogenetic factors have been identified, which are associated with relative risk of complications in Crohn’s disease, but the relative impact of biologics on low- or high-risk patients has yet to be prospectively determined [25].
Maintenance schedules
The maintenance regimens tested in RCTs have confirmed that continued use of biologics is required to maintain remission in responsive patients. Episodic therapy leads, in practice, to higher rates of ADAs and infusion reactions, and lower rates of remission [10, 26]. Patients with Crohn’s disease receiving scheduled infliximab had lower disease activity scores and fewer hospitalizations, but higher response rates, than patients who received episodic therapy [26]. The recommended schedule for administration (every other week, every 4 weeks or every 8 weeks) may in some cases be insufficient to maintain remission, and schedules of administration that are more frequent than the approved dose are commonly used [6, 27]. A number of cohort studies have reported that these ‘escalation strategies' are able to re-capture response in up to 80% of patients who have lost response [28, 29].
Concurrent immunomodulators
In some studies, the administration of concurrent immunomodulators (IMMs), such as azathioprine or methotrexate, has been associated with higher remission rates and lower rates of ADAs. Post-hoc analysis of the RCTs of anti-TNFs, and retrospective review of clinical cohorts, did not demonstrate superior efficacy of combination therapy over monotherapy in Crohn’s disease [30, 31]; however, subsequent prospective trials in both CD and UC concluded that initial combination therapy was beneficial. In the SONIC (Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease) trial in CD, 57% of patients receiving infliximab and thiopurines were in corticosteroid-free clinical remission at week 26, as compared with 44% of those receiving infliximab alone (P = 0.02) [32]. Similarly, in the SUCCESS (Efficacy and Safety of Infliximab, as Monotherapy or in Combination with Azathioprine, versus Azathioprine Monotherapy in Moderate to Severe Ulcerative Colitis) trial in UC, steroid-free remission was achieved by 40% of patients receiving infliximab or azathioprine, compared with 22% receiving infliximab alone (P = 0.017) [33]. The reason for the synergistic effects of immunomodulators may be their ability to reduce the immunogenicity of biologics. In the SONIC trial, ADAs developed in less than 1% of patients receiving azathioprine and infliximab, in contrast to 15% of patients who received infliximab alone [32]. In one small case series, even post-hoc addition of an immunomodulator to a biologic could reduce ADAs, and elevate serum drug levels, in patients with CD [34].
Therapeutic drug monitoring
Many observational studies have linked low serum drug levels to a higher risk of ADA development, and/or loss of response to biologics in IBD [35–37]. In response, reactive measurement of ADAs and serum drug levels using Enzyme Linked Immunosorbent Assay (ELISA) assays, and appropriate adjustment of drug regimen, has been utilized in practice to optimize clinical outcomes [28, 38]. This strategy has been reported to be more cost-effective than empirical escalation of doses of biologics, with similar clinical outcomes in this setting [7, 39]. In Denmark, Steenholdt et al. concluded that individualized infliximab therapy, based on drug levels, was more cost-effective than empirical dose intensification in patients losing response to infliximab; response rates were similar (∼55%), but costs were 34% lower when therapeutic drug monitoring was used [7].
In the light of these findings, other groups have examined proactive adjustment of biological drug levels to prevent relapse of disease. The Trough level Adapted infliXImab Treatment (TAXIT) trial from Europe enrolled patients in remission and adjusted their infliximab dose to obtain a target serum drug level; subsequently this cohort was randomized to either standard care, or continued adjustment of infliximab dose, based on drug levels. After 1 year, overall remission rates were similar in both arms (69% and 72%) [40]. A small retrospective analysis from another group reported that patients who had proactive adjustment of infliximab levels to above 5 µg/mL were less likely to discontinue infliximab than those who were not adjusted (10% vs. 31%, respectively; P = 0.009) [41]. Where commercial assays for biological drug levels are not available, C-reactive protein (CRP) and fecal calprotectin may be surrogate markers to identify patients at risk of relapse due to low drug levels [42–44]. One area of uncertainty is the drug level to aim for when using therapeutic monitoring; many studies have used different cut-offs, so it is unclear what is the ideal therapeutic range (Table 4) [16, 35, 45–54].
