Abstract
This study was carried out to determine some of the biochemical properties of pectin methylesterase (PME) from Alyanak apricot which is an important variety grown in Malatya region of Turkey. The enzyme had high activity in a pH range of 7.0–8.0 with the maximal activity occurring at pH 7.5. However, the enzyme activity at high and low pH values was very low. The optimum temperature for maximal PME activity was found to be 60 °C. The activity of PME has been enhanced by NaCl, particularly at 0.15 M. Km and Vmax values for Alyanak apricot PME using apple pectin as substrate were found to be 1.69 mg/mL (r2 = 0.992) and 3.41 units/mL, respectively. The enzyme was stable at 30–45 °C/10 min whereas it lost nearly all of its activity at 80 °C/10 min. Ea and Z values were found to be 206.1 kJ/mol (r2 = 0.993) and 10.62 °C (r2 = 0.992), respectively.
Keywords: Apricot, Pectin methylesterase, Kinetics, Thermal inactivation kinetics, Heat stability
Introduction
One of the main problems in the fruit and vegetable products industry is maintenance of turbidity in fruit and vegetable juices and of consistency of whole or diced products during processing and storage. Research on non-thermally treated products demonstrated that cloud loss in fruit juices is mainly due to the cooperative action of pectin methylesterase (PME, EC 3.1.1.11) and polygalacturonase (PG). PMEs play an important role in determining the extent to which demethylesterified pectic polysaccharides are accessible to degradation by PG. Demethylation of pectin results in a juice that separates in clear serum and a sediment, which arises from low methoxyl pectin that complexes with calcium ions. Therefore, control of PME activity is crucial for the cloud stability of juices. Cloud loss problem can be easily overcome with thermal processes (Fischer and Bennett 1991; Castaldo et al. 1997; Balogh et al. 2004; Chavez-Sanchez et al. 2013).
PMEs have been detected in plants, pathogenic fungi and bacteria. Multiple forms of PME (basic, neutral, and acidic isoforms) can be present within each species. These isoforms have different biochemical properties. In general, the plant and bacterial PMEs have pH optima between 6 and 8 whereas some fungal PMEs have pH optima between pH 4 and 6. Plant PMEs require NaCl for the optimal catalysis. PMEs are cell wall-bound enzymes which catalyze the hydrolytic cleavage of the methylester moieties on pectin molecules, resulting in the release of methanol and partially de-esterified pectin (Unal and Bellur 2009). The control of PME activity has been a common subject of study because of the implications in the modification of the texture of fruit and vegetables and as a destabilizing agent of pectin materials in fruit juices and concentrates (Balogh et al. 2004; Vivar-Vera et al. 2007; Gupta et al. 2011).
Turkey is the leading apricot producer in the world with an annual production of 695364 tons in 2009 according to FAO (2011), which amounted to 13 % of the world production. Malatya region of Turkey is particularly important for cultivation, production, and processing of apricots, as around 50 % of the fresh apricots and 90 % of the dried apricots in Turkey are produced in this region. The most cultivated apricot varieties in Malatya region are, Hacıhaliloğlu, Hasanbey, Soğancı, Kabaaşı, Alyanak, Çataloğlu, and Çöloğlu (Asma 2000; Akin et al. 2008)
Epidemiological and clinical studies indicate that a diet rich in fruits and vegetable consumption can reduce the risk of several chronic diseases such as cancer, cardiovascular disease, coronary heart disease, and hypertension (Akin et al. 2008). In a study carried out by Parlakpinar et al. (2009) on effects of supplementation of the diet with apricot on myocardial I/R injury in rats, in vivo cardio-protective activity of apricot-feeding related to its antioxidant phenolic contents in rats subjected to myocardial I/R was demonstrated. Vardi et al. (2008) investigated the role of oxidative stress in methotrexate-induced intestinal injury and also explored the protective effect of apricot and/or β-carotene against this injury. They demonstrated that treatment with apricot/or β-carotene may protect the impairment of oxidative stress and ameliorate methotrexate-induced intestine damage at biochemical and histological levels.
There have been numerous research on PME from different sources, e.g. Malatya apricot (Karakus and Pekyardimci, 2012), carrot (Unal and Bellur 2009), green beans (Laats et al. 1997), orange juice (Lee et al. 2003), pepper (Castro et al. 2006), grape (Deytieux-Belleau et al. 2008), strawberry (Draye and Cutsem 2008; Bodelon et al. 2013), black carrot (Jolie et al. 2009), Fragaria chiloensis and Fragaria-ananassa fruits (Figueroa et al., 2010), red carrot and tomato (Jolie et al. 2010), black olives (Cardoso et al. 2010), persimmon (Luiz de Souza et al. 2011), apple (Ortiz et al. 2011), watermelon juice (Liu et al. 2012). However, to the best of our knowledge, no work has been done on PME from Alyanak apricot.
