Abstract
Fresh frozen tea leaves (Camellia assamica L.) were extracted with SC-CO2 to obtain polyphenols rich in EGCG and compared with conventional solvent extraction. Extraction parameters such as temperature, pressure and solvent to material ratio were critical factors in extraction and optimized by response surface methodology (RSM). The maximum yield of extractable solids using SC-CO2 with ethanol entrainer was carried out at pressures 150 to 350 bar, temperatures from 40 °C to 60 °C and solvent to material ratio 100 to 200. The theoretical yield was 3.91 % (w/w), while experimental yield was 4.20 ± 0.27 % (w/w) at temperature of 50 °C, pressure 250 bar and solvent to material ratio of 200. The chemical compositions of extracted solids were investigated by HPLC which showed 722.68–848.09 ± 1.12 mg of EGCG/g of extractable solids were separated in SC-CO2. Also, 54.62 ± 1.19 mg of EGCG/g of extractable solids was separated using conventional extraction which is quantitatively lesser than SC-CO2 extraction yield. Thus, SC-CO2 extraction was proved to be effective technique in obtaining extracts rich in EGCG (>95 %).
Keywords: Camellia assamica L., Supercritical carbon dioxide extraction (SC-CO2), Response surface methodology (RSM), HPLC
Introduction
Tea belongs to family Camelliaceae and all the cultivated tea plants belongs to two distinct species Camellia sinensis L. and Camellia assamica L. (Sato et al. 2007). Tea is among most popular beverages consumed worldwide. The Assam type (Camellia assamica L.) has a high content of polyphenols, which would make green tea taste excessively bitter (Cabrera et al. 2006). Phenolics are secondary metabolites that are involved in a wide range of specialized physiological functions. Young shoots of green tea contains several polyphenolic compounds and extractable solids upto 30–40 % (Kilmartin and Hsu 2003). Green teas and black teas are processed differently during manufacturing. Fresh green tea leaves, which are very rich in catechins, are not fermented; they are withered, and catechin oxidation by polyphenol oxidase is prevented by steaming (Japan) or by panning (China), it is important process to maintain the polyphenols into their monomeric form (Anesini et al. 2008).
Non-fermented tea has various catechins, caffeine, chlorophyll, amino acids and vitamins etc. (Cabrera et al. 2006). Polyphenols constitute the most interesting group of green tea leaf components, particularly flavonoids (approximately 0.5–1.5 %). Flavonoids present in green tea includes four major catechins as (-)-epigallocatechin-3-gallate (EGCG) (approximately 59 %), (-)-epicatechin-3-gallate (ECG) (approximately 13.6 %), (-)-epigallocatechin (approximately 19 %); and (-)-epicatechin (approximately 6.4 %) (Cabrera et al. 2006). Among these, EGCG a flavanoid phytochemical represents the most abundant catechin in green tea leaves (Ho et al. 1997).
The catechin in green tea leaves have wide pharmacological properties including anticancer and antioxidant activities (Gramza et al. 2005). The strong antioxidant potential of catechins is attributed to EGCG, has a higher chemical and biochemical importance is majorly found in non-fermented tea. In addition, catechins possess anti-mutagenic, anti-diabetic, anti-inflammatory, antibacterial and anti-viral properties (Cabrera et al. 2006). The potential health benefit ascribed to EGCG include anti-carcinogenic properties that functions as antioxidant, preventing oxidative damage in healthy cells, but also as anti-angiogenic agent, preventing tumors by inducing apoptosis in cancerous cells by negatively regulating the cell cycle and strong sulfated effects in cosmetics (Gramza et al. 2005; Vinson et al. 1995). It offers several potential clinical advantages and also regulates disease specific molecular targets for these reasons production of highly pure catechin compounds have become an attractive research material in the recent years (Dale et al. 2006). Catechin plays an important role in the reduction of lipid oxidation and accumulation of cholesterol, and thus they have been suggested to partly counteract atherosclerosis and decrease the risk of other cardiovascular diseases (Muzolf et al. 2008). EGCG potently and specifically inhibits a small number of important molecular targets at concentrations that may be achieved by consuming green tea or EGCG-rich dietary supplements (Carlson et al. 2007). EGCG possesses many potential anti-carcinogenic functions and as an antioxidant serving to neutralize free radicals in the body before they damage cells and was confirmed by the influence of catechins on rat eye (Zhen 2002; Kai et al. 2010).
Catechins are usually isolated by extraction with organic solvents, supercritical fluids and solid phase extraction. Extraction with the organic solvent involves lower selectivity, higher temperature treatment, use of large amount of solvent which may be leads to loss or decrease in activity of polyphenols. Also chemical reactions, usually oxidation and epimerization of catechin compounds, may takes place during extraction and purification. For catechin compounds, the epimerization is of concern during operation process, and the operating temperature and extraction time should be taken into consideration carefully (Wang and Keith 2000). Optimization of extraction condition for catechins and L-theanine using water as extracting solvent has been studied by Vuong et al. (2011a, b). Also, Zhang has used RSM technique to optimize the extraction condition for extraction of epigallocatechin gallate and L-theanine (Zhang et al. 2012). In recent years, solid phase extraction (SPE) involving a molecular imprinted polymer (MISPE) leads to high selectivity, ease of synthesis, low cost for preparation and workability under different conditions, especially with harsh pH and organic solvents (Jin and Row 2007). There are some common problems in SPE as there is incomplete removal of interferences and low recovery of analyte. Supercritical state of an element or compound is above its critical pressure and temperature. At this stage Supercritical carbon dioxide (critical temperature 31.2 °C, critical pressure 73.8 bar) is compressible and possesses both the properties of a gas and liquid, with improved solvating power and has edge over conventional liquids (Rodrguez et al. 2008). Supercritical fluid extraction (SC-CO2) may offer efficient, nontoxic and faster extraction process. SC-CO2 has been successfully used for extraction of polyphenols from plant materials such as leaf and flower (Cheah et al. 2006; Pereira et al. 2010). SC-CO2 extraction of catechins from processed green tea has been reported (Chang et al. 2000; Hyong et al. 2007). A decaffeination of dry and ground green tea leaves with SC-CO2 was done at company NATEX Prozesstechnologie GesmbH (Ternitz, Austria) and furthermore, residue material extracted with water in order to obtain a water-soluble caffeine-free green tea extract enriched in major catechins (Perva et al. 2006).
