Abstract
BACKGROUND
Poor sleep quality has been observed in individuals with substance use disorders and is often a trigger for relapse. To date, little research has investigated sleep quality among individuals with prescription opioid (PO) dependence. The present study aimed to address this gap in the literature by examining subjective and objective sleep disturbances among PO dependent individuals.
METHODS
Subjects were 68 non-treatment seeking individuals (33 PO dependent, 35 healthy controls). Subjective sleep was assessed with the Pittsburgh Sleep Quality Index (PSQI) and Insomnia Severity Index (ISI). Subjects were admitted for an overnight inpatient hospital stay during which objective sleep data was collected using an actigraphy device. Self-report pain was measured with the Brief Pain Inventory.
RESULTS
Significant group differences in subjective sleep quality were revealed in the PSQI (p<0.01) and ISI (p<0.01). Poor sleep quality (i.e., PSQI total score > 5) was identified in 80.6% of the PO group, as compared to 8.8% of the control group (p<.001). Significant group differences in sleep quality were identified in five of six actigraphy variables: total time asleep, sleep efficiency, latency of onset of sleep, total time awake and time mobile. Furthermore, significant associations between pain severity and sleep quality were observed.
CONCLUSIONS
Results indicate high rates of sleep impairment and poor sleep quality among PO dependent individuals. Pain severity was significantly correlated with sleep quality. Although preliminary, the findings highlight the importance of assessing and treating sleep disturbances, as well as pain, among patients with PO dependence.
Keywords: Prescription Opioids, Opiates, Sleep, Sleep Disturbance, Actigraphy
1. Introduction
The non-medical use of prescription opioids (PO) is a growing problem in the United States. Data from the National Survey on Drug Use and Health (NSDUH; N = 55,279) showed that 13.6% of respondents endorsed lifetime non-medical PO use and 5.1% endorsed non-medical use in the previous year (Back et al., 2010). Similarly, McCabe and colleagues (2005) found a lifetime prevalence of 12% and past year prevalence of 7% in a nationally representative sample of college students (N = 10,904). Impairment in functioning across a variety of domains (e.g., medical, legal, occupational) is often evident among individuals with PO dependence (Miller, 2004). Additionally, the incidence of emergency room visits, overdoses and unintentional fatalities from non-medical PO misuse have increased significantly over the past two decades (Paulozzi et al., 2006; Strassels, 2009).
Motives for non-medical PO use vary and a significant proportion of individuals report initiating PO use for pain management, but then subsequently using the medication for alternative reasons (Back et al., 2011), such as to improve sleep (Rigg & Ibanez, 2010). Boyd and colleagues (2006) showed in a sample of adolescents (N = 1086) that 12% had engaged in non-medical PO use in the previous year and that of those, over 10% were using POs to aid sleep. Among a sample of adult lifetime non-medical PO users (N = 640) McCabe and colleagues (2007) found that 13.7% used POs to improve sleep.
Poor sleep quality has been observed in individuals with substance use disorders including alcohol (Brower, 2001), nicotine (Jaehne et al., 2009), marijuana (Bolla et al., 2008), and heroin (Hsu et al., 2012) and often serves as a salient trigger for relapse such that substance users reporting poor sleep are at greater risk for relapse and sleep disturbance is predictive of treatment outcome (Brower and Perron, 2010; Wang and Teichtahl, 2007). Sleep problems can persist for weeks and months, and sometimes years, after substance use cessation (Brower, 2003; Peles et al., 2011). One study of 60 alcohol-dependent patients found that poor sleep, specifically sleep latency, was the best predictor of relapse after a 12-week inpatient program (Foster and Peters, 1999). In another study by Brower and colleagues (2001), 60% of alcohol-dependent patients with baseline insomnia had relapsed at 5-months post treatment, as compared to 30% of patients without baseline insomnia. Additionally, significantly higher rates of relapse were observed among patients who endorsed, as compared to those who did not endorse, using alcohol to self-medicate symptoms of insomnia (59.5% vs. 37.8%; Brower et al., 2001).
To date, the research investigating sleep among opioid users has focused on heroin users, primarily in methadone maintenance treatment (MMT) (Sharkey et al., 2011). Stein and colleagues (2004) reported that 83.9% of 225 MMT patients had Pittsburgh Sleep Quality Index (PSQI) scores indicating poor sleep quality (i.e., > 5). In a study of opioid naïve individuals, sleep architecture was significantly altered after a single opioid medication administration, with participants evidencing increases in the percentage of time spent in light sleep stages, and a marked reduction in the percentage of time spent in deep sleep stages (Dimsdale et al., 2007). Multiple mechanisms of action leading to disturbed sleep in those abusing opioids have been theorized, including decreased REM sleep (Lydic and Baghdoyan, 2005), altered GABA functioning (Watson et al., 2007), and lowered levels of adenosine (Trksak et al., 2010). Though sleep has become a focus of substance use research, no known studies to date have utilized actigraphy with a group of current PO dependent individuals. An actigraphy device, usually a watch, collects data about body movement continuously while it is worn thus allowing computer programs to determine sleep-wake cycles (Martin and Hakim, 2011).