Table 4.
Drug thresholds used to categorize patients’ outcomes in observational studies
| Study | Drug | Threshold (μg/mL) | Outcome |
|---|---|---|---|
| Ben-Bassat O [35] | IFX | 2 | Remission |
| Drastich P [45] | IFX | 2 | Remission |
| Murthy S [46] | IFX | 2 | Remission |
| Maser EA [47] | IFX | 1.4 | Remission |
| Reinisch W [48] | IFX | 3 | Remission |
| Seow CH [49] | IFX | 2 | Remission |
| Steenholdt C [50] | IFX | 0.5 | Remission |
| Vande Casteele [16] | IFX | 2.2 | CRP |
| Feagan BG [51] | IFX | 3 | CRP |
| Bortlik M [52] | IFX | 3 | CRP |
| Mazor Y [53] | ADA | 5 | Remission |
| Imaeda H [54] | ADA | 10 | CRP |
IFX = infliximab; ADA = adalimumab; CRP = C-reactive protein
Conclusions
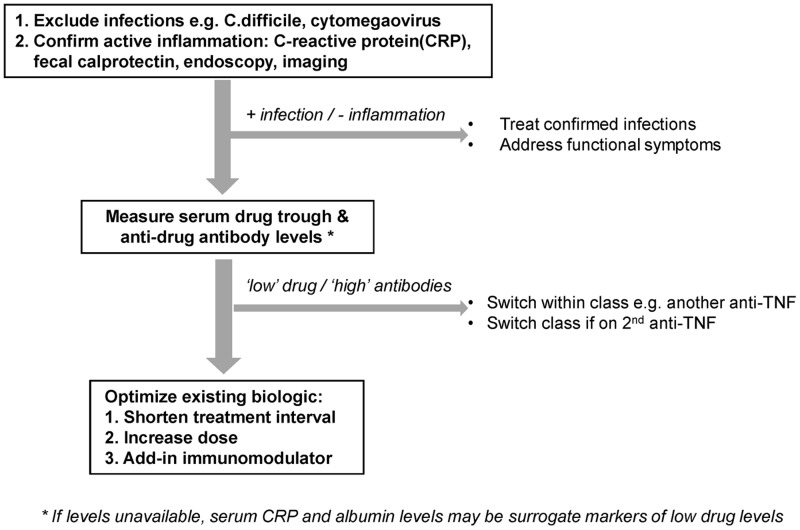
There has been a steep learning curve in the optimal use of biologics in IBD since 1997. The current strategies of patient selection, maintenance schedules, use of concurrent immunomodulators, and therapeutic drug monitoring have generated incremental improvements in the long-term remission rates with this class of drug. We are still not at the point where the majority of patients treated with biologics achieve sustained clinical and/or endoscopic remission. Alterations in immunological pathways over time, persistence of functional intestinal symptoms, and differences in underlying pathogenic processes may prevent this being universally achievable; however, optimal use of biologics in those who initially respond to them will certainly enhance the efficacy of this drug class in the medium term (Figure 1).
Figure 1.
Strategies of biologics use in patients with inflammatory bowel disease
Funding
ACM is supported by NIH grant K23DK084338.
Conflict of interest statement. ACM has consulted for Janssen, Abbott, UCB, Roche and Bayer, and received research support (to BIDMC) from Pfizer.