However, there are no such reports on the extraction of unprocessed green tea leaves and optimization of process parameters using SC-CO2 with ethanol as entrainer. The primary objective of this study was to optimize the SC-CO2 extraction process to maximize yield of extractable solids from green tea leaves by response surface methodology. Response surface methodologies (RSM), a statistical experimental design have been recognized as useful technique to optimize process variables due to its efficient and less data requirement. The present study aims at extraction of unprocessed frozen (−20 °C) green tea leaves using SC-CO2 with ethanol as entrainer, and optimizing the process variables, such as temperature, pressure and solvent material ratio by three-level face-centered central composite design (Praiya et al. 2008). The yield of SC-CO2 extraction was then compared with that of soxtec extraction. The chemical compositions of extracted solids were analyzed, identified and quantified using HPLC gradient type elution system with the help of authentic standards.
Materials and methods
Materials
Fresh green tea leaves (Camellia assamica L.) were procured from M/s Silver Cloud Tea Estate, Gudalore, Tamilnadu (India). After plucking, the leaves were stored at −20 °C within 5 h. Fresh frozen tea leaves were ground in dry ice to an average particle size of 20–60 mesh (BIS) using IKA analytical mill just before extraction. The standard components (-)-Gallocatechingallate (GCG), (-)-Epicatechingallate (ECG), (-)-Epigallocatechingallate (EGCG), (-)- Epicatechin (EC) and (-)-Catechin (C) were purchased from Sigma-Aldrich Company Ltd. (Stein cheim, Germany). Food grade Carbon dioxide (99.0 % pure) was procured from M/s Kiran Corporation, Mysore, India. Absolute ethanol, HPLC grade acetonitrile and acetic acid were purchased from Merck Chemicals, Mumbai.
Supercritical carbon dioxide extraction (SC-CO2)
The samples were extracted on a pilot scale unit, with a high-pressure equipment (NOVA Swiss WERKE AG, EX 1000-1.4-1.2 type, Switzerland) designed for working pressures of up to 1,000 bar and temperature up to 100 °C. SC-CO2 at pressure ranges from 150 to 350 bar and temperature 40–60 °C used as a solvent. The green tea leaves (20–60 mesh) crushed under sub-ambient conditions of about 400 g were loaded into a insert vessel of 1 L capacity equipped with sintered metal plate on both ends, the insert vessel was then introduced into the extraction vessel. To study the influence of the entrainer concentration, extraction was carried out at 250 bar and 50 °C with SC-CO2 along with ethanol at concentration ranging from 1.2 % to 3.6 % (w/w) using dosing HP pump (Milton Roy™ duplex pump, USA) into the extractor system. The average yields of three experiments were taken. After establishing optimized entrainer condition, 17 experiments were carried out in duplicates at temperatures of 40, 50, and 60 °C; pressures of 150, 250, and 350 bar. The extracts were monitored for solvent to material ratio (w/w) at 100, 150, and 200. The flow rate of carbon dioxide was maintained at 1.8–5.5 kg/h. After sometime the programmed time was complete, the extraction vessel was then depressurized and the fractions were collected from the bottom of separation vessel and flash evaporated to remove ethanol and water residue from extracted solids. Each fraction was collected separately after certain interval of time. Then obtained solids were weighed in an analytical balance. All experiments were conducted in duplicates. A more detail description of the equipment is given elsewhere (Uday Sankar 1989; Zarena and Uday Sankar 2009).
Experimental design
Response surface methodology with three-level face-centered central composite design was used to optimize the extraction process of extractable solids from crushed green tea leaves, after determining the preliminary range of the extraction variables through single-factor tests. Three independent variables extraction pressures (X1), extraction temperature (X2), solvent to material ratio (X3) at three levels were performed for statistical calculation; the variables were coded according to:
| 1 |
Where Xi is a coded value of the variable, Ai is the real value of variable, A0 is the real value of the Ai at the center point and ΔA is the step change of the variable. The ranges of independent variables and their levels are given in Table 1. The experimental design involved 17 trials from 3 important variables of the system at 3 levels (Praiya et al. 2008). The three-level face-centered central composite design and the experimental points used are shown in Table 2. Second-order polynomial equation was used to express the yield (response variable, Y) as a function of independent variables (X) and their interactions;
| 2 |
Table 1.
Independent variables and their levels used in the response surface design
| Independent variables | Coded levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: Pressure (bar) | 150 | 250 | 350 |
| X2: Temperature (°C) | 40 | 50 | 60 |
| X3: Solvent: material ratio | 100 | 150 | 200 |
Table 2.
Face-centered central composite design matrix of three variables and the experimentally observed responses values of yield of extractable solids from crushed green tea leaves
| Run | X1/Extraction | X2/Extraction | X3/Solvent to | Experimental | Predicted |
|---|---|---|---|---|---|
| Pressure (bar) | Temperature (°C) | Material ratio | Yield (% w/w) | Yield (% w/w) | |
| 1 | 1 (350) | 1 (60) | 1 (200) | 2.64 ± 0.17 | 2.63 |
| 2 | 1 (350) | 1 (60) | −1 (100) | 0.15 ± 0.03 | 0.13 |
| 3 | 1 (350) | −1 (40) | 1 (200) | 2.31 ± 0.30 | 2.42 |
| 4 | 1 (350) | −1 (40) | −1 (100) | 1.10 ± 0.17 | 0.96 |
| 5 | −1 (150) | 1 (60) | 1 (200) | 1.92 ± 0.15 | 2.07 |
| 6 | −1 (150) | 1 (60) | −1 (100) | 0.70 ± 0.05 | 0.60 |
| 7 | −1 (150) | −1 (40) | 1 (200) | 0.63 ± 0.13 | 0.67 |
| 8 | −1 (150) | −1 (40) | −1 (100) | 0.20 ± 0.02 | 0.23 |
| 9 | 1 (350) | 0 (50) | 0 (150) | 1.88 ± 0.08 | 1.95 |
| 10 | 0 (250) | 1 (60) | 0 (150) | 2.44 ± 0.22 | 2.42 |
| 11 | 0 (250) | 0 (50) | 1 (200) | 4.20 ± 0.27 | 3.91 |
| 12 | −1 (150) | 0 (50) | 0 (150) | 1.42 ± 0.10 | 1.30 |
| 13 | 0 (250) | −1 (40) | 0 (150) | 2.17 ± 0.23 | 2.14 |
| 14 | 0 (250) | 0 (50) | −1 (100) | 2.20 ± 0.11 | 2.44 |
| 15 | 0 (250) | 0 (50) | 0 (150) | 2.90 ± 0.21 | 2.93 |
| 16 | 0 (250) | 0 (50) | 0 (150) | 2.90 ± 0.17 | 2.93 |
| 17 | 0 (250) | 0 (50) | 0 (150) | 2.90 ± 0.56 | 2.93 |
All experiments were carried out in duplicates (n = 2)
Where Y is the predicted response or yield; X1, X2 and X3 are input variables which influence the response variable Y; an is the quadratic coefficients, where n = 0,1,2,3…….,9.
