Abstract
Lal peda is a traditional dairy-based sweet, popular in the Indian subcontinent, but it has a poor shelf life. The lal peda samples were packed in polyethylene bags filled with 3 different gaseous compositions (Air, 70 % N2: 30 % CO2 and 98 % N2) and stored at 10 °C. The shelf life was evaluated on the basis of changes in the microbial status of the product such as total plate count, yeast and molds and coliform counts and also the physico-chemical changes such as hydroxy methyl furfural (HMF), thiobarbituric acid (TBA) and free fatty acid (FFA). The microbial spoilage and the indices of chemical changes increased in all the samples with the progression of storage period. The samples packed with air showed significantly higher chemical deterioration and microbial spoilage as compared to the other two combinations. The results showed that samples packed with 70 % N2: 30 % CO2 combination had better shelf stability as compared to the samples packed under air and 98 % N2.
Keywords: Lal peda, Coliforms, Free fatty acid, Hydroxy methyl furfural, Thiobarbituric acid
Introduction
Traditional dairy products of Indian sub-continent have a limited shelf life as many deteriorative changes take place during the storage. Milk sweets during storage undergo several physical, biochemical and microbiological changes making them unfit for human consumption (Londhe et al. 2012). Sharma et al. (2001) studied the shelf life at room and refrigerated temperatures under controlled conditions for danedar khoa, which has a very poor shelf life under ambient conditions. Several previous studies have reported an increase in the shelf life of traditional dairy foods using hurdle technology, water activity changes, increase in sugar content etc. (Kumar and Srinivasan 1982; Biradar et al. 1985; Thakur et al. 1992; Kumar et al. 1997; Sharma et al. 2001), however, not much scientific literature is available on the use of MAP for extending the shelf life of traditional dairy foods except as reported by Biradar et al. (1985) for peda, Rai et al. (2008) and Thippeswamy et al. (2011) for paneer and Londhe et al. (2012) for brown peda.
Lal peda is one of the most popular indigenous heat desiccated dairy products of India, particularly of eastern India. It has been traditionally made on small scale by local sweetmeat makers. It is made from khoa obtained from either cow milk or buffalo milk or combination of both. Lal peda has a characteristic brown-red colour developed during heat processing due to caramelization of khoa along with sugar. In a previous study, Jha et al. (2012) reported the shelf life of lal peda as 9 and 30 days at 37 and 4 °C, respectively. In the present study, the lal peda samples were stored under different combinations of N2 and CO2 (98 % N2: 0 % CO2; 70 % N2: 30 % CO2) and effects on microbial status, textural and physico-chemical attributes were studied during storage.
The shelf life of a product can be altered by changing its composition and form, the environment to which it is exposed, or by the packaging system (Harte and Gray 1987). The deteriorating agents of the food quality are oxygen contained in the air and microorganisms such as bacteria and mold. Oxygen promotes several types of deteriorative reactions in foods including oxidation of fat, browning reactions and pigment oxidation. Most of the common spoilage bacteria and fungi require oxygen for growth. Therefore, to increase the shelf life of foods, the package atmosphere should contain a low concentration of residual oxygen (Sandhya 2010). In order to increase the shelf life of perishable products, different techniques could be applied, among them modified atmosphere packaging (MAP) could be an interesting possibility because of the high demand for additive-free products (Floros and Matsos 2005). Numerous researches related to the MAP technique on different foods have been reported, where chemical preservatives are used to prevent or minimize the microbial growth (Ponte et al. 1993). However, the addition of chemical additives (preservatives, antioxidants, colorants, etc.) has frequently been associated with certain health problems, including allergies, and other more serious illnesses such as the initiation of carcinogenesis (Halliwell et al. 1995).
By using MAP technique, the need of chemical preservatives can be reduced or eliminated while maintaining a desired shelf life for the packaged food product (Fernandez et al. 2006). MAP technique is based on changing the gas combination of packaging of the products. MAP has been used to preserve the freshness of many food products and can improve the food safety under certain conditions (Hotchkiss 1989; Farber et al. 1990). Compared to traditional product packaging methods, MAP offers many key benefits—the most important of which is extending the shelf life (Farber 1991). MAP of foods has proved to be capable of extending the shelf life of many foods by altering the relative proportions of the surrounding atmospheric gases. MAP can bring about changes in the respiration rate, microbial growth, oxidation reactions and thus affects the shelf life of food products (Mangarj and Goswami 2009). CO2, O2 and N2 are normally used in MAP. These gases are tasteless and have peculiar characteristics such as CO2 inhibits the microbial growth as well as insect infestation in packaged stored products and N2 is an inert, tasteless gas mostly used as a filler gas. However, N2 is being insoluble in water is preferred over CO2 as it is resistant to the collapse of packaging material, commonly seen in CO2 packaged products (Ponte et al. 1993; Smith and Simpson 1995; Kotsianis et al. 2002). Generally O2 concentration must be below atmospheric levels (i.e. < 21 % v/v) (Farber 1991; O’Conner et al. 1992). The most important gas from a microbiological stand point is CO2, which effectively inhibits the growth of spoilage bacteria and molds (Hotchkiss 1989).
