Abstract
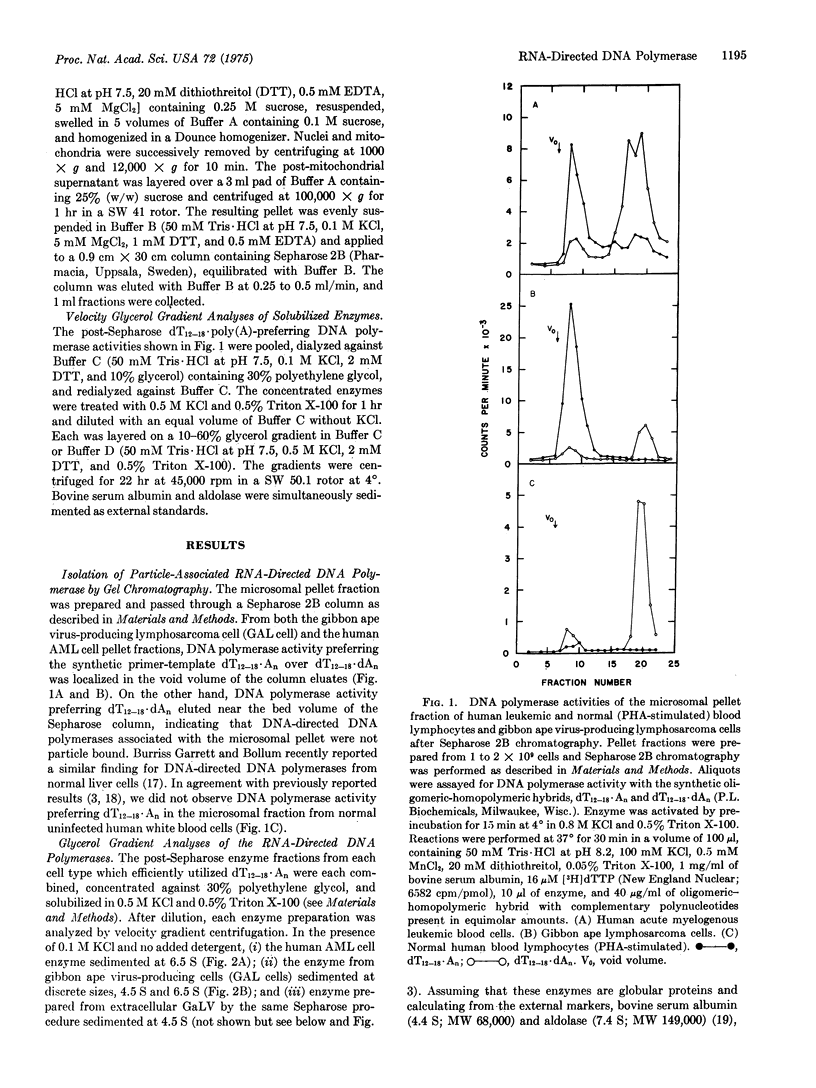
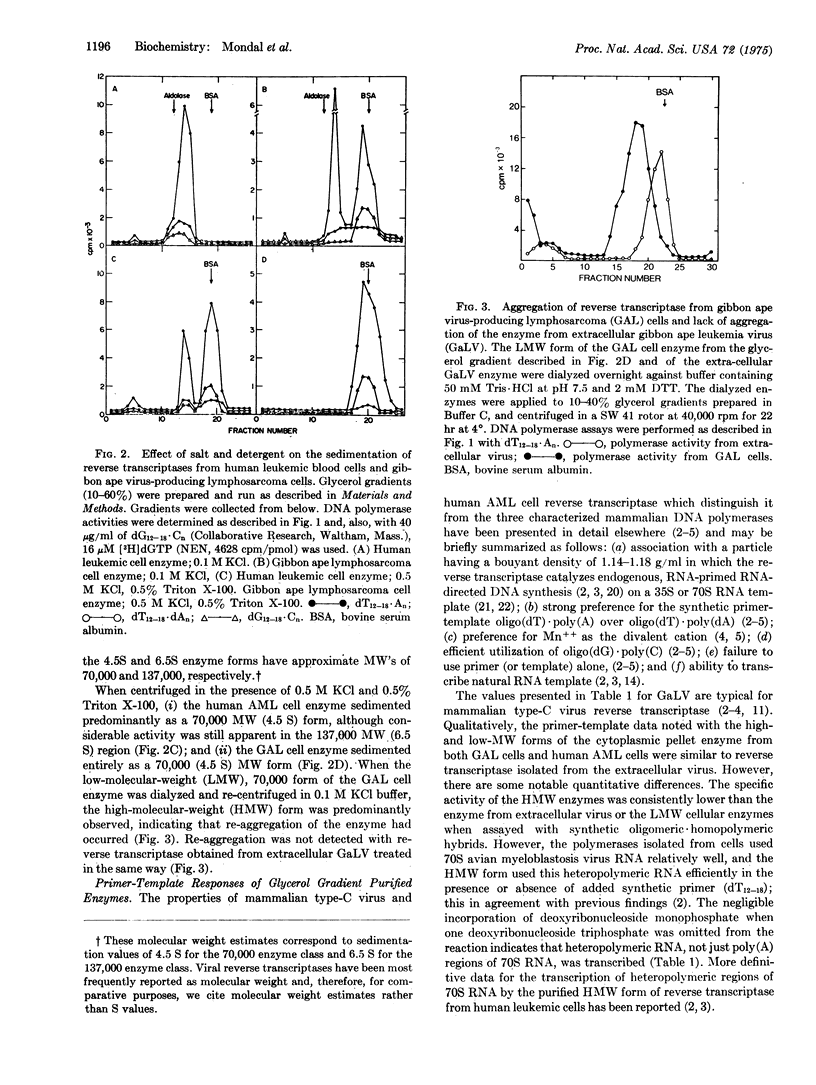
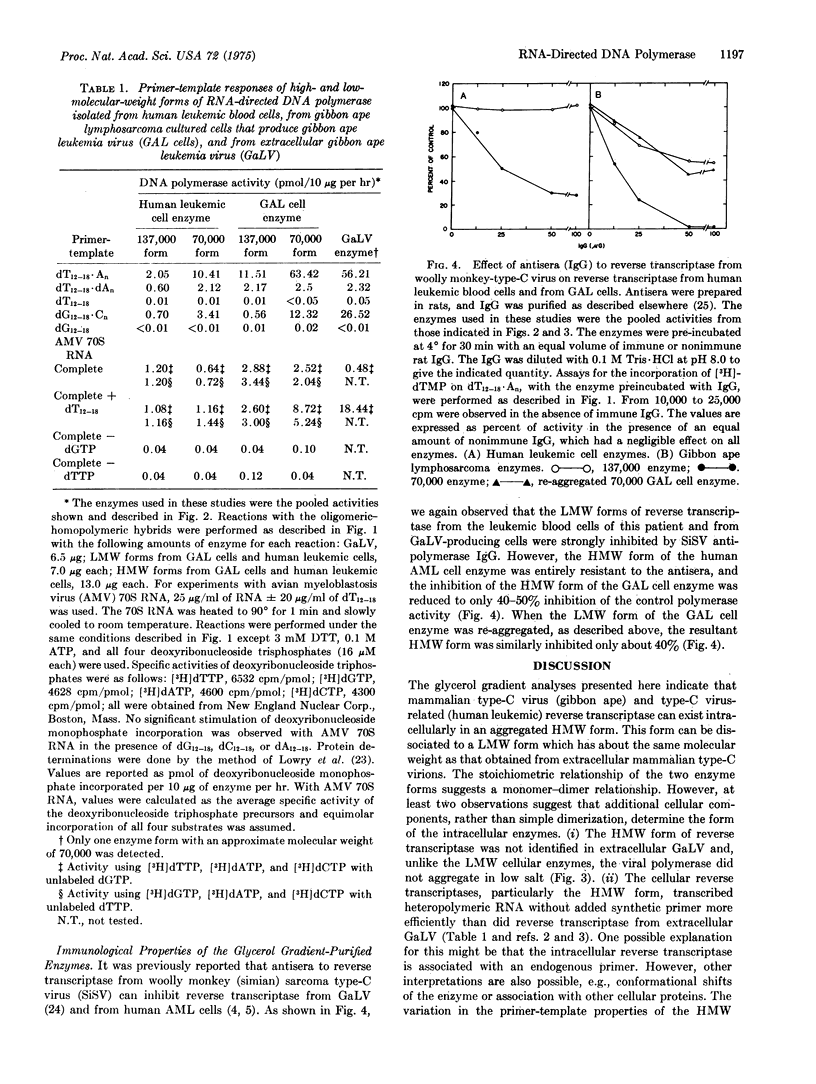
RNA-directed DNA polymerase (reverse transcriptase) from leukocytes of individual leukemic patients can be grouped by velocity gradient analyses into two distinct classes, a low-molecular-weight (LMW) class of approximately 70,000 and a high-molecular-weight (HMW) class of 130,000 to 140,000. The reverse transcriptases from mammalian type-C viruses have with one exception (see text) been isolated as enzymes with molecular weights of 70,000. In this study, the reverse transcriptase from extracellular gibbon ape leukemia virus was also isolated only as the LMW class. However, the enzyme from gibbon virus-producing cells was isolated partially in the HMW form; this form was converted completely to the LMW form by treatment with 0.5 M KC1 and 0.5% Triton X-100 and could be re-converted to the HMW form by lowering the KC1 and Triton X-100 concentrations. A similar conversion from a HMW form to a LMW form was demonstrated with enzyme from human leukemic cells. The LMW form of the human and gibbon ape cellular enzymes utilized synthetic primer-templates in a similar fashion to viral enzyme, and this form was strongly inhibited by antisera (IgG) to reverse transcriptase from simian (woolly monkey) type-C virus. The HMW form of both enzymes utilized synthetic primer-templates less efficiently than the LMW form, and was resistant to inhibition by antipolymerase IgG of simian type-C virus. The HMW form of the cellular reverse transcriptases transcribed viral 70S RNA in the absence of synthetic primer relatively more efficiently than did the extracellular viral form. These data suggest that the HMW form is due in part to aggregation of the LMW form and in part to a cellular factor(s) which may affect both the form and function of intracellular reverse transciptase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Gallo R. C. Purification, characterization, and comparison of the DNA polymerases from two primate RNA tumor viruses. J Virol. 