Coefficients for the above equation were determined by employing Kyplot Software Version 2.0 Beta. Analysis of variance (ANOVA) and three-dimensional response plots were obtained using Kyplot software by varying any two of the variables and maintaining the other variable at +1 coded value. Search for optimum yield within the range of variables was carried using SOLVER function of the MS-Excel® software (2007 version).
Soxtec extraction
To compare the yield and components of SC-CO2 with conventional organic solvent extraction, soxtec extraction (Foss Tecator, Sweden) was carried out using absolute ethanol. Crushed green tea leaves (5.0 g) with mesh size of 20–60 were weighed. The material was then extracted by a soxtec extractor (Soxtec™ HT2 system). The soxtec extraction process took about 2 h (1 h boiling and 1 h rinsing), with an extraction temperature of 85 °C. After extraction, the solvent containing extracted solids were filtered through whatmann filter paper no. 1. Extractable-solids yield was calculated from TSS content in the extract after flash evaporation under vacuum using Rota vapor (Buchi-461, Switzerland) and the amount of crushed green tea used for the extraction. The moisture content of the tea leaves determined using azeotropic distillation using toluene (ASTA 1985).
HPLC analysis of green tea extracts
HPLC analysis was carried out for the fractions obtained from SC-CO2 extraction and Soxtech extraction. Separation was performed on HPLC apparatus (Shimadzu, LC10, Japan.) equipped with a reverse phase C18 (15 μ-Diamonsil) column (250 mm × 4.6 mm) and an UV detector monitored at 280 nm. The mobile phase consisted of Solvent A: acetonitrile/acetic acid/water (6:1:193, v: v: v). and Solvent B: acetonitrile/acetic acid/water (60:1:139, v: v: v). Gradient chromatograph was run at 1 ml/min and the injection volume of extract was 20 μl. Identification and quantification of the catechins were determined according to the method of (Liang et al. 2001, 2003).
Examination of leaves microstructure
SEM analysis of grounded tea leaves were done to study the morphological changes in structure of material before and after SC-CO2 extraction. Samples were subjected to the nitrogen flushing to assure the complete removal of moisture. The particles were fixed on a specimen holder with aluminum tape and then sputtered with gold in sputter-coater. All specimens were examined with a scanning electron microscope (Leo Electron Microscope Ltd., Cambridge, UK) under high vacuum condition and at an accelerated voltage of 15 kV (1000× magnification) (Jun et al. 2011; Wang et al. 2011).
Results and discussions
The effect of entrainer concentration on extractable solids yield
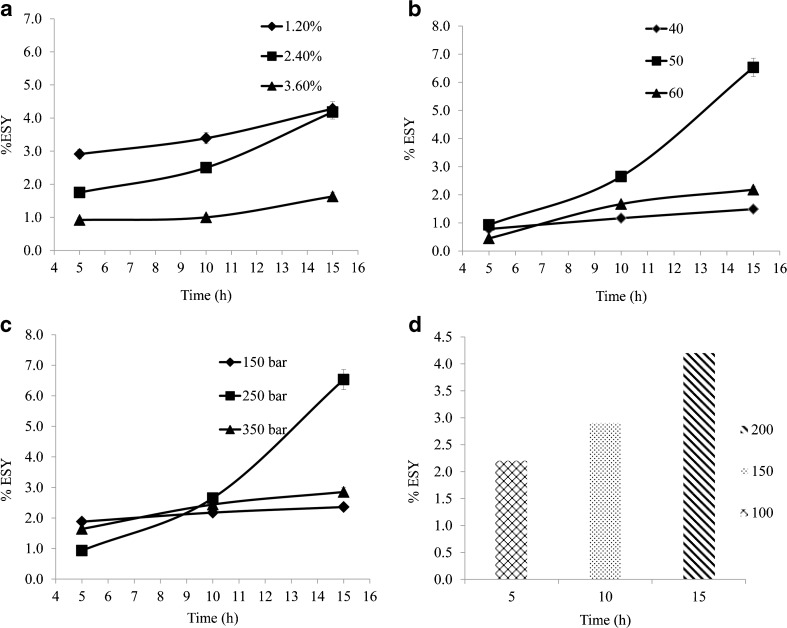
Generally the entrainer concentration is an important parameter in SC-CO2 for extraction of polyphenols. Too much use of entrainer could increase or decrease the density of SC-CO2 which is finally affects on yield of extractable solids. Moreover, entrainer addition always influences the selectivity and solubility of SC-CO2 due to van der waals’ force, H-bond or other interaction formation between entrainer and extractable solids (John et al. 1987). SC-CO2 carbon dioxide is non polar solvent, so as to extract the polar compounds from the crushed green tea leaves, ethanol was pumped into the extractor vessel using dosing pump (Milton Roy TM duplex pump, USA). To study the effect of concentration of ethanol (1.2 to 3.6 %) on yield of extractable solids three experiments were carried out at extraction pressure 250 bar and temperature 50 °C for 15 h. Figure 1a, illustrate that yield of extractable solids is maximum at lower concentration of entrainer (1.2 % of ethanol). 4.20 ± 0.27 % (w/w) yield of extractable solids was obtained at 1.2 % of ethanol concentration in SC-CO2 extraction. Further the yield with time of extraction showed significant differences with entrainer concentration. It was also observed that the moisture in the fresh green tea leaves significantly decreased with extraction time and found to be around 10–15 % after 15 h of extraction. At higher moisture content in the fresh tea leaves there is significant increase in yield of solids with lower ethanol concentration but as the moisture content decreased in the tea leaves, at 2.4 % of ethanol concentration showed an increase in yield is evident from the higher slope of the extraction curve. Similar results were observed on the yield with entrainer in SC-CO2 by Sun et al. (2010). Excess addition of entrainer alters the phase behavior of SC-CO2 leads to decrease in extraction yield of solids. Therefore, 1.2 % of ethanol was determined to be optimal co-solvent concentration in this experiment.