Materials and methods
The present work was carried out in the laboratory of Centre of Food Science and Technology, Banaras Hindu University, Varanasi, India. Lal peda was manufactured using milk standardized to 6 % fat and 9 % SNF. The Milk was procured from the Dairy Farm, Banaras Hindu University, Varanasi, India. Sugar used for the manufacture of lal peda was procured from the local market of Varanasi, India.
Manufacture of lal peda
Milk was concentrated by heating and continuous stirring, upto 67 % total solids for making khoa. Khoa was then kept for 15 min and cooled to 25 ± 3 °C. Khoa and sugar (35 % of khoa) were taken in a jacketed vessel and heated for desiccation by continuous stirring till a product with reddish brown colour (lal peda) was obtained. Lal peda was cooled to 25 ± 3 °C and was cut into 1.5 × 1.5 cm rectangular pieces of 1 cm height, using a sharp edged knife. Detailed process for the manufacture of lal peda as given by Jha et al. (2012) was followed. The average final moisture content of the product was 15.0 ± 0.12 %.
Packaging and storage of lal peda under MAP
The lal peda samples (100 g) were packed in polyethylene pouches (5 layers, dimensions; 26 × 15 cm of 0.1 mm thickness) using MAP equipment Reepack®, VAC-STAR, S 220 MP, Switzerland and MAP mix 9000 Gas mixer, manufactured by PBI DANSENSOR A/S, Ringstead, Denmark. The 5 layers comprised of an alternate layer of low density polyethylene (LDPE) film with bonding layer. The oxygen transmission rate of the LDPE film was 480 cc/0.001 inch/100 inch2/24 h/atm O2 and the water vapour transmission rate of the LDPE film was 1.2 g/1002 inch/24 h at 90 % RH. The packaging material was procured from Suprabha Protective Products Pvt. Ltd., Pune, (India). Two different combinations of gases i.e. 98 % N2 (with 2 % oxygen impurity) and 70 % N2/30 % CO2 were used to pack the lal peda samples. The samples packed under the air were kept as control. The samples were stored in a BOD incubator (Remi Elektrotechnik Ltd., New Delhi, India) at 10 °C at 60 % relative humidity and were analyzed for microbial, textural and physico-chemical changes at an interval of 10 days.
Microbial analysis
All the samples were subjected to microbiological analysis for total plate count (TPC), yeast and mold count (YMC) and coliform counts. The TPC was determined by surface spreading the homogenate (prepared by macerating the lal peda samples in pestle and mortar) with 10−2 dilution on plate count agar (PCA) and incubated at 37 °C for 24–48 h. For mold and yeast detection, 10−2 dilution of sample was spread on potato dextrose agar (PDA) and incubated at 25 °C for 24–48 h. Coliforms in the samples were estimated by plating 10−2 dilution on Violet Red Bile Agar (VRBA) before being incubated at 37 °C for 24–48 h.
Moisture content
The moisture content in lal peda samples was analyzed using the AOAC (2000) method. It was expressed as % moisture content in lal peda.
Texture profile analysis (TPA)
TPA on lal peda samples was performed by using the texture analyser TA.XT plus, Exponent Lite (Stable Micro Systems, Surrey, UK). TPA was done to characterize the hardness, adhesiveness, springiness, cohesiveness, gumminess and chewiness of the product. The sample of lal peda was cut into size of 1.5 cm length, 1.5 cm in breadth and 1 cm in height and their temperature was maintained at 25 °C during the textural analysis. The sample was subjected to monoaxial compression of 5 mm height. The force distance curve was obtained for a two bite compression cycle with the test speed of 1 mm/sec and trigger force of 5 g. The time gap between the two successive bites of TPA test was 11 s.