References
- 1.Antunes O, Filippi J, Hebuterne X, et al. Treatment algorithms in Crohn's: up, down or something else? Best Pract Res Clin Gastroenterol 2014;28:473–83. [DOI] [PubMed] [Google Scholar]
- 2.Mandel MD, Miheller P, Mullner K, et al. Have biologics changed the natural history of Crohn's disease? Dig Dis 2014;32:351–9. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein GR, Feagan BG, Cohen RD, et al. Drug therapies and the risk of malignancy in Crohn's disease: results from the TREAT Registry. Am J Gastroenterol 2014;109:212–23. [DOI] [PubMed] [Google Scholar]
- 4.Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol 2009;104:760–7. [DOI] [PubMed] [Google Scholar]
- 5.Vande Casteele N, Feagan BG, Gils A, et al. Therapeutic drug monitoring in inflammatory bowel disease: current state and future perspectives. Curr Gastroenterol Rep 2014;16:378. [DOI] [PubMed] [Google Scholar]
- 6.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology 2009;137:1628–40. [DOI] [PubMed] [Google Scholar]
- 7.Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. [DOI] [PubMed] [Google Scholar]
- 8.Sauerborn M, Brinks V, Jiskoot W, et al. Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci 2010;31:53–9. [DOI] [PubMed] [Google Scholar]
- 9.Fasanmade AA, Adedokun OJ, Blank M, et al. Pharmacokinetic properties of infliximab in children and adults with Crohn's disease: a retrospective analysis of data from two phase III clinical trials. Clin Ther 2011;33:946–64. [DOI] [PubMed] [Google Scholar]
- 10.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003;348:601–8. [DOI] [PubMed] [Google Scholar]
- 11.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol 2004;2:542–53. [DOI] [PubMed] [Google Scholar]
- 12.O'Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014;20:1–6. [DOI] [PubMed] [Google Scholar]
- 13.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013;108:40–7; quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben Horin S, Mazor Y, Yanai H, et al. The decline of anti-drug antibody titres after discontinuation of anti-TNFs: implications for predicting re-induction outcome in IBD. Aliment Pharmacol Ther 2012;35:714–22. [DOI] [PubMed] [Google Scholar]
- 15.van de Casteele N, Cuypers L, Singh S, et al. Antibodies to Infliximab can either be persistent or transient; a retrospective case-control study in IBD patients treated with Infliximab maintenance therapy. Gastroenterology 2012;143:563. [Google Scholar]
- 16.Vande CN, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962–71. [DOI] [PubMed] [Google Scholar]
- 17.Coates MD, Lahoti M, Binion DG, et al. Abdominal pain in ulcerative colitis. Inflamm Bowel Dis 2013;19:2207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrokhyar F, Marshall JK, Easterbrook B, et al. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis 2006;12:38–46. [DOI] [PubMed] [Google Scholar]
- 19.Gothe F, Beigel F, Rust C, et al. Bile acid malabsorption assessed by 7 alpha-hydroxy-4-cholesten-3-one in pediatric inflammatory bowel disease: correlation to clinical and laboratory findings. J Crohns Colitis 2014;8:1072–8. [DOI] [PubMed] [Google Scholar]
- 20.Nobrega AC, Ferreira BR, Oliveira GJ, et al. Dyspeptic symptoms and delayed gastric emptying of solids in patients with inactive Crohn's disease. BMC Gastroenterol 2012;12:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 22.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–94. [DOI] [PubMed] [Google Scholar]
- 23.Cornillie F, Hanauer S, Diamond RH, et al. Can clinical, biological or pharmacological markers predict sustained response to infliximab? A retrospective analysis of ACCENT 1. Gut 2011;60:A296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Wu EQ, Bensimon AG, et al. Cost per responder associated with biologic therapies for Crohn's disease, psoriasis, and rheumatoid arthritis. Adv Ther 2012;29:620–34. [DOI] [PubMed] [Google Scholar]
- 25.Beaugerie L, Sokol H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J Gastroenterol 2012;18:3806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology 2004;126:402–13. [DOI] [PubMed] [Google Scholar]
- 27.Rostholder E, Ahmed A, Cheifetz AS, et al. Outcomes after escalation of infliximab therapy in ambulatory patients with moderately active ulcerative colitis. Aliment Pharmacol Ther 2012;35:562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afif W, Loftus EV, Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn's disease: a systematic review. Am J Gastroenterol 2011;106:674–84. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26. [DOI] [PubMed] [Google Scholar]