The present study aimed to expand the extant literature on the presence and characteristics of sleep impairment among individuals with PO dependence. Specifically, we examined subjective self-report measures as well as actigraphy data collected during an overnight hospital stay. We hypothesized that PO dependent individuals, in comparison to healthy controls, would demonstrate poorer sleep quality, as measured by subjective and objective assessments. In addition, associations between poor sleep quality and pain severity were assessed.
2. Methods
2.1 Participants
Participants (N = 68) were 33 non-treatment seeking individuals with current (i.e., past 6 months) PO dependence and 35 healthy controls participating in a larger study on stress, the hypothalamic-pituitary-adrenal (HPA) axis, and prescription opioids. Participants were recruited primarily through advertisements (e.g., newspapers, Craigslist) and were initially screened over the telephone for study eligibility. A total of 220 participants were invited to the in person baseline assessment. Of these, 70 continued in the study, 79 were deemed ineligible, and 71 dropped out.
Exclusion criteria for all participants included the following: pregnant or nursing; major medical or psychiatric conditions that could interfere with the HPA axis (e.g. depression, PTSD, significant hematological, endocrine, cardiovascular, pulmonary, renal, or neurological disease, including diabetes); use of antihypertensive medications, beta-blockers, synthetic glucocorticoid therapy, or treatment with other agents in the past month that may interfere with the HPA axis response; BMI > 39; younger than 18 years old. Exclusion criteria specific to the PO group included the use of methadone in the past three months and meeting DSM-IV criteria for current substance dependence on other substances. Individuals who met criteria for abuse on other substances had to identify PO as their primary drug of choice. Exclusion criteria specific to the control group included current or lifetime substance dependence (other than nicotine) and abuse (other than past alcohol abuse). No participants were taking sleep medications during the time of the study.
2.2 Procedure
Participants were informed about all study procedures and IRB-approved written informed consent was obtained before any study procedures occurred. Following a preliminary telephone screen, participants came into the office and completed a baseline visit to determine eligibility. The baseline visit consisted of a structured clinical interview to assess substance use disorders and comorbid psychiatric conditions, self-report measures assessing constructs related to opioid dependence including sleep, a urine drug screen and breathalyzer test, and a history and physical examination. Eligible participants (both PO and healthy controls) were scheduled for a one-night hospital stay at the Medical University of South Carolina (MUSC).
Prior to admission for the overnight stay, three days of abstinence from alcohol and other substances, including PO, as evidenced by self-report, breathalyzer, and urine drug screen, were required. Caffeine and nicotine during the three days prior to the overnight stay were allowed. Participants were admitted to the MUSC hospital at 2000h the evening prior to testing to allow for the control of extraneous variables (e.g., sleep, caffeine intake) that could potentially affect stress reactivity. Opiate withdrawal symptoms were assessed at the time of hospital admission using the 10-item self-report Short Opiate Withdrawal Scale (SOWS; Gossop, 1990). Participants with a SOWS score indicating acute withdrawal were rescheduled. Cigarette smokers were provided with a nicotine patch upon admission. Twenty-four hour nicotine replacement therapy was maintained throughout the hospital stay (≥20 cigarettes/day =21 mg patch; 10–19 cigarettes/day =14 mg patch; 5–9 cigarettes/day =7 mg patch). Participants were provided a standard breakfast at 0730h and then escorted by research staff to laboratory for testing. The current study does not include data from the laboratory testing. Participants were compensated $50 for completing the assessment battery and $150 for completing the hospital overnight.
2.3 Measures
2.3.1 Demographic information
Relevant demographic information (e.g., age, gender, employment status) was assessed with a form created for the purposes of this study.
2.3.2 Substance use
Substance use disorders were assessed with the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002). The Timeline Follow-Back (TLFB; Sobell and Sobell, 1992) was used to assess substance use (e.g., PO, heroin, alcohol, marijuana, and cocaine) in the one month prior to the baseline visit. For each substance assessed, two summary variables were generated: 1) percent days used during the past month, and 2) average amount of substance used per day. The Addiction Severity Index, Lite (ASI-Lite; McLellan et al., 1997) assessed areas of functioning impacted by substance use disorders: 1) medical status, 2) employment status, 3) alcohol use, 4) drug use, 5) legal status, 6) family/social status, and 7) psychiatric status. A recent review of subscale scores by Cacciola and colleagues (2011) demonstrated that internal validity scores for the seven subscales ranged from 0.71 (Family/Social problems) to 0.94 (Drugs). On Track Test Cup® (Roche Diagnostics) multi-panel urine drug screen (UDS) test was used to screens for opiates, oxycodone, THC (Marijuana), cocaine, methamphetamines, methadone, and amphetamines.
