Abstract
The transformation of byproducts and wastes generated by agro-food companies is of high importance since only a small portion of plant material is utilized directly for human consumption. Squash pumpkin is greatly used in Portugal and as by-products of its processing are generated tons of shell and seeds. In this study we aim to evaluate the potential of these wastes as sources of beneficial and bioactive compounds (antioxidants and antimicrobials), studying the effect of different extraction solvents and drying methods. The samples (fresh and cooked) were freeze-dried and oven-dried followed by extraction with different solvents that revealed the following decreasing order of efficiency: 70 % ethanol, 70 % methanol, 70 % acetone, ultra-pure water and 100 % dichloromethane. The oven-dried samples showed higher values of antioxidant activity and phenolic content, with exception of the values of phenolics for the seeds material. The shell samples presented higher values (1.47 – 70.96 % inhibition) of antioxidant activity and total phenolic content (2.00 – 10.69 mg GAE/g DW). A positive correlation was found between these two parameters on the shell samples, however the squash seeds revealed a negative correlation between the phenolic content and the antioxidant activity. The results show that these industrial agro-food residues are potentially good sources of bioactive compounds with health benefits.
Keywords: Cucurbita pepo, Industrial by-products, Antioxidant activity, Antimicrobial activity, Phenolic acids
Introduction
Cucurbita pepo L. (pumpkin, squash, gourd) is an economically important member of the Cucurbitaceae family and is among the 10 leading vegetable crops worldwide, being the extensively grown in temperate and subtropical regions of the world (Paris 1996; Tadmor et al. 2005). This specie is probably the most polymorphic species with respect to fruit characteristics like size, shape, and color, and nearly all of the domesticates have non-bitter fruit flesh that is thicker, more highly colored, and less fibrous than that of their wild relatives. These domesticate types also have larger and fewer vegetative and reproductive parts (Paris et al. 2003). Pumpkin has received considerable attention in recent years because of the nutritional and health protective value of the seeds that are rich in health beneficial compounds like polysaccharides, carotene, mineral salts, vitamins, and others (Fu et al. 2006; Fu et al. 2007; Zhemerichkin and Ptitchkina 1995). Some previous preliminary reports showed that a pumpkin-rich diet could reduce blood glucose (Li et al. 2001; Zhang and Yao 2002) as well as mixtures of flax and pumpkin seeds supplemented in diet of diabetic rats revealed to be helpful in preventing diabetes and its complications (Makni et al. 2010).
Lately more attention has been focused on the utilization of food-processing byproducts and wastes, as well as underutilized agricultural products. Only a small portion of plant material is utilized directly for human consumption, while the remaining portion of this material or part of it may be converted into nutrients for either food or feedstuff or into fertilizers, so an important contribution to food resources or industrial products could be made (Kamel et al. 1982). After the removal of the pulp and flesh from pumpkin squash still remains a large quantity of shell and seeds as waste products. Pumpkin seeds are often utilized directly for human consumption as snacks after salting and roasting in many Arabian countries and these seeds have been reported as excellent sources of protein (25.2–37 %) and oil (37.8–45.4 %) (Al-Khalifa 1996; Lazos 1986). Several previous reports have described the nutritive value of proteins, fatty acids and vitamins from pumpkin seed oils (Murkovic et al. 1996; Stevenson et al. 2007), but there are very few on the antioxidant properties of pumpkin seeds and shell. These by-products are produced in large quantities by economically important agro-food companies in Portugal.
In this study we aim to evaluate the potential of residues from the industrial processing (squash pumpkin shell and seeds) in order to be used as sources of beneficial compounds like nutraceuticals for functional foods (for humans and animals) evaluating the presence of antibacterial agents, as well as of natural antioxidants and therefore having a high potential of being processed into added value co-products.
Material and methods
Chemicals
All chemicals and reagents were of analytical grade and were obtained from various commercial sources (Sigma/Aldrich, Merck, and Pronalab). All solvents were of high-performance liquid chromatography (HPLC) grade, and water was ultrapure. Gallic acid standard were obtained from Sigma-Aldrich.
Plant material
The samples of squash pumpkin seeds and shell used in the present study were provided from a large company (Douromel - Fábrica de Confeitaria, Lda.) localized in the North of Portugal that processes fruits (orange, lemon, fig, cherry, lemongrass, pear, as a well as pumpkin and squash pulp) in order to produce crystalized fruits. At the present moment this company is processing annually 52 140 kg of squash pumpkin, generating about 18 900 kg of primary product and 63.8 % of residues (squash seeds and shell).