Alyanak apricot is an important variety grown in Malatya region of Turkey, which is suitable for both nectar production and fresh consumption (Asma, 2000). It was reported that many producers of fruit and nectar juices complained of consistency loss occurring in as much as 50 % in apricot and peach puree, during storage in tanks (Laratta et al. 1995). PMEs from different sources have different characteristics, and multiple isoenzymes existing in the same source with different molecular weight, isoelectric points, and/or kinetic properties (Zhi et al. 2008). The variability in processing stability of PME is important in many food industries. An understanding of the essential factors controlling the action of PME and kinetic data is necessary in an attempt to control its activity in fruit and vegetables during processing. The present work was undertaken to study the characteristics of PME from Alyanak apricot in terms of pH and temperature optima, thermal stability and inactivation, kinetic parameters and effect of salt.
Materials and methods
Materials
Alyanak apricots used in this study were obtained from the orchard of Apricot Research Institute of Malatya province of Turkey and frozen at −25 °C until used.
Apple pectin (70–75 % esterification degree), trisma base, dialysis bag (cellulose membrane: 76 × 49 mm) were purchased from Sigma (St.louis, USA). Sodium hydroxide, ethanol (99 %), ammonium sulphate, hydrochloric acid, sodium disulfite, polyvinylpolypyrrolidone (PVPP) and sodium chloride were purchased from Merck (Darmstadt, Germany). All the reagents were analytical grade.
Extraction of PME
PME is ionically bound to cell wall and therefore a buffer with a high ionic strength (Tris, NaCl) is needed to extract it from cell wall (Nunes et al. 2006).
The extraction of Alyanak apricot PME was performed according to the method of Denes et al. (2000) with modifications. All extraction steps were performed at 4 °C to prevent enzyme inactivation. The deseeded apricots in batches of 300 g were chopped and then homogenized in 300 ml of cold distilled water containing 500 mg/L sodium disulphite for 2 min at maximum speed by using Waring blender (Model HGB2WTS3, Torrington, Connecticut, USA). The homogenate was centrifuged (10000 x g for 30 min at 4 °C) and the supernatant was discarded. The pellet was mixed with 200 mL of cold distilled water containing 500 mg/L sodium disulphite and then centrifuged at 10000 x g for 30 min at 4 °C. Mixing and centrifuging were performed 3 times in order to eliminate proteins, water-soluble phenolic compounds, soluble sugars and pectins.
The last pellet was resuspended in 200 mL of cold buffer solution of 20 mM Tris–HCl (pH 7.5) buffer containing 1 M NaCl and 500 mg/L Na2O5S2, followed by centrifugation at 10000 x g for 30 min at 4 °C. 1 % (w/v) PVPP (polyvinylpolypyrrolidone) was added to the supernatant and magnetically stirred for 30 min at 4 °C, followed by centrifugation at 5000 x g for 10 min at 4 °C. This procedure was repeated until a clear, colourless supernatant was obtained. Altogether, a total of 1.2 kg of apricots was used in the extraction of PME.
All the supernatants obtained at each extraction step were combined and then subjected to 80 % ammonium sulphate precipitation. The precipitate containing PME was collected by centrifugation at 10000 x g for 60 min at 4 °C and dissolved in 3 mL 10 mM Tris–HCl (pH 7.0). The extract was then dialysed overnight in Tris buffer (pH 7.0) at 4 °C. The extract was used as PME source in the following experiments.
Assay of PME activity
PME activity was measured titrimetrically by determining free carboxyl groups formed as a result of enzyme action on pectin. The reaction mixture was composed of 10 mL of 0.5 % apple pectin solution containing 0.1 M NaCl and 0.5 mL of PME extract. The reaction was carried out at 30 °C in a water-jacketed reaction beaker. The volume of 0.001 N NaOH required to maintain the pH of the reaction mixture at 7.5 for 10 min was measured. One unit of PME was defined as the amount of enzyme that released 1 μmol of carboxyl groups/min, under the aforementioned assay conditions. PME activity was calculated using the following formula:
Kinetic parameters
In order to determine Michaelis constant (Km) and maximum velocity (Vmax), PME activities were measured using apple pectin as substrate at various concentrations (0.125–2 g/l). Km and Vmax values of the enzyme were calculated from a plot of 1/V vs. 1/S by the method of Lineweaver and Burk.
pH optima
The pH dependence of PME activity was assayed in a pH range of 4.0–10.0, using the standard reaction mixture. Blanks without PME extract were made for each determination, and the amount of acid produced due to the spontaneous pectin demethylation was subtracted. PME activity was calculated in the form of percent residual activity at the optimum pH. The optimum pH obtained for this enzyme was used in all other studies.