2.3.3 Sleep
During the overnight hospital stay, subjects wore a Respironics® Actiwatch, which is a small, wristwatch-sized device that collects and stores data at regular intervals during the night (e.g., number of hours in bed, number of times out of bed). The following six summary variables can be calculated using Respironics software: sleep latency (time to fall asleep), total time asleep, sleep efficiency (defined as the ratio of time spent in bed to total time asleep), time mobile, total time awake and number of wake bouts. Actigraphy has been shown to be a valid sleep assessment method for insomnia and other sleep problems (Ancoli-Israel et al., 2003; Vallières and Morin, 2003).
Participants completed psychometrically sound self-report forms assessing sleep, including the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) and the Insomnia Severity Index (ISI; Morin et al., 2011). The PSQI is an instrument that measures sleep quality in seven domains over the past month: 1) subjective sleep quality, 2) latency, 3) sleep duration, 4) habitual sleep efficiency, 5) sleep disturbances, 6) sleep medication and 7) daytime dysfunction. Participants rate each domain on a 0–3 Likert scale, with higher scores indicating greater levels of sleep disturbance. The domain ratings are summed to produce a total score. A total score greater than 5 is indicative of poor sleep quality. The ISI contains 7 items that are rated on a 0–4 Likert scale. The items are summed to produce a total sleep quality rating, with higher scores indicating poorer sleep quality. Total scores of 0–7 indicate no clinically significant insomnia, 8–14 indicate sub-threshold insomnia, 15–21 indicate moderate clinical insomnia, and 22–28 indicate severe clinical insomnia. Cronbach’s alpha for the PSQI and ISI were .82 and .94, respectively.
2.3.4 Pain
The Brief Pain Inventory (BPI; Cleeland & Ryan, 1994) is a 17-item self-report measure used to assess the intensity of pain and the degree to which pain interferes with functioning. Two subscales are generated from the BPI: 1) the pain severity scale, which consists of four items that assess the worst and least amount of pain experienced in the past 24 hours, average pain, and current pain; and 2) the pain interference scale, which consists of nine items that assess whether and how much pain has interfered with functioning (e.g., mood, sleep, ability to walk). The BPI also includes items assessing the location of the pain, and the amount and duration of relief derived from pain treatments. A high degree of internal consistency (Cronbach’s alpha) was evidenced in both the pain severity scale (.94), and pain interference scale (.96).
2.4 Statistical Analyses
Descriptive statistics and measures of central tendency (e.g., means, standard deviations, and frequencies) were used to summarize the demographic characteristics, sleep variables and other assessments. Independent samples t-tests and Pearson’s chi-square tests were used to test differences between the PO and the control group for the PSQI, ISI, and BPI measures. To examine study hypotheses regarding objective sleep quality, a series of ANCOVA analyses were conducted using PROC GLM in SAS Statistical Software. The primary dependent measures were actigraphy outcomes. All models were initially controlled for depression severity, BPI severity, and SOWS scores, however, no covariates were retained in the final model as none controlled for a significant amount of variance in any models. Pearson’s correlation coefficient was used to measure linear relationships between variables. Given the preliminary nature of this study, α=.05 for all analyses.
3. Results
3.1 Demographics
The average age was 34.5 ± 12.0 years (PO group = 36.0 ± 12.5 vs. control group = 33.2 ± 11.5). No significant group differences were observed with regard to age, gender or race. Significant group differences in education, employment, marital and smoking status were revealed (see Table 1).
Table 1.
Demographic and baseline characteristics of prescription opioid (PO) dependent and control participants.