Hydroxy methyl furfural (HMF)
The HMF content in lal peda was determined by the method of Keeney and Bassette (1959) with slight modifications. Lal peda (0.5 g) sample was thoroughly mixed with 9.5 mL distilled water. Then, 5.0 mL of 3 N oxalic acid was added and the tubes were kept in boiling water bath for 60 min. The contents of the tubes were cooled and 5 mL of 40 % trichloroacetic acid solution was added. The precipitated mixture was filtered through Whatman No. 42 filter paper. Filtrate (0.5 mL) was pipetted out into a 5 mL test tube followed by 3.5 mL of distilled water and 1 mL of 0.05 M thiobarbituric acid solution (aq.) and was mixed well. Tubes were kept in water bath at 40 °C for 50 min. A blank was run in the same manner as those for the samples, substituting distilled water for lal peda. The samples and standards were measured against the blank at 443 nm (UV-1800, UV Spectrophotometer, Shimadzu Corporation, Kyoto, Japan). A standard curve of HMF concentration and optical density at 443 nm was drawn by using a range of 1.0–10 μmol/mL (0.5 mL aliquot) HMF concentrations.
Thiobarbituric acid (TBA)
The extent of oxidation of fat in lal peda was measured in terms of thiobarbituric acid-reactive substances (TBARS) value. The extraction method of Strange et al. (1977) was adopted with slight modifications. For estimation, 2 g sample was taken and blended with 50 mL of 20 % TCA (trichloroacetic acid) and 50 mL of distilled water. The contents were left undisturbed for 10 min followed by filtration. Further, 5 mL of the filtrate was pipetted out in each test tube followed by 5 mL of 0.01 M 2-thiobarbituric acid. The test tubes were heated in boiling water bath for 30 min at 95 °C for colour development. The contents were then cooled to 30 °C and absorbance was determined at 532 nm (UV-1800, UV Spectrophotometer, Shimadzu corporation, Japan). Blank determinations were made using distilled water in place of sample. TBA value was expressed as absorbance at 532 nm.
Free fatty acid
The method prescribed by Deeth et al. (1975) was used to determine the free fatty acid (FFA) content of lal peda.
Statistical analysis
One-way analysis of variance (ANOVA) was performed as described by Snedecor and Cochran (1989), to test the significance of data in each trial and parameter. All the data was expressed as mean ± standard deviation from three independent experiments.
Results and discussion
Microbial analysis Lal peda
samples were analyzed at an interval of 10 days of storage for microbial changes. The initial population of total plate count and yeasts and mold count was 4.60 and 2.70 log10 cfu/g, respectively. Coliforms were not detected in the samples throughout the study. Microbial counts in different gas combinations are shown in Table 1. In the samples stored under air as packaging atmosphere at 10 °C, microbial activity reached the critical limits within 30–40 days (5.31 log10 cfu/g). Hence, further storage of samples stored with air was discontinued after 30 days.
Table 1.
Microbial changes in lal peda during storage packaged under air and under MAP
| Storage period (days) | Atmosphere | Total plate count (log10 cfu/g) | Yeast and mold count (log10 cfu/g) | Coliform count (log10 cfu/g) |
|---|---|---|---|---|
| 0 | 4.60 ± 0.05 | 2.70 ± 0.03 | N.D. | |
| 10 | Control | 4.63 ± 0.01a | 2.73 ± 0.02a | N.D. |
| 98:: N2 | 4.59 ± 0.01a | 2.64 ± 0.03b | N.D. | |
| 70:30::N2:CO2 | 4.60 ± 0.01a | 2.70 ± 0.05b | N.D. | |
| 20 | Control | 5.23 ± 0.03a | 2.99 ± 0.04a | N.D. |
| 98:: N2 | 4.59 ± 0.01b | 2.74 ± 0.02b | N.D. | |
| 70:30::N2: CO2 | 4.60 ± 0.01b | 2.75 ± 0.03b | N.D. | |
| 30 | Control | 6.38 ± 0.08a | 3.16 ± 0.02a | N.D. |