- 31.Moss AC, Kim KJ, Fernandez-Becker N, et al. Impact of concomitant immunomodulator use on long-term outcomes in patients receiving scheduled maintenance infliximab. Dig Dis Sci 2010;55:1413–20. [DOI] [PubMed] [Google Scholar]
- 32.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 33.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400e3. [DOI] [PubMed] [Google Scholar]
- 34.Ben Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11:444–47. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Bassat O, Romanova A, Lacono A, et al. Association of serum infliximab and antibodies to infliximab to long-term clinical outcome and mucosal healing in Crohn's disease. Gastroenterology 2013;144 (5 Suppl. 1):S775. [Google Scholar]
- 36.Cesarini M, Katsanos K, Papamichael K, et al. Dose optimization is effective in ulcerative colitis patients losing response to infliximab: a collaborative multicentre retrospective study. Dig Liver Dis 2014;46:135–9. [DOI] [PubMed] [Google Scholar]
- 37.Colombel JF, Sandborn WJ, Allez M, et al. Association Between Plasma Concentrations of Certolizumab Pegol and Endoscopic Outcomes of Patients with Crohn's Disease. Clin Gastroenterol Hepatol 2014;12:423–31e1. [DOI] [PubMed] [Google Scholar]
- 38.Vande Casteele N, Ballet V, Van Assche G, et al. Early serial trough and antidrug antibody level measurements predict clinical outcome of infliximab and adalimumab treatment. Gut 2012;61:321. [DOI] [PubMed] [Google Scholar]
- 39.Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn's disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol 2013;11:654–66. [DOI] [PubMed] [Google Scholar]
- 40.vande Casteele N, Gils A, Ballet V, et al. Randomised controlled trial of drug level versus clinically based dosing of infliximab maintenance therapy in IBD: final results of the TAXIT study. United European Gastroenterol J 2013;1:A1–134. [Google Scholar]
- 41.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 43.Velayos F, Sheibani S, Lockton S, et al. Prevalence of antibodies to adalimumab (ATA) and correlation between ATA and low serum drug concentration on CRP and clinical symptoms in a prospective sample of IBD patients. Gastroenterology 2013;144 (5 Suppl. 1):S490. [Google Scholar]
- 44.Reinisch W, Colombel JF, Sandborn WJ, et al. Infliximab serum trough level and CRP change are associated with corticosteroid-free remission in Crohn's disease: a post-hoc analysis of the sonic trial. Gut 2012;61 (Suppl. 2):A170. [Google Scholar]
- 45.Drastich P, Kozeluhova J, Jaresova M, et al. Infliximab serum trough levels and deep remission in patients with IBD. Gastroenterology 2011;140 (5 Suppl. 1):S292. [Google Scholar]
- 46.Murthy S, Kevans D, Seow CH, et al. Association of serum infliximab and antibodies to infliximab to long-term clinical outcome in acute ulcerative colitis. Gastroenterology 2012;142 (5 Suppl. 1):S388. [Google Scholar]
- 47.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol 2006;4:1248–54. [DOI] [PubMed] [Google Scholar]
- 48.Reinisch W, Feagan BG, Rutgeerts PJ, et al. Infliximab concentration and clinical outcome in patients with ulcerative colitis. Gastroenterology 2012;142 (5 Suppl. 1):S114. [Google Scholar]
- 49.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010;59:49–54. [DOI] [PubMed] [Google Scholar]
- 50.Steenholdt C, Bendtzen K, Brynskov J, et al. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn's disease. Scand J Gastroenterol 2011;46:310–18. [DOI] [PubMed] [Google Scholar]
- 51.Feagan BG, Singh S, Lockton S, et al. Novel infliximab (IFX) and antibody-to-infliximab (ATI) assays are predictive of disease activity in patients with Crohn's disease (CD). Gastroenterology 2012;142 (5 Suppl. 1):S114. [Google Scholar]
- 52.Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohns Colitis 2013;7:736–43. [DOI] [PubMed] [Google Scholar]
- 53.Mazor Y, Almog R, Kopylov U, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn's disease. Aliment Pharmacol Ther 2014;40:620–8. [DOI] [PubMed] [Google Scholar]
- 54.Imaeda H, Takahashi K, Fujimoto T, et al. Accurate determination of serum adalimumab and anti-adalimumab antibodies levels during maintenance therapy for Crohn's disease. Gastroenterology 2013;144 (5 Suppl. 1):S431. [Google Scholar]