| Demographic characteristics | ||||
|---|---|---|---|---|
| Variable | Total | PO Dependent | Control | p Value |
|
| ||||
| N (%) | N=6568 | n=3133 | n=3435 | |
| Male | 34 (50) | 16 (48.5) | 18(51.4) | 0.81 |
| Caucasian | 54 (79.4) | 26 (78.8) | 28 (80) | 0.55 |
| African American | 6 (8.8) | 2 (6.1) | 4 (11.4) | |
| Hispanic | 3 (4.4) | 2 (6.1) | 1 (2.9) | |
| Other | 5 (7.4) | 3 (9.1) | 2 (5.8) | |
| Some College or More | 56 (82.4) | 22 (66.7) | 34 (97.1) | 0.001 |
| Unemployed | 24 (35.3) | 21 (63.6) | 3 (8.6) | <.001 |
| Not Married | 50 (73.5) | 29 (87.9) | 21 (60) | 0.03 |
| Smoke | 33 (48.5) | 29 (87.9) | 4 (11.4) | <.001 |
| Current Axis I Diagnosis | 20 (29.4) | 17 (51.5) | 3 (8.6) | <.001 |
| Mean ± Std | ||||
| Addiction Severity Index | ||||
| Medical | 0.10 ± .23 | 0.15 ± .29 | 0.06 ± .13 | .084 |
| Employment | 0.30 ± .33 | 0.44 ± .41 | 0.17 ± .12 | <.001 |
| Alcohol | 0.07 ± .08 | 0.07 ± .09 | 0.07 ± .68 | .952 |
| Drugs | 0.11 ± .13 | 0.22 ± .11 | 0.00 ± .00 | <.001 |
| Legal | 0.05 ± .12 | 0.10 ± .17 | 0.00 ± .00 | .001 |
| Family/Social | 0.08 ± .12 | 0.13 ± .15 | 0.04 ± .08 | .003 |
| Psychiatric | 0.09 ± .15 | 0.16 ± .19 | 0.02 ± .04 | <.001 |
| BPI Pain Severity | 1.68 ± 2.21 | 3.16 + 2.37 | 0.32 ± .64 | <.001 |
| BPI Pain Interference | 1.28 ± 2.09 | 2.58 ± 2.43 | 0.09 ± .27 | <.001 |
Note: BPI = Brief Pain Inventory
3.2 Substance use
As can be seen in Table 1, significant group differences were revealed in the majority of the ASI subscales. Specifically, the PO group evidenced greater impairment in the employment, drugs, legal, family/social and psychiatric subscales, as compared to the control group.
The PO group reported an average of 12.6 ± 9.2 years of PO use, with the age of first use at 23.5 years old. PO dependent subjects reported using PO on 64.1% of days in the month prior to baseline screening visit, with an average of 3.0 ± 2.8 pills consumed per day. The average number of days since last opioid use before hospital admission was 6.0 ± 3.6 days. The PO group evidenced some history of alcohol, cocaine, and marijuana use disorders (see Table 2). The PO group reported low SOWS score at the time of hospital admission (8.59 ± 7.46).
Table 2.
Substance use history of prescription opioid (PO) dependent and control participants.
| Demographic characteristics | ||||
|---|---|---|---|---|
| Variable | Total | PO Dependent | Control | p Value |
|
| ||||
| N (%) | N=6568 | n=3133 | n=3435 | |
| History Alcohol Dependence | 13 (19.1) | 13 (39.4) | 0 | <.001 |
| History Alcohol Abuse | 13 (19.1) | 7 (21.2) | 6 (17.1) | .675 |
| History Marijuana Dependence | 9 (13.2) | 9 (27.3) | 0 | .002 |
| History Marijuana Abuse | 5 (7.4) | 5 (15.2) | 1 (2.9) | .018 |
| History Cocaine Dependence | 12 (17.6) | 12 (36.4) | 0 | <.001 |
| History Cocaine Abuse | 1 (1.5) | 1 (3.0) | 0 | .307 |
| History Sedative Dependence | 1 (1.5) | 1 (3.0) | 0 | .307 |
| History Sedative Abuse | 1 (1.5 | 1 (3.0) | 0 | .307 |
3.3 Sleep
Table 3 includes the self-report indices of sleep. Among the PO group, 80.6% reported poor sleep quality (as defined as >5 on the PSQI), compared to 8.8% of the control group (p < .001). Mean PSQI total scores for the PO and control groups were 8.58 ± 3.77 and 2.79 ± 1.59, respectively (p < .001). As shown in Figure 1, PO dependent subjects scored a mean ISI total of 10.21 ± 7.64, compared to the control mean of 2.91 ± 3.74 (p < .001). Sub-threshold insomnia was reported in 41.9% of the PO dependent group, compared to 8.8% of the control group (p<.01). Furthermore, 19.4% of PO dependent subjects reported moderate or severe clinical insomnia. The ISI total score for the total sample correlated positively with the PSQI total score (r = .83, p < .001).
Table 3.
Subjective sleep and actigraphy measurements for prescription opioid (PO) dependent and control participants.