| 98:: N2 | 4.78 ± 0.02b | 2.85 ± 0.01b | N.D. | |
| 70:30::N2: CO2 | 4.75 ± 0.03b | 2.87 ± 0.02b | N.D. | |
| 40 | 98:: N2 | 5.31 ± 0.03a | 3.01 ± 0.02a | N.D. |
| 70:30::N2: CO2 | 4.85 ± 0.07b | 2.90 ± 0.02b | N.D. | |
| 50 | 98:: N2 | 5.73 ± 0.06a | 3.03 ± 0.03a | N.D. |
| 70:30::N2: CO2 | 5.17 ± 0.03b | 2.92 ± 0.02a | N.D. | |
| 60 | 98:: N2 | 6.36 ± 0.03a | 3.04 ± 0.02a | N.D. |
| 70:30::N2: CO2 | 6.30 ± 0.06a | 2.93 ± 0.01a | N.D. |
Values represents Mean ± S.D of triplicate (n = 3); Values with different superscripts in each storage period group for each trial and parameter are significantly different (p < 0.05)
N. D., not detected, MAP modified atmosphere packaging
In the samples stored with 98 % N2, there was an increase in the TPC and YMC, within 60 days and no coliforms were detected. Critical limits were reached at 60 days of storage. Similarly, when stored in 70 % N2: 30 % CO2 atmosphere package, the total plate count and yeast and mold populations displayed a continuous increase during 60 days of storage period. However, if compared with the samples containing 98 % N2, the growth of the microbial populations was lower in the case of 70 % N2 : 30 % CO2 atmosphere package during the 60 day storage period. As mentioned above, the microbiological quality of the lal peda at the time of packaging was as good as it is possible, without working under sterile conditions. The control sample supported the growth of microbial flora as the gaseous atmosphere was same as air containing oxygen. The samples containing 98 % nitrogen displayed delayed microbial growth as nitrogen is an inert gas which does not support microbial growth. However, as observed, samples with the gas combination of 70 % N2 : 30 % CO2 displayed increased inhibition of microbial growth when compared to samples containing 98 % N2, probably due to the bacteriostatic effect of CO2, as mentioned in several earlier reports (Daniels et al. 1985; Banks and Annis 1990; Davis 1998; Devlieghere and Debevere 2000). Smith et al. (1986) reported that the gas packaged (40 % N2 : 60 % CO2) crusty rolls with the headspace O2 concentration never increased beyond 0.05 % and the rolls remained mold-free even after 60 days.
Changes in the moisture content
It was observed that there was a gradual loss of moisture during the storage period of 60 days, in control samples as well as samples stored with 98 % N2 and 70 % N2 : 30 % CO2 gas composition (Fig. 1a). However, moisture loss was observed the least in the samples stored at 70 % N2: 30 % CO2, during the 60 days storage period. The sensory evaluation (data not shown) of the lal peda samples revealed that samples with moisture content for control range of 12.5–15.5 % is ideal for good texture of the product. Hence, the packages of the samples stored with 70 % N2: 30 % CO2 offered the desired protection against the moisture loss.
Fig. 1.
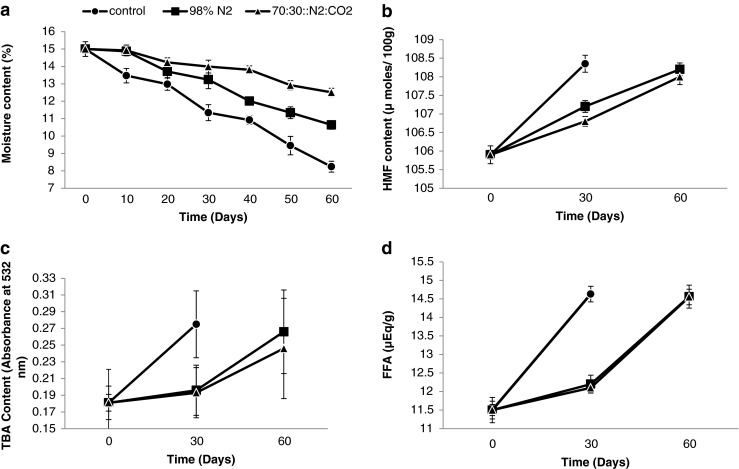
Physico-chemical attributes of the lal peda samples stored under air and under MAP (a) Moisture content (%) (b) HMF (μmoles/100 g) (c) TBA (Absorbance at 532 nm) (d) FFA (μEq/g)
Changes in the textural properties
There was a steep rise in the hardness of the samples stored under air as package atmosphere (Table 2). This could be attributed to the continuous reduction in moisture content or sugar crystallization. This is in accordance with the earlier findings of Gupta et al. (1990), Patel et al. (1990) and Suresh and Jha (1994) who reported that the increased hardness of khoa correlated with the increase in the total solids and that the moisture content of peda had a direct relationship with the hardness.
Table 2.