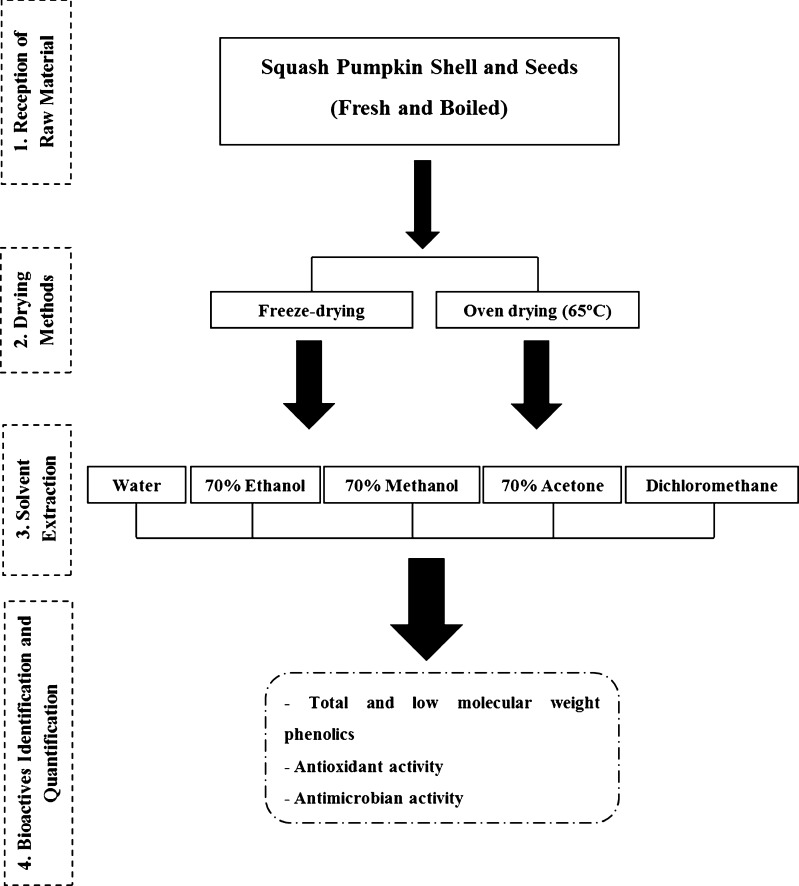
After the reception of the residues from Douromel was first performed the mechanical separation of both types of material (cooked and fresh squash pumpkin seeds and shell), followed by the drying of the samples by two different methods: air-drying in an oven (65 °C) and freeze-drying. These resultant samples of the two drying methods were then powdered and homogenized and later extracted with different solvents (ultra-pure water, 70 % ethanol and 70 % methanol at 70 °C during 30 min, and 70 % Acetone and 100 % dichloromethane at 20 °C during 16 h), in order to evaluate the extraction efficiencies of the different solvents on the samples tested (Fig. 1). These extracts were used for quantification of total phenolics, antioxidant and antimicrobial activities. For the identification of low molecular weight phenolics extracts were analyzed with HPLC (High Performance Liquid Chromatography).
Fig. 1.
Processing scheme of the pumpkin residues after reception from the industry
Extraction and quantification of total and low molecular weight phenolics
Subsamples of the fresh material (3 × 200 mg) were extracted with 5 mL of 70 % methanol at 70 °C for 30 min in 10 mL screw-top tubes, using a vortex mixer every 5 min to optimize extraction. The samples were centrifuged (17,000 g, 4 °C, 20 min), and subsamples of the supernatant (3 × 50 μL) were used for determining total phenolics contents following the Folin-Ciocalteau spectrophotometer method (Javanmardi et al. 2003). For the determination of the low molecular weight phenolics was followed the methods described by Bennett et al. (2006) and it was used an HPLC system from Thermo-Finnigan Surveyor with a Phenomenex Luna C18 (2) 250 × 4.6 mm, 5 μm column and a Phenomenex Security guard C18 pre-column. Data was expressed as Gallic Acid Equivalents (GAE) which was determined by a calibration curve (y = 0.7586x + 0.0694, R2 = 0.9952).