| Subjective Sleep Measurements | Total | PO Dependent | Control | p Value |
|---|---|---|---|---|
|
| ||||
| Mean ± Std | N=6568 | n=3133 | n=3435 | |
| PSQI Total | 5.55 ± 4.10 | 8.58 ± 3.77 | 2.79 ± 1.59 | <.001 |
| ISI Total | 6.51 ± 7.0 | 10.21 ± 7.64 | 2.91 ± 3.74 | <.001 |
| N (%) | ||||
| Poor Sleep Quality (PSQI>5) | 28 (43.1) | 25 (80.6) | 3 (8.8) | <.001 |
| Subthreshold Insomnia (ISI 8–14) | 16 (24.6) | 13 (41.9) | 3 (8.8) | 0.014 |
| Moderate or Severe Clinical Insomnia (ISI>14) | 7 (10.8) | 6 (19.4) | 1 (2.9) | 0.001 |
| Actigraphy Sleep Measurements | ||||
| Mean ± Std | ||||
| Time to Fall Asleep (min) | 39.64 ± 46.90 | 52.09 ± 56.43 | 27.92 ± 32.40 | 0.035 |
| Sleep Efficiency | 77.84 ± 12.59 | 74.69 ± 11.97 | 80.80 ± 12.62 | 0.048 |
| Total Time Asleep (min) | 377.70 ± 54.88 | 360.11 ± 59.11 | 394.26 ± 45.45 | 0.010 |
| # of Wake Bouts | 38.12 ± 16.33 | 40.41 ± 14.30 | 35.97 ± 17.98 | 0.27 |
| Total Time Awake(min) | 38.30 ± 26.27 | 45.72 ± 27.21 | 31.30 ± 23.67 | 0.025 |
| Time Mobile (min) | 33.20 ± 22.45 | 39.57 ± 24.55 | 27.21 ± 18.73 | 0.024 |
Note. PSQI=Pittsburgh Sleep Quality Index, ISI=Insomnia Severity Index
Figure 1.
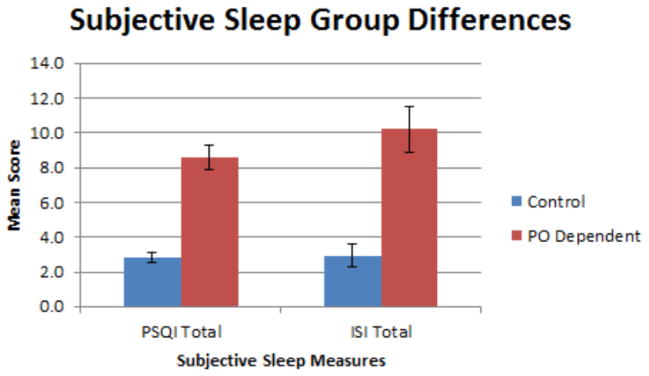
PSQI and ISI subjective sleep measures among PO dependent individuals and controls.
Objective sleep measurements were obtained using an actigraphy device, and the findings revealed significant group differences in five of six sleep variables measured. As shown in Table 3, PO dependent subjects, in comparison to controls, evidenced significantly lower total time asleep, sleep efficiency, greater latency of onset of sleep, total time awake, and time mobile (p < .05).
Correlations between subjective and objective sleep measures were examined. No significant correlations between subjective sleep scores and actigraphy data were observed for PO subjects (r range .04 to .32), controls (r range .04 to .25) or combined groups (r range .03 to .24).
3.4 Pain
Pain severity and pain interference, as assessed using the BPI, were significantly higher in the PO group as compared to the control group (p < .001; see Table 1). Examination of the relationship between subjective sleep and pain revealed significant correlations. The PSQI total score correlated positively with average pain severity (r = .56, p < .01) and pain interference (r = .40, p < .01) for the PO group. Similarly, the ISI total score correlated positively with average pain severity (r = .47, p < .01) and pain interference (r = .46, p < .001) for the PO group. No significant correlations observed for the control group for ISI total score and pain severity (r = .15, p > .05) or pain interference (r = .27, p > 0.5). No significant correlations were revealed between actigraphy data and pain for either the PO group (range .05 to .20, p > .05) or the control group (range .005 to .28, p > .05).
4. Discussion
The association between substance use disorders and poor sleep quality has been well documented, with poor sleep serving as a trigger for relapse. Despite the remarkable rise in the prevalence and deleterious health consequences associated with PO use disorders over the past two decades, little research has examined sleep disturbances among PO dependent individuals. To our knowledge, the current study is the first to report on sleep quality among PO dependent individuals using both objective (actigraphy) and subjective self-report measures. Similar to prior research examining individuals with heroin dependence (Asaad et al., 2011; Sharkey et al., 2011), the current study found significantly poorer objective and subjective quality of sleep among PO dependent individuals in comparison to healthy controls.
Actigraphy data indicated poorer outcomes among PO dependent individuals on five of six sleep indices, including: 1) less total time asleep, 2) longer latency to sleep onset, 3) more total time awake, 4) more time mobile during the night, and 5) sleep efficiency. Similarly, PO dependent individuals exhibited self-reported sleep disturbance (assessed by the PSQI) and sub-threshold insomnia (assessed by the ISI) that were approximately 10 and 4.5 times higher, respectively, than controls. In accordance with these findings, prior research has documented deficits in sleep functioning among other opioid dependent populations, including individuals entering methadone maintenance treatment (Dyer & White, 1997; Puigdollers et al., 2004; Sharkey et al., 2011; Wang et al., 2005), treatment seeking heroin dependent individuals (Burke et al., 2008), and recipients of long-term opioid agonist therapy (Stein et al., 2004; Peles et al., 2006). The importance of continued investigation of the effects of PO dependence on sleep are underscored by data suggesting that the effects of opioids on sleep may be greater than that of other substances of abuse (Casola et al., 2006), and that sleep architecture is negatively affected during each stage of opioid dependence: induction, maintenance, acute abstinence, and protracted abstinence (for review see Wang and Teichtahl, 2007).