Textural changes in lal peda during storage under air and under MAP
| Storage period(days) | Atmosphere | Textural attributes | |||||
|---|---|---|---|---|---|---|---|
| Hardness (g) | Adhesiveness (g.sec) | Springiness | Cohesiveness | Gumminess | Chewiness | ||
| 0 | 1432.39 ± 0.77 | −191.73 ± 0.47 | 0.25 ± 0.02 | 0.24 ± 0.01 | 404.19 ± 0.38 | 112. 39 ± 0.27 | |
| 10 | Control | 1937.65 ± 0.21a | −154.15 ± 0.36a | 0.26 ± 0.05a | 0.26 ± 0.09a | 452.34 ± 0.69a | 114.56 ± 0.65a |
| 98:: N2 | 1822.23 ± 0.75b | −167.78 ± 0.29a | 0.25 ± 0.01a | 0.26 ± 0.01a | 692.11 ± 0.02a | 164.09 ± 0.52a | |
| 70:30::N2:CO2 | 1828.73 ± 0.51b | −168.55 ± 0.84a | 0.28 ± 0.07a | 0.25 ± 0.06a | 463.55 ± 0.31a | 132.67 ± 0.62a | |
| 20 | Control | 3094.20 ± 0.59ab | −137.43 ± 0.49ab | 0.31 ± 0.01ab | 0.31 ± 0.08b | 552.54 ± 0.82b | 178.57 ± 0.45a |
| 98:: N2 | 2977.66 ± 0.85ba | −148.39 ± 0.32b | 0.28 ± 0.50b | 0.28 ± 0.07ab | 837.67 ± 0.07ab | 222.82 ± 0.64a | |
| 70:30::N2:CO2 | 2919.69 ± 0.22ab | −155.62 ± 0.62b | 0.29 ± 0.01b | 0.26 ± 0.01ab | 798.49 ± 0.52ab | 208.17 ± 0.90a | |
| 30 | Control | 5906.72 ± 0.88abc | −67.27 ± 0.27ba | 0.31 ± 0.00ab | 0.29 ± 0.02b | 562.69 ± 0.18b | 168.95 ± 0.75a |
| 98:: N2 | 3151.29 ± 0.73bc | −140.99 ± 0.46c | 0.29 ± 0.60b | 0.23 ± 0.007ab | 1102.62 ± 0.87ab | 249.36 ± 0.28a | |
| 70:30::N2:CO2 | 2937.10 ± 0.11ba | −143.92 ± 0.84c | 0.30 ± 0.01b | 0.26 ± 0.002ab | 1216.19 ± 0.82ab | 281.56 ± 0.76a | |
| 40 | 98:: N2 | 3580.94 ± 0.62ab | −117.06 ± 0.15ac | 0.33 ± 0.00ac | 0.25 ± 0.001ab | 503.95 ± 0.36ab | 168.89 ± 0.16a |
| 70:30::N2:CO2 | 3611.99 ± 0.38ab | −134.76 ± 0.14ab | 0.31 ± 0.00ab | 0.25 ± 0.004ab | 956.86 ± 0.67ab | 204.05 ± 0.08a | |
| 50 | 98:: N2 | 4507.10 ± 0.08abc | −89.76 ± 0.30ab | 0.34 ± 0.02ba | 0.26 ± 0.01ab | 890.66 ± 0.01ab | 237.87 ± 0.97a |
| 70:30::N2:CO2 | 4172.59 ± 0.33bca | −126.03 ± 0.43b | 0.39 ± 0.02bc | 0.26 ± 0.01ab | 930.44 ± 0.42ab | 272.98 ± 0.39a | |
| 60 | 98:: N2 | 5865.23 ± 0.14acb | −86.27 ± 0.27ab | 0.38 ± 0.01bc | 0.27 ± 0.01ab | 792.97 ± 0.43ab | 221.35 ± 0.3a |
| 70:30::N2:CO2 | 5678.4 ± 0.34bac | −97.76 ± 0.77b | 0.39 ± 0.01b | 0.26 ± 0.01ab | 992.69 ± 0.43ab | 281.78 ± 0.68a | |
Values are Mean ± S.D., n = 3; Values with different superscripts in each storage period group for each trial and parameter are significantly different (p < 0.05)
MAP modified atmosphere packaging
Adhesiveness is related to the sensory stickiness. It was observed that the adhesiveness for all the three samples displayed a gradual decline. The adhesiveness for the samples ranged between 67.27–191.73, 86.27–191.73 and 97.76–191.73 g.s for samples containing air, 98 % N2 and 70 % N2 : 30 % CO2, respectively (Table 2). The decline in the adhesion could be due to the decrease in free moisture during storage. The adhesiveness of the lal peda samples were found to be higher than that reported by Londhe et al. (2012) in brown peda. The higher adhesiveness values could be attributed to the higher moisture content in the lal peda.