Antioxidant activity assays (DPPH)
The antioxidant activity (AA) was evaluated by 2.2′-difenil-1-picrilhidrazil radical (DPPH) method (Oliveira et al. 2008) with modifications. Briefly, different concentrations (0.0 to 5.0 mg.mL−1) of methanolic extracts were prepared, diluting the extracts previously obtained in methanol 70 % (methanol: water, v/v). After, 500 μL methanolic extracts, were mixed with 3.5 mL of a methanolic solution containing DPPH• radicals (6*10−5 M in methanol 95 %). This mixture was agitated (vortex) and kept in dark up to 1 h, in order to obtain stable values of absorbance. After, free radical scavenging of DPPH• was evaluated by measuring the absorbance at 517 nm, in U.V. Spectrophotometer (U-2000, serial 121–0120. Hitachi Ltd., Japan). The antioxidant activity of each extract was expressed as (%) DPPH scavenging activity, accordingly to the equation:
(ADPPH is the absorbance of free radical DPPH solution and Asample is the absorbance of the solution containing each extract). The Trolox (Sigma) compound was used as positive control. The experiment was carried out in triplicate.
Antimicrobial activity in vitro assays
Microorganisms and culture conditions
Four reference bacteria were tested: Pseudomonas aeruginosa (ATCC10145), Escherichia coli (CECT434), Staphylococcus aureus (CECT976) and Listeria monocytogenes (ATCC15313). These isolates were selected based on their importance in heath, particularly because they are often associated with human diseases and foodborne disease outbreaks. The bacteria strains were grown from pure cultures preserved in Brain Heart Agar (BHA) and incubated at 37 °C in order to obtain fresh cultures for the in vitro tests.
Antimicrobial susceptibility tests
For the antimicrobial assay were used extracts (10 mg/mL) and pure compounds (standards), analogues to the bioactive compounds quantified in each extract. For positive controls were used the commercial antibiotics (Oxoid): Gentamicin (10 μg disc−1) for Gram-negative and Vancomicin (30 μg disc−1) for Gram-positive.
The test of susceptibility (Bauer et al. 1996) was performed using the disk diffusion method on Mueller-Hinton agar plates (Oxoid), accordingly to procedures of CLSI (CLSI 2005). This methodology is based on the principle of diffusion through agar, of the compound with potential antimicrobial properties deposited on a sterile filter paper disc (Oxoid CT0998B). The antibacterial effects of the tested extracts and pure compounds were assessed by the following equation (Aires et al. 2009; Rojas et al. 2006):
RIZD is the percentage of relative inhibition zone diameter and IZD is the inhibition (mm). All tests were done in triplicate and after overnight incubation the diameter in mm of the inhibitory zones around the disc was recorded.
Statistical analysis
JMP for windows version 7.0.1.0 (JMP, Cary, NC, USA) was used for statistical analysis. All experiments were performed in triplicate and the results were presented as the mean ± SEM (standard error of the mean). The data were analysed using One-Way ANOVA. The differences between the mean values were separated using Student’s t test, at significance level of P < 0.05. Also, the Pearson’s correlation was used to correlate the presence of bioactive compounds and the antioxidant activity.
Results and discussion
Total and low molecular weight phenolics
The yield values found for the samples analyzed in this study revealed that the extraction efficiencies of the solvents used were, in decreasing order, as follows: 70 % ethanol, 70 % methanol, 70 % acetone, ultra-pure water and 100 % dichloromethane (data not shown). The total phenolic content (Table 1) found in the samples of squash pumpkin shell and seeds, revealed the highest values for the extraction with water (2.34 – 10.69 mg GAE/g DW) and 70 % ethanol (1.62 – 8.99 mg GAE/g DW), while the 100 % dichloromethane showed the lowest values (0.69 – 3.31 mg GAE/g DW) for pumpkin shell samples. The shell samples presented higher values (2.00 – 10.69 mg GAE/g DW) of total phenolic content than the seeds samples (0.95 – 3.43 mg GAE/g DW), with exception of the values obtained with the extraction solvent 100 % dichloromethane that showed higher values for the seeds samples (1.32 – 6.12 mg GAE/g DW), either fresh or cooked. The values of total phenolics of the pumpkin shell samples revealed higher values in the oven-dried material (water, 70 % Ethanol and 70 % acetone), with exception of the contents found for the cooked samples and extracted with 70 % ethanol and 70 % methanol. Previous reports showed that heat treatments may increase the level of free flavonols (Stewart et al. 2000). On the other hand, regarding the pumpkin seeds there were no significant differences between the two drying methods, with exception to the cooked pumpkin seeds extracted with 70 % ethanol and the fresh seeds extracted with 100 % dichloromethane. A previous study (Turkmen et al. 2005) on the effect of cooking methods on several vegetables revealed a total phenolic content of fresh and cooked squash flesh (8.33 and 4.97 mg GAE/g DW, respectively) that are close to the values found in the present study for fresh and cooked squash (shell and seeds). Another study (Xanthopoulou et al. 2009) presented lower values of total phenolics found in pumpkin oilseeds (0.00005 to 0.01100 mg GAE/g). Other reports (Peschel et al. 2006) on antioxidant compounds of vegetable and fruit wastes extracted with different solvents found higher values of total phenolics content (11.65 to 181.88 mg GAE/g DW), showing greatest efficiency of extraction with the solvents methanol and acetone.