1973 Sep;12(3):431–439. doi: 10.1128/jvi.12.3.431-439.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt W., Hehlmann R., Spiegelman S. Human leukaemic cells contain reverse transcriptase associated with a high molecular weight virus-related RNA. Nat New Biol. 1972 Nov 15;240(98):72–75. doi: 10.1038/newbio240072a0. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J., Xuma M., Reitz M., Sarin P. S., Gallo R. C. Utilization of mammalian 70S RNA by a purified reverse transcriptase from human myelocytic leukemic cells. Biochem Biophys Res Commun. 1973 Sep 5;54(1):324–334. doi: 10.1016/0006-291x(73)90926-1. [DOI] [PubMed] [Google Scholar]
- De Paoli A., Johnsen D. O. Granulocytic leukemia in whitehanded gibbons. J Am Vet Med Assoc. 1973 Sep 15;163(6):624–628. [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Gallagher R. E., Mondal H., Miller D. P., Todaro G. J., Gillespie D. H., Gallo R. C. Relatedness of RNA and reverse transcriptase from human acute myelogenous leukemia cells and from RNA tumor viruses. Hamatol Bluttransfus. 1974;14:185–196. [PubMed] [Google Scholar]
- Gallagher R. E., Todaro G. J., Smith R. G., Livingston D. M., Gallo R. C. Relationship between RNA-directed DNA polymerase (reverse transcriptase) from human acute leukemic blood cells and primate type-C viruses. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1309–1313. doi: 10.1073/pnas.71.