Springiness refers to a food’s ability to return to its original form after compression. It is the height that the sample recovers between the first and second compression. Springiness values of lal peda during storage with air, 98 % N2 and 70 % N2 : 30 % CO2 ranged between 0.25–0.31, 0.25–0.38 and 0.25–0.39, respectively. The lal peda samples in all trials showed an increasing trend in springiness. Current findings were in accordance with Palit (1998). Cohesiveness is the ratio of area under the second bite curve before reversal compression to that under the first bite curve. It is the measure of the extent to which the lal peda structure was disrupted during the first compression. Cohesiveness of the samples packed with air, 98 % N2 and 70 % N2 : 30 % CO2 was in the range of 0.24–0.31, 0.24–0.28 and 0.24–0.26, respectively. Cohesiveness of the product initially increased significantly (p < 0.05) and then decreased insignificantly (p < 0.05) during the storage period (Table 2). However, the cohesiveness of the MAP packed product remained fairly constant throughout the storage. Londhe et al. (2012) have reported that cohesiveness of MAP packed brown peda remained constant upto 20–30 days. Current findings are in accordance with this observation. Loss in moisture content may be responsible for the decrement in the cohesiveness with the progression of storage. Similar findings have been reported by Gupta et al. (1990), where cohesiveness of khoa tended to decline with increasing the total solids.
Gumminess is related to the primary parameters such as hardness and cohesiveness and it is obtained by multiplication of these two parameters. Chewiness refers to the energy required to masticate food into a state ready for swallowing and is a product of gumminess and springiness. The gumminess of the samples followed the same trend as that of cohesiveness. There was a sharp increase in gumminess for all the samples followed by a gradual decline during the storage. Chewiness did not show much variation throughout the storage period.
Changes in the HMF content
HMF is an index of browning in the dairy products. It is more important in the case of lal peda, as the product is recognised and is popular due to its characteristic brown colour and caramelized flavour. The HMF values (μ moles/100 g) of the 3 samples are depicted in Fig. 1b. The HMF content of the samples increased gradually with the storage period. The increase in the HMF content of the sample containing 98 % N2 and 70 % N2 : 30 % CO2 was similar ranging from 105.90108.20 μ moles/100 g to 108.20 μ moles/100 g in the storage period of 60 days. On the other hand, the HMF content of the lal peda stored with air as package atmosphere increased from 105.90 μ moles/100 g to 108.35 μ moles/100 g during the storage period of 30 days. It is evident from Fig. 1b that rate of formation of HMF in the lal peda samples reduced with the alteration of gaseous composition of the package. The control sample showed the highest HMF content during storage. While, the HMF content was lowest in the samples packed with 70 N2 : 30 CO2 of gas. Londhe et al. (2012) studied the effect of packaging techniques on the shelf life of brown peda, a milk-based confection. They reported that during storage, the highest increase in the HMF was in the control samples packaged in cardboard boxes on the 20th day of storage and the lowest content of the HMF was in the MAP packaged (40 % CO2 : 55 % N2 and 60 % CO2 : 40 % N2) samples. Current findings were in agreement with these observations.
Changes in the TBA value
The values of TBA measured in terms of absorbance at 532 nm varied from 0.181 to 0.275 during the 30 day storage period, in the case of samples stored under air as package atmosphere (Fig. 1c). The samples stored in both the modified atmospheric packages displayed slow deteriorative changes as compared to the control samples, with TBA values ranging from 0.181–0.266 to 0.181–0.246 for samples with 98 % N2 and 70 % N2: 30 % CO2 gas composition, respectively. This shows that the packaging of lal peda samples with both the combinations of 70 % N2: 30 % CO2 and 98 % N2 had an inhibitory effect on the Maillard reaction.
Changes in the FFA
FFA is the measure of free fatty acids liberated from the fat globules because of processing or fat degradation occurring prior or after manufacturing. The rate of increase of FFA content was maximum in the samples stored with air as packaging atmosphere, where the FFA increased from an initial value of 11.5 to 14.63 (μ eq/g) during the storage period of 30 days (Fig. 1d). Jha et al. (1977) and Kumar et al. (2010) also reported that FFA in khoa increased significantly with the progression of storage period. The FFA content of the lal peda stored under MAP was in the range of 11.5–14.56 μ eq/g during the storage for 60 days. The reason for the delayed lipolysis of the lal peda during storage under MAP could be attributed to the absence of oxygen in the package during storage. Oxygen is the causative factor for the occurrence of lipid oxidation in foods and in this case, elimination of oxygen from the package reduced the deteriorative changes. Similar findings have been reported by Hong et al. (1995) where lipid oxidation in cheeses was significantly reduced by the removal of oxygen from the package environment.