Table 1.
Total phenolics content of fresh and boiled squash pumpkin residues (shell and seeds)
| Material | Drying method | Extraction solvent | ||||
|---|---|---|---|---|---|---|
| Water | 70 % Ethanol | 70 % Methanol | 70 % Acetone | 100 % Dicholomethane | ||
| Shell (fresh) | FD | 7.41 ± 0.49 d, A | 5.71 ± 0.07 c, A | 2.99 ± 0.38 b, A | 6.31 ± 0.22 c, A | 0.86 ± 0.25a, A |
| 65 °C | 10.69 ± 0.37 c, B | 8.99 ± 0.19 c, B | 4.69 ± 0.82 ab, A | 8.69 ± 0.25 bc, B | 3.31 ± 2.73 a, A | |
| Shell (cooked) | FD | 4.37 ± 0.39 c, A | 5.11 ± 0.22 c, B | 2.78 ± 0.09 b, B | 5.19 ± 0.37 c, A | 0.69 ± 0.08 a, A |
| 65 °C | 8.36 ± 5.89 a, A | 4.18 ± 0.08 a, A | 2.00 ± 0.08 a, A | 4.04 ± 0.44 a, A | 0.69 ± 0.02 a, A | |
| Seeds (fresh) | FD | 2.34 ± 0.06 c, A | 1.66 ± 0.05 b, A | 0.97 ± 0.06 a, A | 1.83 ± 0.14 b, A | 6.12 ± 0.22 d, B |
| 65 °C | 2.39 ± 0.19 b, A | 1.62 ± 0.10 ab, A | 0.95 ± 0.10 a, A | 1.41 ± 0.10 ab, A | 3.81 ± 0.76 c, A | |
| Seeds (cooked) | FD | 2.79 ± 0.03 b, A | 2.78 ± 0.12 b, A | 1.70 ± 0.04 a, A | 2.78 ± 0.12 ab, A | 1.96 ± 0.36 a, A |
| 65 °C | 3.43 ± 0.28 c, A | 3.23 ± 0.19 c, B | 1.45 ± 0.08 a, A | 2.64 ± 0.05 b, A | 1.32 ± 0.22 a, A | |
Expressed as mg Gallic Acid Equivalent (GAE) / g DW
Data are expressed as means ± SE of triplicate experiments. Mean values in a row with different letters are significantly different at p < 0.05
The small sized letters compare the values for each row while the cap sized letter compare the values of each sample for each drying method (column – FD and 65 °C)
FD freeze-dried material
The qualitative identification of the low molecular weight phenolics found in the squash pumpkin seeds is visible in Fig. 2 where the main compounds were classified in comparison with previous studies (Pericin et al. 2009). According to this study the main compounds found in the cooked pumpkin seeds extracted with 70 % methanol at 70 °C were p-hydroxybenzoic acid, p-hydroxybenzaldehyde, cafeic acid and trans-p-coumaric acid (Fig. 2). The same previous study reported low values of p-hydroxybenzoic acid, p-hydroxybenzaldehyde and trand-p-coumaric acid (0.02, 0.0012 and 0.001 mg/g DW respectively).
Fig. 2.