Fig. 1.
Effect of various extraction parameters on the yield of extractable solids. a Extractable solids yield versus time with different entrainer concentration (at constant pressure 250 bar and temperature 50 °C); b Extractable solids yield versus time with different extraction temperatures (at constant pressure 250 bar); c Extractable solids yield versus time with different extraction pressures (at constant temperature 50 °C); d Extractable solids yield versus time with different solvent to material ratio (at constant pressure 250 bar and temperature 50 °C)
The effect of different extraction temperatures on extractable solids yield
Different extraction temperatures 40, 50 and 60 °C were studied at constant pressure of 250 bar to investigate the effect of temperature on the yield of solids. Other operating conditions were set as follows: 400 g crushed green tea leaves (20–60 mesh particle size), 1.2 % of entrainer concentration, extraction pressure 250 bar and 15 h of extraction time. Figure 1b, indicates that the yield of extractable solids increased with the increasing extraction temperature until reaching a peak value around 50 °C, but higher temperature had adverse effect on yield of solids. The solubility curve of solute in the SC-CO2 phase at different temperatures evidenced crossover that were balanced by density and vapour pressure effect. So when the density effect is prominent, at a higher temperature, a decrease in the solvent density results in the reduction of dissolving power of the supercritical carbon dioxide (Park et al. 2007). Therefore, 50 °C of SC-CO2 was determined to be the optimal extraction temperature in this experiment.
The effect of different extraction pressure on extractable solids yield
Critical pressure of carbon dioxide (≥73 bar) is the main parameter that influences the extraction competence and yield (Pourmortazavi and Hajimirsadeghi 2007). An elevation of the pressure at a given temperature leads to an increase in the fluid density, resulting in an increased solubility of the solute. However, at higher the extraction pressure, higher the dissolution of solute of crushed green tea leaves. Therefore, a suitable extraction pressure should be selected for extraction of crushed green tea leaves.
In present study to investigate the effect of different extraction pressures on yield of extractable solids, extraction pressures were set to 150, 250 and 350 bar. Other extraction circumstances were as: 400 g crushed green tea leaves (20–60 mesh particle size), 1.2 % of entrainer concentration, 15 h of extraction time and extraction temperatures 50 °C. In Fig. 1c, yield of extractable solids increases with increase in extraction pressure from 150 to 250 bar. The increasing extraction pressure may increase diffusivity of the solvent into cells, hence enhancing desorption of the solids from the cells. On the other hand, the results indicate that when the pressure is higher than 250 bar, the yield of extractable solids was decreased. An increase of pressure (≥250 bar) can result in an increase in the fluid density, which alters solute solubility. Gomes studies have indicated that a higher recovery of volatile fractions and a lower recovery of non-volatile fractions are obtained at high pressure (Gomes et al. 2007). It is interesting to control the composition of the extract using intermediate pressure. Therefore, 250 bar was used as an optimum extraction pressure in this work.
The effect of different solvent to material ratio on extractable solids yield
In this work, the effect of ratio of solvent to raw material on yield of extractable solids from crushed green tea leaves was investigated, and the results are illustrated in Fig. 1d. Other extraction condition such as follows: 400 g crushed green tea leaves (20–60 mesh particle size), 1.2 % of co-solvent concentration, pressure 250 bar and temperatures 50 °C. It was found that yield of extractable solids can be increased using higher solvent to material ratio. However, use of large amount of solvent resulted flavonoids content in the extract was unnecessary low (Wei et al. 2009). Therefore, solvent to material ratio of 200 was sufficient for the yield of solids. If higher extraction rate is needed, more amount of solvent should be used (Jin et al. 2002). So use of high ratio of solvent to material (200) could help to achieve high extraction yield extractable solids.
Optimization of the SC-CO2 carbon dioxide extraction process
The objective of the present study was to optimise the operating conditions required to accomplish an efficient yield of extractable solids from crushed green tea leaves. The SC-CO2 extraction conditions (extraction pressure, extraction temperature and solvent to material ratio) of crushed green tea leaves were analysed using RSM. A maximum experimental extraction yield of 4.20 ± 0.27 % was achieved at a temperature of 50 °C, a pressure of 250 bar and a solvent to material ratio of 200.
Statistical analysis and the model fitting
Extractable solids yield obtained from 17 experiments are listed in Table 2. The experimental data were used to calculate the coefficients of the second-order polynomial equation. The application of RSM offered, based on parameter estimates, an empirical relationship between the response variable, and the test variables under consideration. By applying multiple regression analysis on the experimental data, the response variable and the test variables were related by the following second-order polynomial equation:
| 3 |
Where, Y is the yields of extractable solids and X1, X2, X3 are the extraction pressure, the extraction temperature and the solvent to material ratio, respectively. Coefficients for the above equation were determined by employing Kyplot Software Version 2.0 Beta. Analysis of variance (ANOVA) and three-dimensional response plots were obtained using Kyplot software by varying any two of the variables and maintaining the other variables at +1 level.