Conclusion
From the studies carried out, it can be concluded that the shelf life of lal peda can be enhanced significantly by the application of MAP. The critical limits of the microbial load were achieved after the storage of 60 days for the samples stored under MAP as compared to the control samples which was unfit for consumption after 30 days. The study indicates that although both the samples under MAP displayed shelf stability of lal peda upto 60 days, the combination of 70 % N2 : 30 % CO2 depicts higher acceptability in comparison to 98 % N2, thereby making it more desirable for storage. This study also assumes significance for the preservation of many more indigenous dairy foods using MAP and will lead to many more researches on similar lines.
References
- AOAC . Official methods of analysis of AOAC International. 17. Washington: AOAC International; 2000. [Google Scholar]
- Banks HJ, Annis PC. Comparative advantages of high CO2 and low O2 types of controlled atmospheres for grain storage. In: Calderon M, Barkai-Golan R, editors. Food preservation by modified atmospheres. Boca Raton: CRC Press Inc; 1990. pp. 93–122. [Google Scholar]
- Biradar US, Dev DK, Ingle UM. Shelf life extension of pedha by packaging. J Food Sci. 1985;50:51–55. doi: 10.1111/j.1365-2621.1985.tb13275.x. [DOI] [Google Scholar]
- Daniels JA, Krishnamurthi R, Rizvi SSH. A review of effect of carbon dioxide on microbial growth and food quality. J Food Prot. 1985;48:532–537. doi: 10.4315/0362-028X-48.6.532. [DOI] [PubMed] [Google Scholar]
- Davis HK. Fish and shellfish. In: Blakistone BA, editor. Principles and applications of modified atmosphere packaging of foods. 2. London: Blackie Academic & Professional; 1998. pp. 194–239. [Google Scholar]
- Deeth HC, Fitz-Gerald CH, Wood AF. A convenient method for determining the extent of lipolysis in milk. Aus J Dairy Technol. 1975;30:109–111. [Google Scholar]
- Devlieghere F, Debevere J. Influence of dissolved carbon dioxide on the growth of spoilage bacteria. LWT-Food Sci Techol. 2000;33:531–537. doi: 10.1006/fstl.2000.0705. [DOI] [Google Scholar]
- Farber JM. Microbiological aspects of modified-atmosphere packaging technology: a review. J Food Prot. 1991;54:58–70. doi: 10.4315/0362-028X-54.1.58. [DOI] [PubMed] [Google Scholar]
- Farber JM, Warburton DW, Gour L, Milling M. Microbiological quality of foods packaged under modified atmospheres. Food Microbiol. 1990;7:327–334. doi: 10.1016/0740-0020(90)90037-I. [DOI] [Google Scholar]
- Fernandez U, Vodovotz Y, Courtney P, Pascall MA. Extended shelf-life of soy bread using modified atmosphere packaging. J Food Prot. 2006;69:693–698. doi: 10.4315/0362-028x-69.3.693. [DOI] [PubMed] [Google Scholar]
- Floros JD, Matsos KI. Introduction to modified atmosphere packaging. In: Han JH, editor. Innovations in food packaging. London: Elsevier Ltd; 2005. pp. 159–172. [Google Scholar]
- Gupta SK, Patil GR, Patel AA, Garg FC, Rajorhia GS. Instron texture profile parameters of khoa as influenced by composition. J Food Sci Technol. 1990;27:209–213. [Google Scholar]
- Halliwell B, Murcia M, Chirico S, Aruoma OI. Free radicals and antioxidants in food and in vivo: what they do and how they work? Crit Rev Food Sci Nutr. 1995;35:7–20. doi: 10.1080/10408399509527682. [DOI] [PubMed] [Google Scholar]
- Harte BR, Gray JI. The influence of packaging on product quality. In: Gray JI, Harte BR, Miltz J, editors. Food product package compatibility proceedings. Lancaster: Technomic Publishing Co. Inc.; 1987. p. 17. [Google Scholar]
- Hong CM, Wendorff WL, Bradley JRRL. Effects of packaging and lighting on pink discoloration and lipid oxidation of annatto-coloured cheeses. J Dairy Sci. 1995;78:1896–1902. doi: 10.3168/jds.S0022-0302(95)76814-X. [DOI] [Google Scholar]
- Hotchkiss JH. Microbiological hazards of controlled/modified atmosphere food packaging. J Assoc Food Drug Off. 1989;53:41–49. [Google Scholar]
- Jha YK, Singh S, Singh S. Effect of antioxidants and antimicrobial substances on keeping quality of khoa. Indian J Dairy Sci. 1977;30:1–6. [Google Scholar]