Chronic pain is common among individuals with PO dependence and may help explain the association between PO dependence and poor sleep quality. In comparison to controls, PO dependent participants in the current study reported experiencing greater pain severity and pain interference, and their pain was significantly correlated with the PSQI and ISI subjective measures of sleep quality. These findings are consistent with literature demonstrating significant interrelations between chronic pain conditions, sleep disruption, and PO dependence (Onen et al., 2005). Of note, a substantial proportion of opioid dependent individuals report regulation of sleep as a significant motivator for use (Burke et al., 2008; McCabe et al., 2009; Rigg & Ibanez, 2010). Conversely, neurobiological research indicates that use of exogenous opioids that block endogenous opioid peptide receptors are also active in the regulation of sleep, thereby impacting sleep architecture and contributing to increased latency of sleep onset, decreased REM sleep, increased waking bouts, and greater sleep fragmentation (Aghajanian, 1978; Dimsdale et al., 2007; Lord et al., 1977; Wang & Teichtahl, 2007). Research examining the unique contributions of pain versus opioid use on sleep quality is limited. Shaw and colleagues (2005) found that acute administration of opioids to healthy, pain-free, non-dependent adults resulted in significant disruption of sleep architecture, suggesting that opioid use accounts for significant variance in sleep quality even in the absence of chronic pain. Further, both opioid use and increased pain intensity have been implicated in exacerbation of central sleep apneic events, although opioid use contributed significantly more variance to the frequency of apneic events than pain intensity (Jungquist et al., 2012).
Although the current findings document a consistent association between PO dependence and poorer sleep assessed via both objective and subjective methods, subjective measures of sleep were not significantly correlated with objective measures of sleep for either the PO or control group subjects. This pattern of results is consistent with a growing literature documenting discrepancies between objective and subjective measures of sleep quality, as well as differential predictors of sleep quality by method of assessment (Silva et al., 2007; van den Berg et al., 2008). Of note, both modes of sleep assessment have limitations: actigraphy-based data are best interpreted as a proxy assessment of sleep quality rather than a direct measure of the depth or quality of sleep, and self-report data are subject to recall biases (Krystal &Edinger, 2008). Whereas factors such as depressed mood, elevated stress, poor overall health status, and low social support are stronger predictors of self-reported sleep efficiency, factors such as BMI, employment, sleep apnea, medication use, and sleep/wake times are stronger predictors of actigraphy-based assessments of sleep quality (Dhurva et al., 2012; Jackowska et al., 2011; Sharkey et al., 2010; Tworoger et al., 2005). Given the unique limitations associated with each method of sleep assessment, obtaining both subjective and objective data has been recommended (van den Berg et al., 2008).
This study has several limitations that warrant consideration. A relatively small sample size may limit the generalizability of the findings. The study design also necessitated an overnight stay in the hospital, potentially increasing participants’ anxiety. The change of environment and evening routine may have also increased sleep disturbances. The data are cross-sectional in nature and some assessments employed in the study assessed different timeframes (e.g., substance use was assessed over the past 30 days, pain was assessed over the past 24 hours). Therefore, the current study cannot address the temporal order of onset of PO dependence and sleep disruption, or the role of poor sleep quality in maintaining PO dependence. The use of both subjective and objective assessments of sleep is a notable strength of the current study; however, objective measurement of sleep consisted of actigraphy-based data that was limited to a single, in-lab, overnight observation. Although actigraphy is an accepted and commonly used method for sleep assessment and is endorsed for use in community sleep research by the American Academy of Sleep Medicine (Kushida et al., 2001; Lichtenstein et al., 2006; Morgenthaler et al., 2007; Onen et al., 2005), this methodology has limitations. For example, actigraphy may be less accurate in measuring sleep latency and is prone to first night effects. Future research would benefit from the collection of more in-depth sleep quality data utilizing polysomnographic methods that alleviate first night effects and allow for assessment of sleep apneic events (Krystal & Edlinger, 2008; Kurth et al., 2009; Sharkey et al., 2011; Stein et al., 2012). Finally, future research may also be informed by the extension of the assessment timeframe and environment (i.e., to naturalistic home environment).
In summary, the results of the current study indicate marked reductions in sleep quality among non-treatment seeking PO dependent individuals, as well as a significant association between pain and sleep disruption. Given the complex roles that poor sleep quality and chronic pain may play in the initiation, maintenance and relapse to substance use (Brower et al., 2001), the current findings highlight the importance of assessing and treating sleep disturbances as well as pain conditions among PO dependent individuals as these may increase risk for relapse as patients attempt to self-medicate with opioids. Much remains to be investigated in future research, including increased understanding of the discrepancy in self-report and objective measures, the role of chronic PO use in sleep disruption, the role of pain in the maintenance of PO use, and the association of pain and sleep disruption in PO users.