Temperature optima
Temperature optima of PME activity was tested under standard assay conditions at temperatures ranging from 20 °C to 80 °C. The temperature was controlled by means of a circulating water bath. Blanks without PME sample were made for each determination, and the amount of acid produced due to the spontaneous pectin demethylation was subtracted. PME activity was calculated in the form of percent residual activity at the optimum temperature.
Thermal stability
Thermal stability of apricot PME was determined at temperatures ranging from 30 °C to 80 °C, using screw-cap tubes. The screw-cap tubes were pre-heated to the selected temperature to prevent temperature lag before addition of enzyme solution. The enzyme samples were removed from water bath after 10 min and were immediately transferred to ice bath to stop thermal inactivation. The residual activity was determined within 60 min as described above.
Thermal inactivation kinetics
The enzyme samples were incubated for 2, 5 and 8 min at 60 °C and 2, 4 and 6 min at 65 °C and 70 °C in screw-cap tubes. The screw-cap tubes were pre-heated to the selected temperature to prevent temperature lag before the addition of enzyme solution. The enzyme samples were removed from water bath after pre-set times and were immediately transferred to ice bath to stop thermal inactivation. After the enzyme sample was cooled in ice bath, the residual activity (A) was determined within 60 min as described previously. A non-heated enzyme sample was used as blank (Ao). The percentage residual activity was calculated by comparison with the unheated sample. First order inactivation constant (kD) was calculated from the slope of the natural logarithm (ln) of A/Ao vs. time graph. Half-life of the enzyme (t1/2) was calculated by using the following equation: t1/2 = 0.693/ kD.
Decimal reduction time (D value) was estimated from the relationship between kD and D value: D = ln (10)/ kD. The Z value, which is the temperature increase required for a one-log10 reduction (90 % decrease) in D value, was determined from a plot of log10D versus temperature. The slope of the graph is equal to 1/Z value. The energy of activation of denaturation (Ea) was calculated by multiplying the slope of Arrhenius plot (i.e. natural logarithm of kD values vs. reciprocal of absolute temperatures (1/T)) with universal gas constant, R (kJ/molK) (Marangoni 2003).
Effect of NaCl
Effect of salt on PME activity was determined using 0.5 % apple pectin solution containing NaCl in a range of 0–0.5 M.
Statistical analyses
Average values of duplicates (which differed less than 5 %) were calculated. The data obtained from the studies were analyzed using linear regression (Amaral et al. 2005).
Results and discussion
pH optima
The activities of many enzymes vary with the pH of the medium because the active sites generally contain important acidic or basic groups. It is to be expected that if only one protonic form of the acid or base is catalytically active, the catalysis should depend on the concentration of the active form. Like all proteins, enzymes have a native tertiary structure that is sensitive to pH, and in general denaturation of enzymes occurs at extremely low and high pH values (Fersht 1977; Copeland 2000).
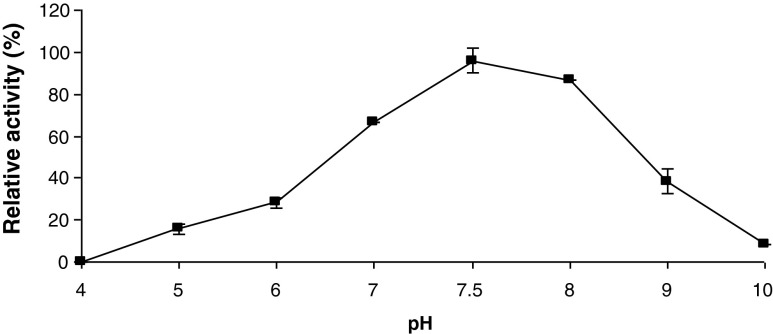
PME activity as a function of pH was determined in a pH range of 4.0–10.0, and the results are depicted in Fig. 1. As the pH increased from 4.0 to 7.5, the enzyme activity increased, with the maximal activity occurring at pH 7.5. The enzyme had high activity in a pH range of 7.0–8.0, after which a sharp drop in enzyme activity was observed. The enzyme retained only 8.3 % activity at pH 10. The enzyme activity at acidic pHs was very low, e.g. there was no activity observed at pH 4.0, which means that the enzyme is expected to show no activity in apricot nectar since pH of Alyanak apricots varies between 3.5 and 3.9 (Asma 2000).
Fig. 1.