- Jha A, Kumar A, Jain P, Om H, Singh R, Bunkar DS. Physico-chemical and sensory changes during the storage of lal peda. J Food Sci Technol. 2012 doi: 10.1007/s13197-012-0613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney M, Bassette R. Detection of intermediate compounds in early stages of browning reaction in milk products. J Dairy Sci. 1959;42:945–960. doi: 10.3168/jds.S0022-0302(59)90678-2. [DOI] [Google Scholar]
- Kotsianis IS, Giannou V, Tzia C. Production and packaging of bakery products using MAP technology. Trends Food Sci Technol. 2002;13:319–332. doi: 10.1016/S0924-2244(02)00162-0. [DOI] [Google Scholar]
- Kumar G, Srinivasan MR. Effect of packaging and storage on the sensory characteristics of khoa samples. Indian J Dairy Sci. 1982;35:132–137. [Google Scholar]
- Kumar R, Bandyopadhyay P, Punjrath JS. Shelf life extension of peda using different packaging techniques. Indian J Dairy Sci. 1997;50:40–49. [Google Scholar]
- Kumar M, Beniwal BS, Rai DC. Effect of antioxidant on shelf life of khoa under refrigerated conditions. Egyptian J Dairy Sci. 2010;38:211–218. [Google Scholar]
- Londhe GK, Pal D, Raju PN. Effect of packaging techniques on shelf life of brown peda, a milk-based confection. LWT Food Sci Technol. 2012;47:117–125. doi: 10.1016/j.lwt.2011.12.025. [DOI] [Google Scholar]
- Mangarj S, Goswami TK. Modified atmospheric packaging-an ideal food preservation technique. J Food Sci Technol. 2009;46:399–410. [Google Scholar]
- O’Conner RE, Skarfhewski P, Phrower SJ. Modified atmosphere packaging of fruits, vegetables, seafood and meat: state of the art. ASEAN Food J. 1992;7:127–136. [Google Scholar]
- Palit C (1998) Application of selected unit processes for commercial production of burfi. M. Tech. Thesis. National Dairy Research Institute, Karnal, India
- Patel AA, Patil GR, Garg FC, Rajorhia GS (1990) Texture of peda as measured by instron. In: Proceedings of XXIII Int. Dairy Cong., Brief Communication. Montreal, Canada
- Ponte JG, Payne JD, Ingelin ME. The shelf life of bakery foods. In: Charalambous G, editor. Shelf-life studies of foods and beverages. Amsterdam: Elsevier Ltd; 1993. pp. 1176–1193. [Google Scholar]
- Rai S, Goyal GK, Rai GK. Effect of modified atmospheric packaging (MAP) and storage on the chemical quality of paneer. J Dairying Foods Home Sci. 2008;27(1):33–37. [Google Scholar]
- Sandhya Modified atmosphere packaging of fresh produce: current status and future needs. LWT Food Sci Technol. 2010;43:381–392. doi: 10.1016/j.lwt.2009.05.018. [DOI] [Google Scholar]
- Sharma HK, Singhal RS, Kulkarni PR. Effect of under vacuum or under nitrogen on the keeping quality of danedar khoa. Int J Dairy Technol. 2001;40(5):543–545. [Google Scholar]
- Smith JP, Simpson BK. Modified atmosphere packaging of bakery and pasta products. In: Farber I, Dodds KL, editors. Principles of modified atmosphere packaging and Sous-Vide processing. Manchester: Technomic Publ. Co.; 1995. pp. 207–242. [Google Scholar]
- Smith JP, Ooraikul B, Koersen WJ, Jackson ED, Lawrence RA. Novel approach to oxygen control in modified atmosphere packaging of bakery products. Food Microbiol. 1986;3:315–320. doi: 10.1016/0740-0020(86)90015-8. [DOI] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 8. Ames: Iowa State University Press; 1989. [Google Scholar]
- Strange ED, Benedict RC, Smith JL, Swift CE. Evaluation of rapid tests for monitoring alterations in meat quality during storage. J Food Prot. 1977;10:843–847. doi: 10.4315/0362-028X-40.12.843. [DOI] [PubMed] [Google Scholar]
- Suresh I, Jha YK. Sensory, biochemical and microbiological qualities of kalakand. J Food Sci Technol. 1994;31:330–332. [Google Scholar]
- Thakur BR, Semwal AD, Arya SS. Packaging requirements and stability of sorbate preserved khoa sweets (pedas) Indian Food Packer. 1992;46:53–56. [Google Scholar]
- Thippeswamy L, Venkateshaiah BV, Patil SB. Effect of modified atmospheric packaging on the shelf stability of paneer prepared by adapting hurdle technology. J Food Sci Technol. 2011;48(2):230–235. doi: 10.1007/s13197-010-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