High-performance liquid chromatograms (at 280 nm) of free phenolic acids in cooked squash pumpkin seeds extracted with 70 % methanol at 70 °C. Peaks identification: (a) p-hydroxybenzoic acid, (b) p-hydroxybenzaldehyde, (c) caffeic acid and (d) trand-p-coumaric acid
Antioxidant activity (DPPH)
The antioxidant activity results (Table 2) revealed that the most efficient solvent with the highest values was 70 % ethanol (18.92 – 70.96 % inhibition of DPPH radicals), while the 100 % dichloromethane showed the lowest values (1.47 – 4.16 % inhibition of DPPH radicals) for all the samples of shell and seeds of squash pumpkins. The shell samples presented higher values (1.47 – 70.96 % inhibition of DPPH radicals) of antioxidant activity than the seeds samples (1.47 – 49.74 % inhibition of DPPH radicals). Regarding the pumpkin shell samples (fresh and cooked) the values obtained for each solvent tended to be significantly different especially for water, 70 % ethanol and dichloromethane, with exception of the oven-dried (65 °C) fresh shell samples. On the other hand the seed samples values presented no significant differences between the extraction solvents 70 % ethanol and 70 % methanol, while the values found with the extractions with water, 70 % acetone and 100 % dichloromethane were significantly different. When comparing the two drying methods used it was visible higher values on the oven-dried (65 °C) samples (2.65 – 72.36 % inhibition of DPPH radicals) than on the frieze-dried (FD) samples (1.47 – 52.41 % inhibition of DPPH radicals) for almost all samples and extraction solvents. However there were no significant differences in the values obtained with the two drying methods (65 °C and FD) for the samples of fresh pumpkin seeds and the samples of fresh pumpkin shell extracted with 100 % dichloromethane. A previous report (Turkmen et al. 2005) on the effect of cooking methods on different green vegetables revealed antioxidant activity values on fresh and cooked squash flesh (15.80 and 19.70 % inhibition, respectively) that are similar to the values found in this study for the fresh seeds but lower than the values presented for the fresh shell, with exception of the data obtained with extraction made with dichloromethane. On the other hand, in the same study the values presented for the boiled squash flesh are lower than the ones found in our analysis for the cooked samples (shell and seeds). Wu et al. (2004) reported values of total antioxidant capacity in pumpkin flesh higher (4.83 μmol/g TE) than the ones in the present study (0.04 to 0.84 μmol/g TE) for shell and seeds, while other authors (Tuberoso et al. 2007) presented very close values (0.95 μmol/g TE) of antioxidant activity in pumpkin oilseeds. Other previous reports (Peschel et al. 2006) show values of antioxidant activity of several fruits and vegetables wastes extracts revealing similar and higher values (27.41 to 90.03 % inhibition), which reveals that the samples analyzed in the present are also good sources of antioxidant compounds. Another work (Verma et al. 2010) studied the antioxidant properties of Cluster fig revealing values of antioxidant activity within the range (20.36 to 54.81 % inhibition) of the values found for the pumpkin shell samples in the present study.
Table 2.
Antioxidant activity of fresh and boiled squash pumpkin residues (shell and seeds)
| Material | Drying method | Extraction solvent | ||||
|---|---|---|---|---|---|---|
| Water | 70 % Ethanol | 70 % Methanol | 70 % Acetone | 100 % Dicholomethane | ||
| Shell (fresh) | FD | 24.01 ± 0.17 b, A (383.43 ± 1.65) | 52.2 ± 0.73 d, A (655.8 ± 7.11) | 47.5 ± 0.44 c, A (609.1 ± 4.29) | 44.9 ± 1.86 c, A (592.0 ± 17.90) | 3.9 ± 0.73a, A (133.9 ± 7.48) |