Activity of apricot PME as a function of pH. Each data point is the mean of two determinations. The vertical bars represent standard deviations
In a study carried out by Karakus and Pekyardimci (2012) on comparison of covalent and noncovalent immobilization of pectinesterase isolated from Malatya apricot, it was found that the pH optima of covalently-immobilized and free PME were 8.0 and 9.0, respectively, which is slightly higher than the one found for Alyanak PME. Some of the reported optimum pH values for PME from different sources are 7.5 for black carrot (Daucus carota L.) (Unal and Bellur 2009), green pepper (Castro et al. 2004), plum (Nunes et al. 2006) and hawthorn (Vivar-Vera et al. 2007), 8.0 for orange (Amaral et al. 2005), 8.0 for peach (Javeri and Wicker 1991), 9.0 for guava (Carvalho et al. 2009). The pH optimum of 7.5 found in this study was in accord with these values.
Temperature optima
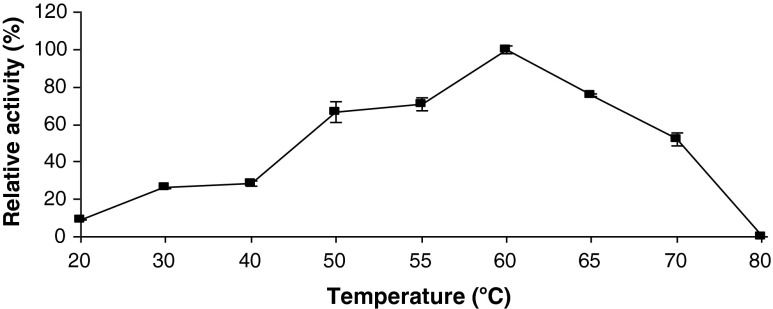
Temperature affects both the velocity of enzymatic reactions and stability of the enzyme. Temperature also affects the equilibria of all association/dissociation reactions, solubility of substrates and ionization of prototropic groups in the catalytic centre of the enzyme and enzyme-substrate complex (Whitaker 1996). Effect of temperature on PME activity was investigated in the range 20–80 °C and the results are shown in Fig. 2. As the temperature increased from 20 °C to 60 °C, the enzyme activity increased. The optimum temperature for maximal PME activity was found to be 60 °C, after which the enzyme activity dropped sharply. The enzyme had no activity at 80 °C.
Fig. 2.
Activity of apricot PME as a function of temperature. Each data point is the mean of two determinations. The vertical bars represent standard deviations
Karakus and Pekyardimci (2012) reported temperature optimum of free PME from Malatya apricot as 60 °C which is in accord the one found in this study. Reported temperature optima for PMEs from different sources include 55 °C for black carrot (Unal and Bellur 2009), 55 °C for hawthorn (Vivar-Vera et al. 2007), between 52.5 and 55.0 for green pepper (Castro et al. 2004), 55 °C for tomato (Van Den Broeck et al. 2000), 65 °C for plum (Prunus domestica) (Nunes et al. 2006), which are similar to the one obtained in this study. However, higher temperature optima were reported for PME from guava, e.g. 75–80 °C (Psidium guajava L.) (Leite et al. 2006) and 95 °C (Carvalho et al. 2009).
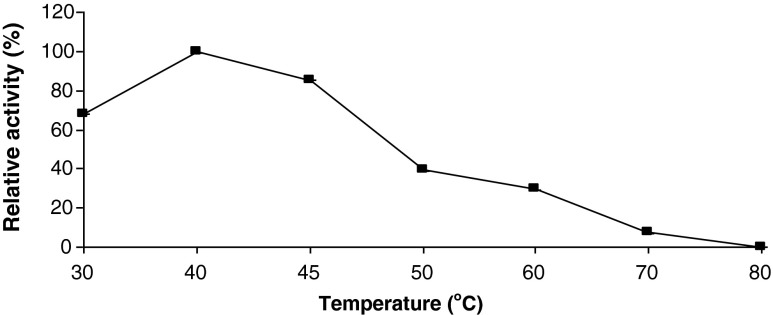
Temperature stability
The heat stability of apricot PME was studied by incubating the enzyme in preheated glass tubes for 10 min in the range 30–80 °C. As can be seen from Fig. 3, the enzyme was stable between the temperatures of 30–40 °C, which indicated that the PME from Alyanak apricot was heat sensitive. The critical temperature for inactivation of PME was 40 °C because above this temperature the inactivation rate increased. The enzyme completely lost its activity at 80 °C. From these results it can be concluded that Alyanak apricot PME can easily be inactivated by pasteurization since apricot nectar is pasteurized at 88 to 93 °C (Bates et al. 2001). It was reported that the noncovalently immobilized enzyme exhibited better thermostability than the free and covalently immobilized Malatya Apricot PE (Karakus and Pekyardimci 2012).
Fig. 3.