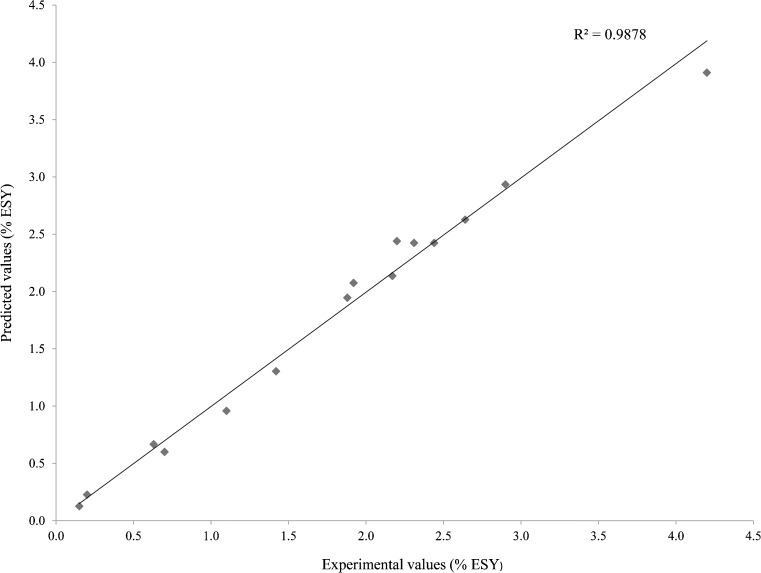
Statistical testing of the model was performed in the form of ANOVA. Tables 3 and 4 shows the test statistics (F-test and P-value) for the green tea extraction model. The determination coefficient (R2 = 0.98), as shown by ANOVA of the quadratic regression model. The value of the adjusted determination coefficient (Adj R2 = 0.97) also confirmed that the model was significant. Figure 2, shows the grant satisfactory predictions within the range of experimental used.
Table 3.
ANOVA table for refined models
| Degrees of freedom | Sum of square | Mean square | F | Significance F | |
|---|---|---|---|---|---|
| Regression | 9 | 19.0952 | 2.1217 | 63.4804 | 7.03E-06* |
| Residual | 7 | 0.2340 | 0.0334 | ||
| Total | 16 | 19.3292 |
*Significant
Table 4.
Regression coefficients of polynomial functions of response surfaces of extractable solids yield
| Coefficients | Standard error | t Stat | P-value | |
|---|---|---|---|---|
| a0 | −21.1051 | 2.7141 | −7.7761 | 0.0001* |
| a1 | 0.0760 | 0.0068 | 11.2418 | 0.0000* |
| a2 | 0.6657 | 0.1147 | 5.8067 | 0.0007* |
| a3 | −0.0529 | 0.0153 | −3.4673 | 0.0104* |
| a4 | −0.0003 | 0.0001 | −4.6607 | 0.0023 |
| a5 | 0.0001 | 0.0000 | 3.9645 | 0.0054 |
| a6 | 0.0005 | 0.0001 | 4.0032 | 0.0052 |
| a7 | −0.0001 | 0.0000 | −11.7169 | 0.0000 |
| a8 | −0.0065 | 0.0011 | −5.8525 | 0.0006 |
| a9 | 0.0001 | 0.0000 | 2.1608 | 0.0675** |
*Significant, **not significant. R2 = 0.98, Adjusted R2 = 0.97
Fig. 2.
Experimental values vs. predicted values for yield of extractable solids
The P-value was used as a tool to check the significance of each coefficient, which indicated a pattern of interactions between the variables. The smaller the P-value, the more significant the corresponding coefficient. Pressure, temperature and solvent to material ratio, had highly significant effects on the yield extractable solids at the 1 % level (P < 0.01). The interactions between pressure and temperature, temperature and solvent to material ratio as well as pressure and solvent to material ratio, had significant effects on the yield extractable solids at the 5 % level (P < 0.05). The ‘lack of fit F-value of 63.48 measured the models’ fit. It illustrates that the model is suitable for analysis of mentioned factors in this experimental work.
Optimization of process parameters for extraction process
The 3D surface plots were obtained using Kyplot Software Version 2.0 Beta and predicting the optimal extraction yield of extractable solids during the SC-CO2 extraction process. Yield extractable solids from crushed green tea leaves were obtained by varying process parameters such as extraction pressure, temperature and solvent to material ratio. The coefficients of determination (R2) 0.9878 indicates a high degree of correlation between observed and predicted values (Fig. 2) thus indicating adequacy of the fitted models.
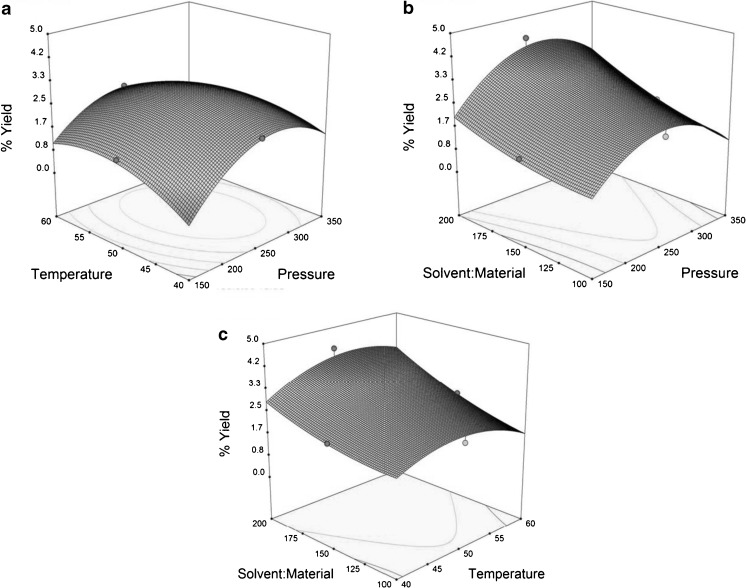
Figure 3a, gives the yield of extractable solids, where solvent to material ratio was kept constant at 0 level, extraction pressure and extraction temperatures are variables. Based on 3D surface plots, it can be seen that extraction pressure has significant effect on yield of extractable solids. But extraction pressure above 250 bar has not much effect on yield of extractable solids. Also Gomes studies indicated that a higher recovery of volatile fractions and a lower recovery of non-volatile fractions are obtained at high pressure (Gomes et al. 2007). In Fig. 3b, extraction temperature was kept constant at 0 level, extraction pressure and solvent to material ratio are variables. Solvent to material ratio along with extraction pressure has shown greater effect on yield of extractable solids from crushed green tea leaves. It can be seen that the extractable solids has greater solubility at extraction pressure 250 bar using the same property at higher solvent to material ratio maximum yield of solids obtained. Wei et al. has studied that use of large amount of solvent in extraction resulted flavonoid content in the extract was unnecessary low (Wei et al. 2009). So a study on use of solvent to material ratio has limited upto 200. In Fig. 3c, extraction pressure was kept constant at 0 level, extraction temperature and solvent to material ratio are variables. Both the parameters have significant effect on yield of extractable solids. As explanation given for effect of solvent to material ratio for Fig. 3b use of solvent to material ratio was limited to 200. A decrease in yield was then observed as extraction temperature above 50 °C this is due to decrease in the solvent density with increase in temperature results in the reduction of dissolving power of the SC-CO2 and was explained by Park et al.(2007).