| 65 °C | 72.36 ± 1.25 a, B (842.95 ± 12.60) | 71.0 ± 0.97 a, B (830.6 ± 9.72) | 53.1 ± 0.92 b, B (656.8 ± 9.05) | 55.0 ± 2.10 b, B (655.3 ± 21.70) | 2.7 ± 0.72 a, A (106.7 ± 7.48) | |
| Shell (cooked) | FD | 33.91 ± 0.39 b, A (632.48 ± 2.90) | 52.4 ± 0.77 d, A (767.7 ± 5.71) | 45.7 ± 0.99 c, A (709.6 ± 7.48) | 52.1 ± 0.76 d, B (754.9 ± 5.77) | 1.5 ± 0.75 a, A (323.9 ± 6.08) |
| 65 °C | 43.48 ± 0.98 b, B (552.95 ± 9.84) | 58.0 ± 0.83 d, B (701.5 ± 8.34) | 64.5 ± 0.84 e, B (769.6 ± 8.30) | 49.5 ± 0.17 c, A (598.2 ± 1.72) | 4.2 ± 0.04 a, B (122.5 ± 0.48) | |
| Seeds (fresh) | FD | 5.28 ± 0.22 a, A (111.05 ± 2.38) | 18.9 ± 0.49 b, A (333.0 ± 4.76) | 17.0 ± 0.54 b, A (311.5 ± 5.24) | 15.9 ± 4.71 b, A (313.0 ± 45.25) | 4.0 ± 0.37 a, A (134.9 ± 3.78) |
| 65 °C | 14.12 ± 0.11 b, B (118.19 ± 1.26) | 20.5 ± 0.83 c, A (326.3 ± 8.25) | 18.9 ± 0.71 c, A (318.7 ± 7.01) | 12.6 ± 0.08 b, A (216.3 ± 0.82) | 3.8 ± 0.57 a, A (119.1 ± 5.95) | |
| Seeds (cooked) | FD | 12.83 ± 0.28 b, B (476.76 ± 2.08) | 37.4 ± 0.76 d, A (656.8 ± 5.62) | 37.1 ± 1.81 d, A (644.2 ± 13.70) | 31.7 ± 0.39 c, A (599.1 ± 2.97) | 1.5 ± 0.33 a, A (323.9 ± 2.65) |
| 65 °C | 7.51 ± 0.60 b, A (41.05 ± 7.02) | 49.7 ± 0.86 d, B (618.7 ± 8.59) | 47.5 ± 2.14 d, B (602.0 ± 21.41) | 37.0 ± 0.88 c, B (468.7 ± 9.09) | 3.6 ± 0.08 a, B (116.3 ± 0.83) | |
% inhibition of DPPH radicals, the extracts for antioxidant activity were tested at concentration of 5 mg/ml on dry basis
Data are expressed as means ± SE of triplicate experiments. Mean values in a row with different letters are significantly different at p < 0.05. The small sized letters compare the values for each row while the cap sized letter compare the values of each sample for each drying method (column – FD and 65 °C). Data in brackets is expressed as micromoles of Trolox equivalents per gram (μímol of TE/g). FD – Freeze-dried material
Antimicrobial activity in vitro assays
The extracts of the pumpkin shell and seeds revealed in the in vitro antimicrobial assays no inhibition halos, 0.00 mm around the disc impregnated with each of the extracts tested, which means no antibacterial activity against the tested bacterial strains. Regarding the positive controls (antibiotics) the results found (in mm of inhibition halo) for the Gram negative bacteria, using gentamicin, were of 16.00 and 18.00 for the P. aeruginosa (ATCC10145) and E. coli (CECT434), respectively, while for the Gram positive strains the values were of 18.00 and 22.00 for S. aureus (CECT976) and L. monocytogenes (ATCC15313), respectively. For the negative control used, DMSO, the value obtained was of 0.00 mm of inhibition halo. Despite these results, this approach meant to evaluate a possible alternative use of the bio-active compounds present in the industrial by-products, for example in the treatment of bacterial infections and of antibiotic resistant strains. Further studies are necessary with higher concentrations and bacterial isolates.
Correlation analysis
The Pearson correlation matrix between the values obtained of total phenolics and antioxidant activity (Table 3) revealed a higher correlation for the squash pumpkin shell samples especially the fresh dried at 65 °C and the cooked frieze-dried (R = 0.7836, p = 0.0005 and R = 0.8550, p < 0.0001, respectively). These values show that there was a directly proportional relationship between the two data compared, so higher amounts of phenolics mean higher antioxidant activity for these samples. On the other hand the seeds samples showed a much lower correlation or even negative for the seeds samples which may be explained by the presence of other compounds with antioxidant activity like fatty acids, tryacilglycerols and tocopherols (Makni et al. 2008; Stevenson et al. 2007).
Table 3.