Thermal stability of apricot PME extract. Each data point is the mean of two determinations. The vertical bars represent standard deviations
In a study carried out by Amaral et al. (2005) on orange PME, the enzyme retained 88.9 % of its specific activity after 50 min of incubation at 50 °C. The enzyme was completely inactivated after 60 min incubation at 90 °C. PME from banana lost 50 % of its activity at 70 °C/5 min, whereas more than 90 % at 75 °C/5 min (Ly-Nguyen et al. 2002). PME from guava retained 75.4 %, 86.2 % and 90.4 % of its specific activity after 60 min of incubation respectively, at 80 °C, 90 °C and 98 °C. When submitted to higher times of incubation at 90 °C, the guava PME showed 96.8 % of its specific activity after 300 min of incubation (Carvalho et al., 2009).
Thermal inactivation kinetics
The thermal inactivation parameters of Alyanak apricot PME are presented in Table 1. The first order inactivation constants (kD) increased with increasing temperature, indicating that the enzyme was less thermostable at higher temperatures. The half-life (t1/2) is another important parameter used in the characterization of enzyme stability. Increasing the temperature from 60 °C to 70 °C resulted in a decrease in t1/2 values (Table 1). Unal and Bellur (2009) who investigated the thermal inactivation of black carrot PME reported kD values of 0.040, 0.133 and 0.337 min at 55, 60 and 65 °C, respectively, which are similar to those obtained in this study.
Table 1.
Thermal inactivation parameters of Alyanak apricot pectin methylesterase
| Temperature (°C) | k D (min−1) | r 2 | t 1/2 (min) | D (min) |
|---|---|---|---|---|
| 60 | 0.0367 | 1.000 | 18.9 | 62.7 |
| 65 | 0.1289 | 0.996 | 5.4 | 17.9 |
| 70 | 0.3210 | 0.986 | 2.2 | 7.2 |
Each data point is the mean of two determinations
The decimal reduction time (D value) is the time, at a given temperature and needed for 90 % reduction of the initial activity. D values obtained in this study ranged between 62.7 and 7.2 min at the temperatures studied (Table 1). Unal and Bellur (2009) reported D values of 57.7 min at 55 °C and 6.8 min at 65 °C for black carrot PME, which are similar to those obtained in this study.
The temperature dependence of the decimal reduction time is characterized by Z value, which is the temperature increase needed for a one log10 reduction (90 % decrease) in the D value. The Z value obtained in this study was 10.62 °C (r2: 0.992). Denes et al. (2000) reported a Z value of 9.2 °C for apple PME, which is very close to the value obtained in this study. Ea value for thermal inactivation of Alyanak apricot PME was found to be 206.1 kJ/ mol (r2: 0.993). Unal and Bellur (2009) reported an Ea value of 196.8 kJ/mol for black carrot PME, which is close to the one calculated in this study.
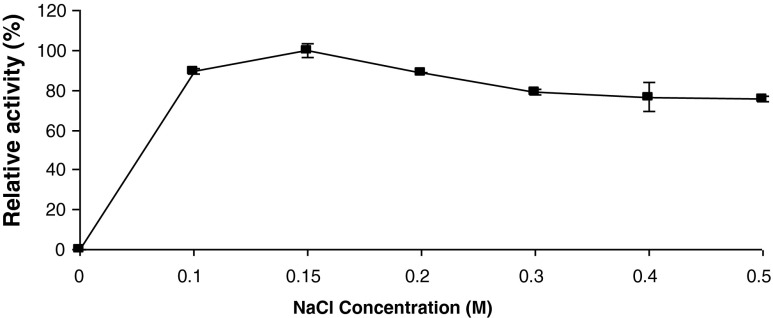
Effect of NaCl concentration
The apricot PME activity was dependent on NaCl concentration as seen in Fig. 4. As the NaCl concentration in the assay mixture increased the PME activity increased gradually and reached the maximum level at 0.15 M. On further increase, the activity declined. Na+ dependence of PME varies depending on the enzyme source. The reported NaCl concentration for maximal PME activity include 0.13 M for green pepper (Castro et al. 2004), 0.15 M for orange (Amaral et al. 2005), 0.2 M for black carrot (Unal and Bellur 2009), 0.25 for kiwi, 0.3 M for guava (Carvalho et al. 2009), 1.27 M for grapefruit (Guivarc’h et al. 2005). Stimulatory effect of Na+ ions is attributed to the interactions of Na+ ions with the substrate (Mc Neil et al. 1984). Furthermore, it is believed that Na+ ions bind to the enzyme, inducing conformational changes, favouring reaction of the enzyme with its substrate (Nari et al. 1991).
Fig. 4.