Fig. 3.
3D surface plots showing the effects of variables (extraction temperature; extraction pressure; solvent to material ratio) on the yield of extractable solids, a 3D surface plot of interaction between extraction pressure and temperature; b 3D surface plot of interaction between solvent to material ratio and extraction pressure; c 3D surface plot of interaction between solvent to material ratio and extraction temperature
Verification of optimized model
The fitness of the model equations for predicting optimum response values was tested under the conditions: extraction pressure 250 bar, extraction temperature 50 °C and solvent to material ratio of 200, which were used to predict the value of responses using the model. An experimental mean value of 4.20 ± 0.27 % (n = 2) was obtained, and the theoretical value was found to be 3.91 %. Although the difference between the theoretical value and experimental value is about 0.29 %, this study has confirmed the validation of the RSM model, indicating that the model is ample for the extraction process.
Comparison of SC-CO2 extraction with soxtec extraction
A comparison study on yield extractable solids was carried out using SC-CO2 extraction and soxtec extraction. The yield of extractable solids was 7.1 ± 0.65 % (w/w) (n = 2) by using Soxtec extraction. The yield of extractable solids in soxtec extraction was found to be elevated than the SC-CO2 extraction method. However, the drawbacks of soxtec extraction were a use of higher extraction temperature leads to oxidation and epimerization of polyphenols, lower selectivity and a more solvent consumption. Also the selective extraction of catechins can be possible using SC-CO2 along with ethanol as an entrainer. Therefore, the SC-CO2 extraction process remains a good choice to obtain solvent free extract from crushed green tea leaves.
Chemical composition of extractable solids
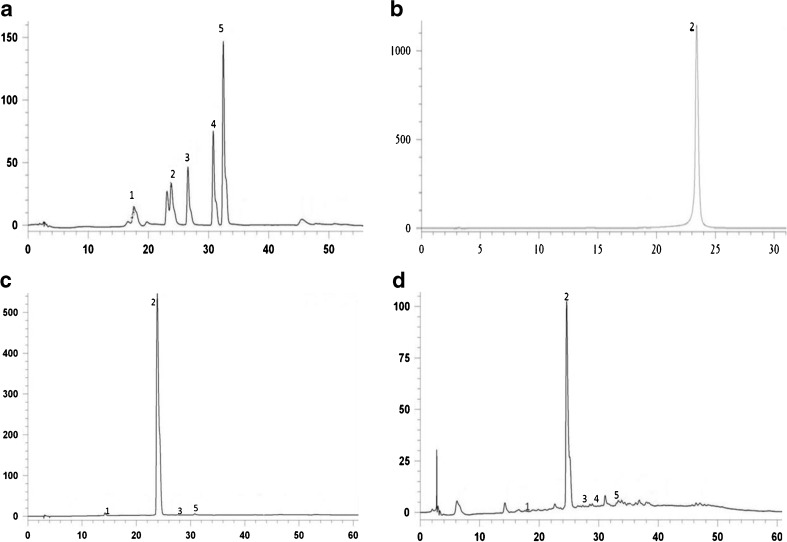
The chemical composition of extracted solids from crushed green tea leaves using both the SC-CO2 and Soxhlet extraction process were studied in gradient elution HPLC system. The chromatograms are obtained as shown in Fig. 4 and results are summarized in Table 5. It was observed that there is a selective separation of EGCG at higher pressures such as 250 bar and 350 bar in SC-CO2 extract (Fig. 4c). The earlier fractions (before 5 h.) collected in SC-CO2 shows some amount of caffeine content but as extraction time increases, in the collected fractions there was selective separation of EGCG in SC-CO2. On the other side, solvent extraction shown poor selective extraction of EGCG because there were other compounds such as pigments and caffeine which interferes in the process of extraction. Also the quantity of EGCG extracted in SC-CO2 is quite high as compare to ethanol extract. So SC-CO2 along with ethanol as an entrainer can be effective solvent system for extraction of EGCG.
Fig. 4.
HPLC chromatograms showing composition of catechins a Standards of catechins,1-Catechin, 2-Epigallocatechin gallate, 3- Epicatechin, 4- Epicatechin gallate, 5- Gallocatechin gallate; b Epigallocatechin gallate; c Fraction obtained at 350 bar, 50 °C in SC-CO2; d Fraction obtained using soxtec extraction
Table 5.
Amount of some catechin compounds in SC-CO2 extracts and Soxtec extract
| Fractions | C | EGCG | EC | ECG | GCG |
|---|---|---|---|---|---|
| 350 bar, 50 °C | 0.03 ± 0.50 | 848.09 ± 1.12 | 3.60 ± 0.78 | ND | 0.11 ± 0.39 |
| 350 bar, 40 °C | ND | 807.27 ± 1.23 | ND | 0.15 ± 0.35 | 0.19 ± 0.89 |
| 250 bar, 60 °C | ND | 811.16 ± 1.23 | 0.57 ± 0.65 | ND | 0.30 ± 0.29 |
| 250 bar, 50 °C | 18.3 ± 0.98 | 722.68 ± 0.48 | ND | ND | 0.68 ± 0.78 |
| Ethanol | 0.16 ± 0.25 | 54.62 ± 1.19 | 0.21 ± 1.54 | 0.52 ± 0.69 | 0.86 ± 0.90 |
ND (Not detectable), Values are expressed as means in mg/g ± SD (n = 2)
SEM analysis of leaves structure
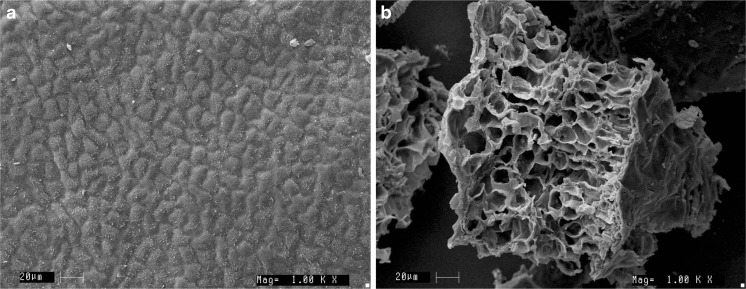
In order to explain the micro mechanism of SC-CO2 extraction of active ingredients from the matrix of the green tea leaves, the microstructure of the unprocessed and SC-CO2 processed samples were examined by SEM (Fig. 5).