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Miller N. R., Saxinger W. C., Gillespie D. Primate RNA tumor virus-like DNA synthesized endogenously by RNA-dependent DNA polymerase in virus-like particles from fresh human acute leukemic blood cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3219–3224. doi: 10.1073/pnas.70.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C. Reverse transcriptase and neoplasia. Biomedicine. 1973 Nov;18(6):446–452. [PubMed] [Google Scholar]
- Gallo R. C., Yang S. S., Ting R. C. RNA dependent DNA polymerase of human acute leukaemic cells. Nature. 1970 Dec 5;228(5275):927–929. doi: 10.1038/228927a0. [DOI] [PubMed] [Google Scholar]
- Garrett R. J., Bollum F. J. DNA polymerase activities in agarose column fractions of rat liver cytoplasm. Biochim Biophys Acta. 1973 Jun 8;312(1):45–51. doi: 10.1016/0005-2787(73)90051-8. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. Ribonuclease H: a ubiquitous activity in virions of ribonucleic acid tumor viruses. J Virol. 1972 Dec;10(6):1136–1142. doi: 10.1128/jvi.10.6.1136-1142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Kawakami T. G., Huff S. D., Buckley P. M., Dungworth D. L., Synder S. P., Gilden R. V. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972 Feb 9;235(58):170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Specific serological relationships among partially purified DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and avian cells. J Virol. 1974 May;13(5):1020–1029. doi: 10.1128/jvi.13.5.1020-1029.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick D. H., Gallo R. C. Correlation of transfer RNA methylase activity with growth and differentiation in normal and neoplastic tissues. Cancer Res. 1970 Oct;30(10):2484–2492. [PubMed] [Google Scholar]