Effect of NaCl on apricot PME activity. Each data point is the mean of two determinations. The vertical bars represent standard deviations
Kinetic parameters
Km and Vmax values for apricot PME using apple pectin as substrate were found to be 1.69 mg/mL (r2: 0.992) and 3.41 units/mL, respectively. Km value is a measure of affinity of the enzyme for the substrate, with smaller values representing greater affinity. Some of the reported Km values of PME, isolated from different sources, include 2.14 mg/mL for black carrot (Unal and Bellur 2009), 0.098 mg/mL for apple (Golden sp) (Denes et al. 2000), 0.152 mg/mL for banana (Ly-Nguyen et al. 2002), 0.274 mg/mL for grapefruit (Guivarc’h et al. 2005). As can be seen Km value changes depending on the enzyme source. The Km value obtained in this study is similar to that reported for black carrot PME. However, it is difficult to compare the Km values because the Km values are dependent on temperature, salt concentration, pectin source, and pH of the reaction medium (Goldberg et al. 1992).
Conclusions
PME from Alyanak apricot had a high activity in a narrow pH range of 7.0–8.0, with the maximal activity occurring at pH 7.5 whereas enzyme activity at low and high pH values was very low, which means that the enzyme is expected to be inactive in apricot nectar which has acidic pH. The enzyme was stable between the temperatures of 30–40 °C/10 min and lost all of its activity at 80 °C/10 min, implying that PME can easily be inactivated by pasteurization since apricot nectar is pasteurized at 88 to 93 °C. The Alyanak apricot PME activity was dependent on NaCl concentration in the assay mixture.
Acknowledgement
This study (ZF2010BAP34) was funded by the Research Fund of the University of Cukurova, Turkey.
References
- Akin EB, Karabulut I, Topcu A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008;107:939–948. doi: 10.1016/j.foodchem.2007.08.052. [DOI] [Google Scholar]
- Amaral SH, Assis SA, Faria Oliveira OMM. Partial purification and characterization of pectin methylesterase from orange (Citrus sinensis) J Food Biochem. 2005;29:367–380. doi: 10.1111/j.1745-4514.2005.00036.x. [DOI] [Google Scholar]
- Asma M. Growing of apricot. Turkey: Evin Ofset, Malatya; 2000. p. 45. [Google Scholar]
- Balogh T, Smout CS, Nguyen BL, Loey AMV, Hendrickx ME. Thermal and high pressure inactivation kinetics of carrot pectinmethylesterase: from model system to real foods. Innovat Food Sci Emerg Tech. 2004;5:429–436. doi: 10.1016/j.ifset.2004.06.002. [DOI] [Google Scholar]
- Bates RP, Morris JR, Crandall PG. Principles and practices of small- and medium-scale fruit juice processing. Italy: FAO of the U.N.; 2001. p. 168. [Google Scholar]
- Bodelon OG, Avizcuri JM, Zurbano FC, Dizy M. Pressurization and cold storage of strawberry puree: colour, anthocyanins, ascorbic acid and pectin methylesterase. LWT-Food Sci Technol. 2013;52:123–130. doi: 10.1016/j.lwt.2012.08.025. [DOI] [Google Scholar]
- Cardoso SM, Mafra I, Reis A, Nunes C, Saraiva JA, Coimbra MA. Naturally fermented black olives: effect on cell wall polysaccharides and on enzyme activities of Taggiasca and Conservolea varieties. Food Sci Tech. 2010;43:153–160. [Google Scholar]
- Carvalho AB, De Asisi SA, Cerqueira Leite KMS, Bach EE, De Faria OMM. Pectin methylesterase activity and ascorbic acid content from guava fruit, cv. predilecta, in different phases of development. Int J Food Sci Nutr. 2009;60:255–265. doi: 10.1080/09637480701752244. [DOI] [PubMed] [Google Scholar]
- Castaldo D, Laratta B, Loiudice R, Giovane A, Ouagliuolo L, Servillo L. Presence of residual pectin methylesterase activity in thermally stabilized industrial fruit preparations. Lebensm Wiss Technol. 1997;30:479–484. doi: 10.1006/fstl.1996.0211. [DOI] [Google Scholar]
- Castro SM, Van Loey AM, Saraiva JA, Smout C, Hendrickx ME. Activity and process stability of purified green pepper (Capsicum annum) pectin methylesterase. J Agric Food Chem. 2004;52:5724–5729. doi: 10.1021/jf0352071. [DOI] [PubMed] [Google Scholar]
- Castro SM, Van Loey AM, Saraiva JA, Smout C, Hendrickx ME. Inactivation of pressure/temperature combinations for optimal pepper (Capsicum annum) pectin methylesterase activity. Enzyme Microb Technol. 2006;38:831–838. doi: 10.1016/j.enzmictec.2005.08.009. [DOI] [Google Scholar]
- Chavez-Sanchez I, Carrillo-Lopez A, Vega-Garcia M, Yahia EM (2013) The effect of antifungal hot-water treatments on papaya postharvest quality and activity of pectinmethylesterase and polygalacturonase. J Food Sci Technol [DOI] [PMC free article] [PubMed]
- Copeland RA. Enzymes: A practical introduction to structure, mechanism and data analysis. New York: Wiley-VCH; 2000. pp. 241–248. [Google Scholar]
- Denes JM, Baron A, Drilleau JF. Purification, properties and heat inactivation of pectin methylesterase from apple (cv Golden Delicious) J Sci Food Agric. 2000;80:1503–1509. doi: 10.1002/1097-0010(200008)80:10<1503::AID-JSFA676>3.0.CO;2-U. [DOI] [Google Scholar]
- Deytieux-Belleau C, Vallet A, Doneche B, Geny L. Pectin methylesterase and polygalacturonase in the developing grape skin. Plant Physiol Bioch. 2008;46:638–646. doi: 10.1016/j.plaphy.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Draye M, Cutsem PV. Pectin methylesterases induce an abrupt increase of acidic pectin during strawberry fruit ripening. J Plant Physiol. 2008;165:1152–1160. doi: 10.1016/j.jplph.2007.10.006. [DOI] [PubMed] [Google Scholar]
- FAO (2011) http://faostat.fao.org/site/339/default.aspx.