Fig. 5.
Scanning electron micrographs of the unprocessed leaves and SC-CO2 processed leaves. a Untreated leaves; b SC-CO2 processed leaves (at pressure 350 bar)
Figure 5 shows the micrographs obtained using SEM of the control/unprocessed sample (A) and the SC-CO2 processed sample (B) respectively. In unprocessed sample, the surface pores of the leaf matrix were closed while in SC-CO2 processed sample, the leaves tissues were found remarkably cracked and pores were opened. Therefore, the observation suggest that SC-CO2 extraction has strongly effect on the structure of tea leaf (tissue, cell wall and organelles) which enhances high mass transfer of the co-solvents into the leaves and thereby release the active compound from the matrix of the leave.
Conclusions
SC-CO2 carbon dioxide extraction of fresh frozen green tea leaves was performed and the effects of variables such as entrainer concentration, extraction pressure, extraction temperature and solvent to material ratio were studied. Considering environmental safety into account, SC-CO2 extraction process is an effective method for extraction of catechins from crushed green tea leaves. Three-level face-centered central composite design was used to screen the key variables and identify the optimization conditions. The polynomial functions that illustrate the effects of three independent variables (extraction pressure, extraction temperature and solvent to material ratio). The optimal experimental yield of extractable solids 4.20 ± 0.27 % was observed using optimum conditions (extraction pressure 250 bar, extraction temperature 50 °C and solvent to material ratio of 200). Extraction pressure, extraction temperature and solvent to material ratio were found to be the most significant factors. Under these optimized conditions, the experimental yield of extractable solids approved closely with the predicted yield. The results suggested that the statistical design methodology offers an efficient and feasible approach for SC-CO2 extraction optimization and also demonstrates that SC-CO2 is a promising alternative over conventional solvent extraction for obtaining EGCG rich catechins from fresh frozen green tea leaves. Also 722.68–848.09 ± 1.12 mg of EGCG/g of extractable solids were separated in SC-CO2 which is quite high and is not possible if any type of conventional methods of extraction. .
Acknowledgments
The authors thank the Director of CFTRI, Mysore. The first author, Mr. Pravin Vasantrao Gadkari acknowledges the CSIR, India for the award of Senior Research Fellowship.
References
- Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis L.) in Argentina. J Agric Food Chem. 2008;56:9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- ASTA . Official analytical method of the American spice trade association. New Jersey: Englewood Clliffes; 1985. p. 3. [Google Scholar]
- Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea-A review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Carlson RJ, Bauer BA, Vincent A, Limburg PJ, Wilson T. Reading the tea leaves: anti-carcinogenic properties of (-)-Epigallocatechin-3-Gallate. Mayo Clin Proc. 2007;82:725–732. doi: 10.1016/S0025-6196(11)61193-2. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Chiu KL, Chen YL, Chang CY. Separation of catechins from green tea using carbon dioxide extraction. Food Chem. 2000;68:109–113. doi: 10.1016/S0308-8146(99)00176-4. [DOI] [Google Scholar]
- Cheah ELC, Chan LW, Heng PWS. Supercritical CO2 and its application in extraction of active principles from plant materials. Asian J Pharm Sci. 2006;1:59–71. doi: 10.1002/jps.3080010123. [DOI] [Google Scholar]
- Dale NG, Ferreira D, Zhou Y. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–1855. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes PB, Mata VG, Rodrigues AE. Production of rose geranium oil using supercritical fluid extraction. J Supercrit Fluids. 2007;41:50–60. doi: 10.1016/j.supflu.2006.08.018. [DOI] [Google Scholar]
- Gramza A, Pawlak-Lemanska K, Korezak J, Wasowicz E, Rudzinska M. Tea extract as radical scavengers. Pol J Environ Stud. 2005;14:861–867. [Google Scholar]
- Ho CT, Chen CW, Wanasundara UN, Shahidi F. Natural antioxidants from tea in natural antioxidants. Champaign: AOCS Press; 1997. pp. 213–223. [Google Scholar]
- Hyong SP, Lee HJ, Shin MH, Lee KW, Lee H, Kim YS, Kim KO, Kim KH. Effect of cosolvent on the decaffeination of green tea by supercritical carbon dioxide. Food Chem. 2007;105:1011–1017. doi: 10.1016/j.foodchem.2007.04.064. [DOI] [Google Scholar]
- Jin Y, Row KH. Solid-phase extraction of caffeine and catechin compounds from green tea by caffeine molecular imprinted polymer. Bull Korean Chem Soc. 2007;28:276–280. doi: 10.5012/bkcs.2007.28.2.276. [DOI] [Google Scholar]
- Jin YH, Han W, Huang SD, Xue BY, Deng X. Microwave-assisted extraction of artemisinin from Artemisia annua L. Sep Purif Technol. 2002;28:191–196. doi: 10.1016/S1383-5866(02)00043-6. [DOI] [Google Scholar]
- John MW, Ikonomou GD, Donohue MD. Supercritical phase behaviour: the entrainer effect. Fluid Phase Equilib. 1987;33:295–814. doi: 10.1016/0378-3812(87)85042-2. [DOI] [Google Scholar]