Pearson correlation matrix between total phenolics and antioxidant activity of squash pumpkin by-products (shell and seeds)
| DPPH | ||||||||
|---|---|---|---|---|---|---|---|---|
| Shell (Fresh) | Shell (Cooked) | Seeds (Fresh) | Seeds (Cooked) | |||||
| FD | 65 °C | FD | 65 °C | FD | 65 °C | FD | 65 °C | |
| TPC | 0.1456 (0.6047) | 0.7836 (0.0005) | 0.8550 (<0.0001) | 0.6414 (0.0100) | −0.6653 (0.0068) | −0.7233 (0.0023) | 0.3495 (0.2016) | 0.0955 (0.7349) |
Value in brackets denotes P value
DPPH 2,2-diphenyl-1-picrylhydrazyl, TPC total phenolic content
Conclusion
The solvents 70 % ethanol and 70 % acetone were the most efficient in the extraction of total phenolics, also with highest antioxidant activity values. The pumpkin shells showed higher amounts of these two parameters and a positive correlation, which explains that the radicals scavenging capacity found is due to the phenolic content. The lower correlation values present in the seeds might be explained by the presence of other compounds. The oven-dried samples showed higher phenolics and antioxidant activity values, maybe due to increase of bioavailability under high temperatures. This work shows that the residues produced from agro-food industries, like pumpkin shells and seeds are potentially good sources of antioxidant compounds like polyphenols, beneficial for human health. Therefore it is of high interest to develop low cost/effective methods of processing to transform them in added value co-products.
Acknowledgments
The authors acknowledge the company Douromel (Confitaria Lda.) for the kind supply of the pumpkin residues samples. The authors also acknowledge the financial support provided by QREN (Quadro de Referência Estratégico Nacional) to the project Nutridouro “Healthy crystalised fruits and jams with a traditional flavour” (I&DT in Co-Promoção Nº 13111).
Contributor Information
M. J. Saavedra, Email: saavedra@utad.pt
A. Aires, Email: alfredoa@utad.pt
C. Dias, Email: cdias@utad.pt
J. A. Almeida, Email: jaalmeida@utad.pt
M. C. B. M. De Vasconcelos, Email: mcarmov@utad.pt
P. Santos, Email: pilar.santos@douromel.com
E. A. Rosa, Email: erosa@utad.pt
References
- Aires A, Mota VR, Saavedra MJ, Rosa EAS, Bennett RN. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J Appl Microbiol. 2009;106:2086–2095. doi: 10.1111/j.1365-2672.2009.04180.x. [DOI] [PubMed] [Google Scholar]
- Al-Khalifa AS. Physicochemical characteristics, fatty acid composition and lipoxygenase activity of crude pumpkin and melon seed oils. J Agric Food Chem. 1996;44:964–966. doi: 10.1021/jf950519s. [DOI] [Google Scholar]
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1996;45:493–496. [PubMed] [Google Scholar]
- Bennett RN, Rosa EAS, Mellon FA, Kroon PA. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish Rocket) J Agric Food Chem. 2006;54:4005–4015. doi: 10.1021/jf052756t. [DOI] [PubMed] [Google Scholar]
- CLSI . Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline M45-A. Wayne: Clinical and Laboratory Standards Institute; 2005. [Google Scholar]
- Fu CL, Shi H, Li QH. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum Nutr. 2006;61:70–77. doi: 10.1007/s11130-006-0016-6. [DOI] [PubMed] [Google Scholar]
- Fu CL, Tian HJ, Cai TY, Liu Y, Li QH. Some properties of an acidic protein-bound polysaccharide from the fruit of pumpkin. Food Chem. 2007;100:944–947. doi: 10.1016/j.foodchem.2005.10.049. [DOI] [Google Scholar]
- Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83:547–550. doi: 10.1016/S0308-8146(03)00151-1. [DOI] [Google Scholar]
- Kamel BS, Deman JM, Blackman B. Nutritional fatty acid and oil characteristics of different agricultural seed. Food Technol. 1982;17:263–267. doi: 10.1111/j.1365-2621.1982.tb00181.x. [DOI] [Google Scholar]
- Lazos E. Nutritional, fatty acid and oil characteristics of pumpkin and melon seeds. J Food Sci. 1986;51:1382–1383. doi: 10.1111/j.1365-2621.1986.tb13133.x. [DOI] [Google Scholar]
- Li QH, Tian Z, Cai TY. Study on the hypoglycemic action of pumpkin extract in diabetic rat. Acta Nutrimenta Sinica. 2001;25:34–36. [Google Scholar]