- Fersht A (1977) Enzyme structure and mechanism. W. H. Freeman and Company, Reading, p 134
- Figueroa CR, Rosli HG, Civello PM, Martinez GA, Herrera R, Moya-Leon MA. Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria x ananassa fruits. Sci Hortic. 2010;124:454–462. doi: 10.1016/j.scienta.2010.02.003. [DOI] [Google Scholar]
- Fischer RL, Bennett AB. Role of cell-wall hydrolases in fruit ripening. Annu Rev Plant Phys. 1991;42:675–703. doi: 10.1146/annurev.pp.42.060191.003331. [DOI] [Google Scholar]
- Goldberg R, Pierron M, Durand L, Mutaftschiev S. In vitro and in situ properties of cell wall pectin methylesterases from mung bean hypocotyls. J Exp Bot. 1992;43:41–46. doi: 10.1093/jxb/43.1.41. [DOI] [Google Scholar]
- Guivarc’h Y, Segiova O, Van Loey AM, Hendrickx ME. Purification, characterisation, thermal and high-pressure inactivation of a pectin methylesterase from grapefruit (Citrus paradisi), electric field treatments. Innovat Food Sci Emerg Tech. 2005;6:363–371. doi: 10.1016/j.ifset.2005.06.003. [DOI] [Google Scholar]
- Gupta N, Jawandha SK, Gill PS. Effect of calcium on cold storage and post-storage quality of peach. J Food Sci Technol. 2011;48:225–229. doi: 10.1007/s13197-010-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javeri H, Wicker L. Partial purification and characterisation of peach pectinesterase. J Food Biochem. 1991;15:241–252. doi: 10.1111/j.1745-4514.1991.tb00159.x. [DOI] [Google Scholar]
- Jolie RP, Duvetter T, Houben K, Clynen E, Sila DN, Van Loey AM, Hendrickx ME. Carrot pectin methylesterase and its inhibitor from kiwi fruit: Study of activity, stability and inhibition. Innovat Food Sci Emerg Tech. 2009;10:601–609. doi: 10.1016/j.ifset.2009.02.003. [DOI] [Google Scholar]
- Jolie RP, Duvetter T, Houben K, Vandevenne E, Van Loey AM, Declerck PJ, Hendricks ME, Gils A. Plant pectin methylesterase and its inhibitor from kiwi fruit: interaction analysis by surface plasmon resonance. Food Chem. 2010;121:207–214. doi: 10.1016/j.foodchem.2009.11.073. [DOI] [Google Scholar]
- Karakus E, Pekyardimci S. Comparison of covalent and noncovalent immobilization of Malatya apricot pectinesterase (Prunus armeniaca L.) Artif Cells Blood Substit Immobil Biotechnol. 2012;40:132–141. doi: 10.3109/10731199.2011.611471. [DOI] [PubMed] [Google Scholar]
- Laats MM, Grosdenis F, Recourt K, Voragen AGJ, Wichers HJ. Partial purification and characterisation of pectin methylesterase from green beans. J Agric Food Chem. 1997;22:5572–5577. [Google Scholar]
- Laratta B, Fasanaro G, Sio FD, Castaldo D, Palmieri A, Giovane A, Servillo L. Thermal inactivation of pectin methylesterase in tomato puree: implications on cloud stability. Process Biochem. 1995;30:251–259. doi: 10.1016/0032-9592(95)85006-6. [DOI] [Google Scholar]
- Lee J-Y, Lin Y-S, Chang H-M, Chen W, Wu M-C. Temperature-time relationships of pectinesterases in orange juice. J Sci Food Agric. 2003;83:681–684. doi: 10.1002/jsfa.1360. [DOI] [Google Scholar]
- Leite KMSC, Tadiotti AC, Baldochi D, Oliveira OMMF. Partial purification, heat stability and kinetic characterization of the pectinmethylesterase from Brazilian guava Plauma cultivars. Food Chem. 2006;94:565–572. doi: 10.1016/j.foodchem.2004.12.008. [DOI] [Google Scholar]
- Liu Y, Hu X, Zhao X, Song H. Combined effect of high pressure carbon dioxide and mild heat treatment on overall quality parameters of watermelon juice. Innovat Food Sci Emerg Tech. 2012;13:112–119. doi: 10.1016/j.ifset.2011.11.001. [DOI] [Google Scholar]