- Jun X, Shen D, Li Y, Zhang R. Micro mechanism of ultrahigh pressure extraction of active ingredients from green tea leaves. Food Control. 2011;22:1473–1476. doi: 10.1016/j.foodcont.2011.03.008. [DOI] [Google Scholar]
- Kai OC, Chan KP, Wang CC, Chu CY, Li WY, Choy KW, Rogers MS, Pang CP. Green tea catechins and their oxidative protection in the rat eyes. J Agric Food Chem. 2010;58:1523–1534. doi: 10.1021/jf902156k. [DOI] [PubMed] [Google Scholar]
- Kilmartin PA, Hsu CF. Characterization of polyphenols in green, oolong, black teas and in coffee, using cyclic voltammetry. Food Chem. 2003;82:501–512. doi: 10.1016/S0308-8146(03)00066-9. [DOI] [PubMed] [Google Scholar]
- Liang Y, Weiyang M, Jianliang L, Ying W. Comparison of chemical composition of Llex latifolia Thumb and Camellia sinensis. L. Food Chem. 2001;75:339–343. doi: 10.1016/S0308-8146(01)00209-6. [DOI] [Google Scholar]
- Liang Y, Lu L, Zhang L, Wu S, Wu Y. Estimation of black tea quality by analysis of chemical composition and colour difference of tea infusions. Food Chem. 2003;80:283–290. doi: 10.1016/S0308-8146(02)00415-6. [DOI] [Google Scholar]
- Muzolf M, Szymusiak H, Ska-Świglo AG, Rietjens IMCM, Tyrkowska BE. pH-dependent radical scavenging capacity of green tea catechins. J Agric Food Chem. 2008;56:816–823. doi: 10.1021/jf0712189. [DOI] [PubMed] [Google Scholar]
- Park HS, Choi HK, Lee SJ, Park KW, Choi SG, Kim KH. Effect of mass transfer on the removal of caffeine from green tea by supercritical carbon dioxide. J Supercrit Fluids. 2007;42:205–211. doi: 10.1016/j.supflu.2007.03.002. [DOI] [Google Scholar]
- Pereira CG, Angela M, Meireles A. Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic aspects. Food Bioprocess Technol. 2010;3:340–372. doi: 10.1007/s11947-009-0263-2. [DOI] [Google Scholar]
- Perva UA, Skerget M, Knez Z, Weinreich B, Otto F, Gruner S. Extraction of active ingredients from green tea (Camellia sinensis L.): extraction efficiency of major catechins and caffeine. Food Chem. 2006;96:597–605. doi: 10.1016/j.foodchem.2005.03.015. [DOI] [Google Scholar]
- Pourmortazavi SM, Hajimirsadeghi SS. Supercritical fluid extraction in plant essential and volatile oil analysis. J Chromatogr A. 2007;1163:2–24. doi: 10.1016/j.chroma.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Praiya T, Machmudah S, Goto M, Sasaki M, Pavasant P, Shotipruk A. Response surface methodology to supercritical carbondioxide extraction of asthaxanthin from Haematococcus pluvialis. Bioresour Technol. 2008;99:3110–3115. doi: 10.1016/j.biortech.2007.05.062. [DOI] [PubMed] [Google Scholar]
- Rodrguez DG, Antonia MD, Rosa AL, Ferreira Supercritical fluid extraction of polyhalogenated pollutants from aquaculture and marine environmental samples: a review. J Sep Sci. 2008;31:1333–1345. doi: 10.1002/jssc.200700637. [DOI] [PubMed] [Google Scholar]
- Sato D, Ikeda N, Kinoshita T. Home-processing black and green tea (Camellia sinensis L.) Food Saf Technol. 2007;26:1–2. doi: 10.1111/j.1365-2621.1991.tb01136.x. [DOI] [Google Scholar]
- Sun QL, Shu H, Jian HY, Jian LL, Xin QZ, Yue RL. Decaffeination of green tea by supercritical carbon dioxide. J Med Plants Res. 2010;4:1161–1168. [Google Scholar]
- Uday Sankar K. Studies on physicochemical characteristics of volatile oil from papper (Piper nigrum) extracted by supercritical carbon dioxide. J Sci Food Agric. 1989;48:483–493. doi: 10.1002/jsfa.2740480411. [DOI] [Google Scholar]
- Vinson JA, Dabbagh YA, Serry MM, Jan J. Plant flavonols are powerful antioxidants using an in-vitro oxidation model for heart disease. J Agric Food Chem. 1995;43:2800–2802. doi: 10.1021/jf00059a005. [DOI] [Google Scholar]
- Vuong QV, John BG, Costas ES, Min HN, Paul DR. Optimum conditions for the water extraction of L-theanine from green tea. J Sep Sci. 2011;34:2468–2474. doi: 10.1002/jssc.201100401. [DOI] [PubMed] [Google Scholar]
- Vuong QV, John BG, Costas ES, Minh HN, Paul DR. Optimizing conditions for the extraction of catechins from green tea using hot water. J Sep Sci. 2011;34:3099–3106. doi: 10.1002/jssc.201000863. [DOI] [PubMed] [Google Scholar]
- Wang H, Keith H. Epimerisation of catechins in green tea infusions. Food Chem. 2000;70:337–344. doi: 10.1016/S0308-8146(00)00099-6. [DOI] [Google Scholar]
- Wang Y, Xu P, Feng L, Yang X, Qian L. Impact of instantaneous controlled pressure drop on microstructural modification of green tea and its infusion quality. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Tao L, Tang K. Flavonoids from mulberry leaves by microwave-assisted extract and anti-fatigue activity. Afr J Agric Res. 2009;4:898–902. [Google Scholar]
- Zarena AS, Uday Sankar K. Supercritical carbon dioxide extraction of xanthones with antioxidant activity from Garcinia mangostana: characterization by HPLC/LC-ESI-MS. J Supercrit Fluids. 2009;49:330–337. doi: 10.1016/j.supflu.2009.03.004. [DOI] [Google Scholar]
- Zhang X, Fei X, Yuan G, Jing W, Yi S, Xiaoxiong Z. Optimizing the extraction of tea polyphenols, (−)-epigallocatechin gallate and theanine from summer green tea by using response surface methodology. Int J Food Sci Technol. 2012;47:2151–2157. doi: 10.1111/j.1365-2621.2012.03082.x. [DOI] [Google Scholar]
- Zhen YS (2002) Tea bioactivity and therapeutical potential. CRC Press: 1–17