- Makni M, Fetoui H, Gargouri NK, Garoui EM, Jaber H, Makni J, Boudawara T, Zeghal N. Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in x-3 and x-6 fatty acids in hypercholesterolemic rats. Food Chem Toxicol. 2008;46:3714–3720. doi: 10.1016/j.fct.2008.09.057. [DOI] [PubMed] [Google Scholar]
- Makni M, Sefi M, Fetoui H, Garoui EM, Gargouri NK, Boudawara T, Zeghal N. Flax and Pumpkin seeds mixture ameliorates diabetic nephropathy in rats. Food Chem Toxicol. 2010;48:2407–2412. doi: 10.1016/j.fct.2010.05.079. [DOI] [PubMed] [Google Scholar]
- Murkovic M, Hillebrand A, Winkler H, Pfannhauser W. Variability of vitamin E content in pumpkin seeds (Cucurbita pepo L.) Z Lebensm Unters Forsch J. 1996;202:275–278. doi: 10.1007/BF01206096. [DOI] [PubMed] [Google Scholar]
- Oliveira I, Sousa A, Morais JS, Ferreira ICFR, Bento A, Estevinho L, Pereira JA. Chemical composition, and antioxidant and antimicrobial activities of three hazelnut (Corylus avellana L.) cultivars. Food Chem Toxicol. 2008;46:1801–1807. doi: 10.1016/j.fct.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Paris HS. Summer squash: history, diversity, and distribution. Hort Tech. 1996;6:6–13. [Google Scholar]
- Paris HS, Yonash N, Portnoy V, Mozes-Daube N, Tzuri G, Katzir N. Assessment of genetic relationships in Cucurbita pepo (Cucurbitaceae) using DNA markers. Theor Appl Genet. 2003;106:971–978. doi: 10.1007/s00122-002-1157-0. [DOI] [PubMed] [Google Scholar]
- Pericin D, Krimer V, Trivic S, Radulovic L. The distribution of phenolic acids in pumpkin’s hull-less seed, skin, oil cake meal, dehulled kernel and hull. Food Chem. 2009;113:450–456. doi: 10.1016/j.foodchem.2008.07.079. [DOI] [Google Scholar]
- Peschel W, Sanchez-Rabaneda F, Diekmann W, Plescher A, Gartzıa I, Jimenez D, Lamuela-Raventos R, Buxaderas S, Codina C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006;97:137–150. doi: 10.1016/j.foodchem.2005.03.033. [DOI] [Google Scholar]
- Rojas J, Ochoa JV, Ocampo SA, Muñoz JF. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: a possible alternative in the treatment of non-nosocomial infections. BMC Compl Alt Med. 2006;6:1–6. doi: 10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson DG, Eller FJ, Wang L, Jane J-L, Wang T, Inglett GE. Oil and Tocopherol content and composition of Pumpkin seed oil in 12 cultivars. J Agric Food Chem. 2007;55:4005–4013. doi: 10.1021/jf0706979. [DOI] [PubMed] [Google Scholar]
- Stewart AJ, Bozonnet S, Mullen W, Jenkins GI, Michael EJ, Crozier A. Occurrence of flavonols in tomatoes and tomato-based products. J Agric Food Chem. 2000;48:2663–2669. doi: 10.1021/jf000070p. [DOI] [PubMed] [Google Scholar]
- Tadmor Y, Paris HS, Meir A, Schaffer AA, Lewinsohn E. Dual role of the pigmentation gene B in affecting carotenoid and vitamin E content in squash (cucurbita pepo) mesocarp. J Agric Food Chem. 2005;53:9759–9763. doi: 10.1021/jf0520591. [DOI] [PubMed] [Google Scholar]
- Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007;103:1494–1501. doi: 10.1016/j.foodchem.2006.08.014. [DOI] [Google Scholar]
- Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–718. doi: 10.1016/j.foodchem.2004.12.038. [DOI] [Google Scholar]
- Verma AR, Vijayakumar M, Rao CV, Mathela CS. In vitro and in vivo antioxidant properties and DNA damage protective activity of green fruit of Ficus glomerata. Food Chem Toxicol. 2010;48:704–709. doi: 10.1016/j.fct.2009.11.052. [DOI] [PubMed] [Google Scholar]
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52:4026–4037. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- Xanthopoulou MN, Nomikos T, Fragopoulou E, Antonopoulou S. Antioxidant and lipoxygenase inhibitory activities of pumpkin seed extracts. Food Res Int. 2009;42:641–646. doi: 10.1016/j.foodres.2009.02.003. [DOI] [Google Scholar]
- Zhang Y, Yao H. Study on effect of hypoglycemia of different type pumpkin. J Chin Food Sci. 2002;23:118–120. [Google Scholar]
- Zhemerichkin DA, Ptitchkina NM. The composition and properties of pumpkin and sugar beet pectins. Food Hydrocolloids. 1995;9:147–149. doi: 10.1016/S0268-005X(09)80277-4. [DOI] [Google Scholar]