- Luiz de Souza E, Kulkamp de Souza AL, Tiecher A, Girardi CL, Nora L, Silva JA, Argenta LC, Rombaldi CV. Changes in enzymatic activity, accumulation of proteins and softening of persimmon (Diospyros kaki Thunb.) flesh as a function of pre-cooling acclimatization. Sci Hortic. 2011;127:242–248. doi: 10.1016/j.scienta.2010.09.025. [DOI] [Google Scholar]
- Ly-Nguyen B, Van Loey AM, Fachin D, Verlent I, Indrawati HME. Purification, characterisation, thermal and high-pressure inactivation of pectin methylesterase from bananas (cv. Cavendish) Biotechnol Bioeng. 2002;78:683–691. doi: 10.1002/bit.10249. [DOI] [PubMed] [Google Scholar]
- Marangoni AG. Enzyme kinetics: A modern approach. New Jersey: Wiley; 2003. pp. 140–158. [Google Scholar]
- Mc Neil M, Davill AG, Fry SC, Albersheim P. Structure and function of the primary cell wall of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Nari J, Noat G, Richard J. Pectin methylesterase, metal ions and plant cell wall extension. Hydrolysis of pectin by plant cell-wall pectin methylesterase. Biochem J. 1991;279:343–350. doi: 10.1042/bj2790343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes CS, Castro SM, Saraiva JA, Coimbra MA, Hendrickx ME, Van Loey AM. Thermal and high-pressure stability of purified pectin methylesterase from plums (Prunus domestica) J Food Biochem. 2006;30:138–154. doi: 10.1111/j.1745-4514.2006.00057.x. [DOI] [Google Scholar]
- Ortiz A, Graell J, Lara I. Preharvest calcium applications inhibit some cell wall-modifying enzyme activities and delay cell wall disassembly at commercial harvest of Fuji Kiku-8 apples. Postharvest Biol Tec. 2011;62:161–167. doi: 10.1016/j.postharvbio.2011.04.014. [DOI] [Google Scholar]
- Parlakpinar H, Olmez E, Acet A, Ozturk F, Tasdemir S, Ates B, Gul M, Otlu A. Beneficial effects of apricot-feeding on myocardial ischemia-reperfusion injury in rats. Food Chem Toxicol. 2009;47:802–808. doi: 10.1016/j.fct.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Unal U, Bellur E. Extraction and characterisation of pectin methylesterase from black carrot (Daucus carota L.) Food Chem. 2009;116:836–840. doi: 10.1016/j.foodchem.2009.03.031. [DOI] [Google Scholar]
- Van Den Broeck I, Ludikhuyze LR, Van Loey AM, Hendrickx ME. Effect of temperature and/or pressure on tomato pectinesterase activity. J Agric Food Chem. 2000;48:551–558. doi: 10.1021/jf990569n. [DOI] [PubMed] [Google Scholar]
- Vardi N, Parlakpinar H, Ozturk F, Ates B, Gul M, Cetin A, Erdogan A, Otlu A. Potent protective effect of apricot and b-carotene on methotrexate-induced intestinal oxidative damage in rats. Food Chem Toxicol. 2008;46:3015–3022. doi: 10.1016/j.fct.2008.05.039. [DOI] [PubMed] [Google Scholar]
- Vivar-Vera MA, Salazar-Montoya JA, Calva-Calva G, Ramos-Ramirez EG. Extraction, thermal stability and kinetic behaviour of pectinmethylesterase from hawthorn (Crataegus pubescens) Fruit. Lebensm Wiss Technol. 2007;40:278–284. doi: 10.1016/j.lwt.2005.10.005. [DOI] [Google Scholar]
- Whitaker JR. Enzymes. In: Fennema OR, editor. Food Chem. New York: Marcel Dekker; 1996. pp. 431–522. [Google Scholar]
- Zhi X, Zhang Y, Hu X, Wu J, Liao X. Inactivation of apple pectin methylesterase induced by dense phase carbon dioxide. J Agric Food Chem. 2008;56:5394–5400. doi: 10.1021/jf800260c. [DOI] [PubMed] [Google Scholar]