Highlights.
We examined sleep functioning in prescription opioid (PO) dependent individuals and controls.
Subjective sleep quality was reportedly less for the PO group than controls.
As measured by actigraphy, objective sleep quality was less for PO group on 4 of 6 measures.
This significant sleep impairment indicates the need for close assessment and treatment.
Acknowledgments
This work was supported by NIDA grants DA021228 (PI: Back, SE) and T32 DA07272-21.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276(5684):186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Assad TA, Ghanem MH, Abdel Samee AM, El-Habiby MM. Sleep profile in patients with chronic opioid abuse: A polysomnographic evaluation in an Egyptian sample. Addict Disord Their Treat. 2011;10:21–28. [Google Scholar]
- Back SE, Lawson KM, Singleton LM, Brady KT. Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav. 2011;36:829–834. doi: 10.1016/j.addbeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: Findings from the National Survey on Drug Use and Health. Addict Behav. 2010;35:1001–1007. doi: 10.1016/j.addbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, David PM, Verdejo-Garcia A, Benbrook AR. Sleep disturbance in heavy marijuana users. Sleep. 2008;31:901–908. doi: 10.1093/sleep/31.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd CJ, McCabe SE, Cranford JA, Young A. Adolescents’ motivations to abuse prescription medications. Pediatrics. 2006;118:2472–2480. doi: 10.1542/peds.2006-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young Adults. Biol Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower KJ, Perron B. Sleep disturbances as a universal risk factor for relapse in addictions to psychoactive substances. Medical Hypotheses. 2010;74:928–933. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CK, Peirce JM, Kidorf MS, Neubauer D, Punjabi NM, Stoller KB, Brooner RK. Sleep problems reported by patients entering opioid agonist treatment. J Subst Abuse Treat. 2008;35(3):328–333. doi: 10.1016/j.jsat.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, Habing B, McLellan AT. Recent status scores for version 6 of the Addiction Severity Index (ASI-6) Addiction. 2011;106:1588–1602. doi: 10.1111/j.1360-0443.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola PG, Goldsmith RJ, Daiter J, Varenbut M. Sleep in the Substance Using Population. Psychiatric Annals. 2006;36(12):6. [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- Dhruva A, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Dunn LB, Swift PS, Wara W, Miaskowski C. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2012;44(2):215–228. doi: 10.1016/j.jpainsymman.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–36. [PubMed] [Google Scholar]
- Dyer KR, White JM. Patterns of symptom complaints in methadone maintenance patients. Addiction. 1997;92:1445–1455. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders Research Version Patient Edition (SCID-I/P) [Google Scholar]
- Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res. 1999;23:1044–1051. [PubMed] [Google Scholar]
- Gossop M. The development of a short opiate withdrawal scale (SOWS) Addict Behav. 1990;15:487–490. doi: 10.1016/0306-4603(90)90036-w. [DOI] [PubMed] [Google Scholar]
- Hsu W-Y, Chiu N-Y, Liu J-T, Wang C-H, Chang T-G, Liao Y-C, Kuo P-I. Sleep quality in heroin addicts under methadone maintenance treatment. Acta Neuropsychiatrica. 2012 doi: 10.1111/j.1601-5215.2011.00628.x. [DOI] [PubMed] [Google Scholar]
- Jackowska M, Dockray S, Hendrickx H, Steptoe A. Psychosocial factors and sleep efficiency: discrepancies between subjective and objective evaluations of sleep. Psychosom Med. 2011;73:810–816. doi: 10.1097/PSY.0b013e3182359e77. [DOI] [PubMed] [Google Scholar]
- Jaehne A, Loessl B, Bárkai Z, Riemann D, Hornyak M. Effects of nicotine on sleep during consumption, withdrawal and replacement therapy. Sleep Med Rev. 2009;13:363–377. doi: 10.1016/j.smrv.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Jungquist CR, Flannery M, Perlis ML, Grace JT. Relationship of chronic pain and opioid use with respiratory disturbance during sleep. Pain Manag Nurs. 2012;13:70–79. doi: 10.1016/j.pmn.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10–17. doi: 10.1016/S1389-9457(08)70011-X. [DOI] [PubMed] [Google Scholar]
- Kurth ME, Sharkey KM, Millman RP, Corso RP, Stein MD. Insomnia among methadone maintained persons: The feasibility of collecting home PSG recordings. J Addict Dis. 2009;28(3):219–225. doi: 10.1080/10550880903014155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, Lester KW, Aguillard RN. Actigraphy validation with insomnia. Sleep. 2006;29:232–239. [PubMed] [Google Scholar]
- Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Sleep, anesthesiology and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- Martin JL, Hakim AD. Wrist actigraphy. CHEST Postgraduate Education. 2011;139:1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Cranford JA, Teter CJ. Motives for nonmedical use of prescription opioids among high school seniors in the United States: self-treatment and beyond. Arch Pediatr Adolesc Med. 2009;163:739–744. doi: 10.1001/archpediatrics.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Boyd CJ, Teter CJ. Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addict Behav. 2007;32:562–575. doi: 10.1016/j.addbeh.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Knight JR, Wechsler H. Nonmedical use of prescription opioids among U.S. college students: Prevalence and correlates from a national survey. Addict Behav. 2005;30:789–805. doi: 10.1016/j.addbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Cacciola JS, Zanis D. The Addiction Severity Index-“Lite” (ASI-“Lite”) Center for the Studies of Addiction, University of Pennsylvania/Philadelphia VA Medical Center; 1997. [Google Scholar]
- Miller NS. Prescription opiate medications: Medical uses and consequences, laws and controls. Psychiatr Clin North Am. 2004;27:689–708. doi: 10.1016/j.psc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A, Coleman J, Lee-chiong T, Pancer J, Swick TJ. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clin J Pain. 2005;21:422–431. doi: 10.1097/01.ajp.0000129757.31856.f7. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15:618–627. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug Alcohol Depend. 2006;82:103–110. doi: 10.1016/j.drugalcdep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Hamburger RB, Adelson M. No change of sleep after 6 and 12 months of methadone maintenance treatment. J Addict Med. 2011;5:141–147. doi: 10.1097/ADM.0b013e3181e8b6c4. [DOI] [PubMed] [Google Scholar]
- Puigdollers E, Domingo-Salvany A, Brugal MT, Torrens M, Alvaros J, Castillo C, Magri N, Martin S, Vasquez JM. Characteristics of heroin addicts entering methadone maintenance treatment: Quality of life and gender. Subst Use Misuse. 2004;39:1353–1368. doi: 10.1081/ja-120039392. [DOI] [PubMed] [Google Scholar]
- Rigg KK, Ibanez GE. Motivations for non-medical prescription drug use: a mixed methods analysis. J Subst Abuse Treat. 2010;39:236–247. doi: 10.1016/j.jsat.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108:78–83. doi: 10.1016/j.drugalcdep.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Assessing sleep in opioid dependence: A comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug Alcohol Depend. 2011;113:245–248. doi: 10.1016/j.drugalcdep.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw IR, Lavigne G, Mayer P, Choiniere M. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary study. Sleep. 2005;28:677–682. doi: 10.1093/sleep/28.6.677. [DOI] [PubMed] [Google Scholar]
- Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, Walsleben JA, Baldwin CM, Quan SF. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007;3:622–630. [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Stein MD, Herman DS, Bishop S, Lassor JA, Weinstock M, Anthony J, Anderson BJ. Sleep disturbances among methadone maintained patients. J Subst Abuse Treat. 2004;26:175–180. doi: 10.1016/S0740-5472(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Stein MD, Kurth ME, Sharkey KM, Anderson BJ, Corso RP, Millman RP. Trazodone for sleep disturbance during methadone maintenance: a double-blind, placebo-controlled trial. Drug Alcohol Depend. 2012;120:65–73. doi: 10.1016/j.drugalcdep.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassels SA. Economic burden of prescription opioid misuse and abuse. Journal of Managed Care Pharmacy. 2009;15:556–562. doi: 10.18553/jmcp.2009.15.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trksak GH, Jense JE, Plante DT, Penetar DM, Tartarini WL, Maywalt MA, Brendel M, Dorsey CM, Renshaw PF, Lukas SE. Effects of sleep deprivation on sleep homeostasis and restoration during methadone-maintenance: A [31]P MRS brain imaging study. Drug Alcohol Depend. 2010;106:79–91. doi: 10.1016/j.drugalcdep.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tworoger SS, Davis S, Vitiello MV, Lentz MJ, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. J Psychosom Res. 2005;59:11–19. doi: 10.1016/j.jpsychores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Vallières A, Morin CM. Actigraphy in the assessment of insomnia. Sleep. 2003;26:902–906. doi: 10.1093/sleep/26.7.902. [DOI] [PubMed] [Google Scholar]
- Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, Neven AK, Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev. 2007;11:35–46. doi: 10.1016/j.smrv.206.03.006. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H, Drummer O, Goodman C, Cherry G, Cunnington D, Kronbork I. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128:1348–1356. doi: 10.1378/chest.128.3.1348. [DOI] [PubMed] [Google Scholar]
- Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of the rat pontine reticular formation are decreased by local and systematic administration of morphine. Neuroscience. 2007;144:375–386. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