- Sarngadharan M. G., Sarin P. S., Reitz M. S., Gallo R. C. Reverse transcriptase activity of human acute leukaemic cells: purification of the enzyme, response to AMV 70S RNA, and characterization of the DNA product. Nat New Biol. 1972 Nov 15;240(98):67–72. doi: 10.1038/newbio240067a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Todaro G. J. Reverse transcriptases of primate viruses as immunological markers. Science. 1972 Sep 22;177(4054):1119–1121. doi: 10.1126/science.177.4054.1119. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Lieber M. M., Benveniste R. E., Todaro G. J. Endogenous baboon type C virus (M7): biochemical and immunologic characterization. Virology. 1974 Apr;58(2):492–503. doi: 10.1016/0042-6822(74)90083-x. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Gallo R. C. DNA-dependent DNA polymerases I and II from normal human-blood lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2879–2884. doi: 10.1073/pnas.69.10.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Todaro G. J., Gallo R. C. Immunological relationship of DNA polymerase from human acute leukaemia cells and primate and mouse leukaemia virus reverse transcriptase. Nature. 1973 Jul 27;244(5413):206–209. doi: 10.1038/244206a0. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Scolnick E. M., Parks W. P. Reversible inactivation of the deoxyribonucleic acid polymerase of Rauscher leukemia virus. J Virol. 1972 Oct;10(4):885–888. doi: 10.1128/jvi.10.4.885-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Fan H., Baltimore D. Hamster leukemia virus: lack of endogenous DNA synthesis and unique structure of its DNA polymerase. J Virol. 1974 May;13(5):1075–1082. doi: 10.1128/jvi.13.5.1075-1082.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L. G., Deinhardt F., Theilen G. H., Rabin H., Kawakami T., Bustad L. K. Induction of tumors in marmoset monkeys by simian sarcoma virus, type 1 (Lagothrix): a preliminary report. J Natl Cancer Inst. 1971 Nov;47(5):1115–1120. [PubMed] [Google Scholar]


