Abstract
More than half of the world populations are affected by micronutrient malnutrition and one third of world’s population suffers from anemia and zinc deficiency, particularly in developing countries. Iron and zinc deficiencies are the major health problems worldwide. Phytic acid is the major storage form of phosphorous in cereals, legumes, oil seeds and nuts. Phytic acid is known as a food inhibitor which chelates micronutrient and prevents it to be bioavailabe for monogastric animals, including humans, because they lack enzyme phytase in their digestive tract. Several methods have been developed to reduce the phytic acid content in food and improve the nutritional value of cereal which becomes poor due to such antinutrient. These include genetic improvement as well as several pre-treatment methods such as fermentation, soaking, germination and enzymatic treatment of grains with phytase enzyme. Biofortification of staple crops using modern biotechnological techniques can potentially help in alleviating malnutrition in developing countries.
Keywords: Phytic acid, Phytase, Dephytinization, Micronutrients, Monogastric animals
Introduction
Micronutrient malnutrition affects more than half of the world population, particularly in developing countries. Iron, zinc and vitamin A deficiencies are the most serious health constraints worldwide (Jorge et al. 2008). In developing countries plants are the major source of food. In unrefined cereal and legumes foods the low bioavailability of Fe, Zn causes metabolic disorder related to these nutritional factors. So improving the nutritional value of such type of food will improve the nutritional status of entire population. Mineral, phosphorous and phytate content is much higher in the bran than the whole grain (Iskander and Morad 1986; Guttieri et al. 2004; Steiner et al. 2007).
Food fortification programs depend on widely distributed, industrially processed food items usually not affordable for half of the world’s population (Jorge et al. 2008). More than one third of the world’s population suffers from anemia, half of it caused by iron deficiency. Iron deficiency adversely affects cognitive development, resistance to infection, work capacity, productivity and pregnancy. Zinc is involved in cellular growth and differentiation. While mild to moderate zinc deficiency is common throughout the world (Sandsted 1995) one third of world’s population at high risk live in low income countries. Zinc deficiency causes impaired growth, immune dysfunction, increased morbidity and mortality, adverse pregnancy outcomes and abnormal neurobehavioral development. The in vitro bioaccessibility of minerals varied significantly, depending on the mineral and the type of the food matrix. In general the best sources of bioaccessible Fe and Zn were found to be pulses and nuts (Joanna and Zbigniew 2011). The limited bioavailability of cereals mineral content due to relatively low mineral levels and the presence of phytic acid and other antinutritional factors that reduce their bioavailability to 5–15 % offers challenges in nutrition point of view (Das et al. 2011). This study focuses on sources of phytic acid in food and different strategies to reduce such phytic acid food inhibitor in major food grain to improve nutritional quality of food.
Phytic acid and its sources in foods
The Phytic acid is myoinositol 1,2,3,4,5,6-hexakis dihydrogen phosphate. Phytic acid is the major storage form of phosphorous comprising 1–5 % by weight in cereals, legumes, oil seeds and nuts (Vats and Banerjee 2004). It represents 50–85 % of total phosphorous in plants (Reddy et al. 1982). Phytate rapidly accumulates in seeds during the ripening period. It is stored in leguminous seeds and oil seeds in the globoid crystal within the protein bodies (Erdman 1979). In cereal grains, rice and wheat, it is found in bran fraction such as aleurone layer and pericarp, in corn it is seen in endosperm (O’Dell et al. 1972). Monogastric animals including poultry and humans are unable to metabolize phytic acid due to the lack of sufficient level of phytate degrading enzymes activity in their digestive tract (Wodzinski and Ullah 1996; Schroder et al. 1996; Maenz and Classen 1998; Boling et al. 2000; Singh et al. 2011) and it is largely excreted in their manure. Hence food products have to supplement with inorganic phosphate to meet the phosphorous requirement (Reddy et al. 1982; Vats and Banerjee 2004). About 70 % of total P in feed is released in excreta due to inefficient uptake of phosphorous by monogastric animals (Milko et al. 2008). Hence the presence of phytic acid in animal feed for chickens and pigs are undesirable. Such high levels of phytate and inorganic phosphate through leaching or surface run-off, can lead to the eutrophication of surface water and algal blooms (Boesch et al. 2001; Turner and Haygarth 2000; Milko et al. 2008), hypoxia, death of fish and aquatic animals and production of nitrous oxide, a potent green house gas (Mallin 2000; Naqvi et al. 2000). The projected growth of livestock industry is expected to accelerate such environmental problems on a global scale. Supplementation of animal feeds with phytase improves the phosphorus bioavailability and reduces the amount of phosphorus excreted (Yano et al. 1999). It reduces phosphorus excretion by 30 % to 50 % (Lie and Porres 2003; Haefner et al. 2005; Greiner and Carlsson 2006; Selle and Ravindran 2007). Thus, for both industrial and ecological reason phytase and phytase producing microbes have attracted significant interest. Furthermore, phytic acid acts as antinutritive agent by blocking the absorption of minerals such as Fe, Zn, and Ca. The binding results in insoluble salt with poor bioavailability of minerals (Zhou and Erdman 1995; Urbano et al. 2000; Feil 2001).
Sources of phytic acid in food are cereals, legumes, oilseeds and nuts which are important for human nutrition (Table 1). It represents approximately 40 % and 60 % of total calorie intake for human in developed and developing countries respectively (Schlemmer et al. 2009). Cereals are rich in phytate and cereal food products show higher phytic acid content (Wise 1983). The phytic acid concentration reported in wheat germs and wheat bran are 1.1–3.9 % and 2.0–5.3 % respectively (Kasim and Edwards 1998). In rice bran, the phytic acid content is present upto 8.7 % (Lehrfeld 1994). In the semi refined pearl millet flour phytic acid content is significantly (P < 0.05) lower while, bran rich fraction retained significant (P < 0.05) amounts (Suma and Urooj 2011). In legume seeds, phytate is located in protein bodies of endosperm. Phytic acid content in whole seed ranged from 0.2 to 2.9 % and is higher (greater than 3.7 %) in cotyledons (Ravindran et al. 1995; Harland and Prosky 1979; Lestienne et al. 2005). The wild type legume seeds contain 0.98–3.14 g/100 g DM of phytic acid. Phytic acid content is drastically reduced during soaking plus cooking (Vellingiri and Hans 2010).
Table 1.
Content of phytic acid in major cereals, legumes, oilseeds and nuts (Schlemmer et al. 2009)
| Name | Phytic acid g/100 g(dw) | References |
|---|---|---|
| Cereals | ||
| Maize germ | 6.39 | Kasim and Edwards 1998 |
| Wheat bran | 2.1–7.3 | Harland and Oberleas 1986; Wise 1983 |
| Wheat germ | 1.14–3.91 | Wise 1983 |
| Rice bran | 2.56–8.7 | Kasim and Edwards 1998; Lehrfeld 1994 |
| Barley | 0.38–1.16 | Kasim and Edwards 1998 |
| Sorghum | 0.57–3.35 | Kasim and Edwards 1998 |
| Oat | 0.42–1.16 | Harland and Oberleas 1986 |
| Rye | 0.54–1.46 | Harland and Prosky 1979 |
| Millet | 0.18–1.67 | Lestienne et al. 2005 |
| Legumes | ||
| Kidney beans | 0.61–2.38 | Lehrfeld 1994 |
| Peas | 0.22–1.22 | Ravindran et al. 1994 |
| Chickpeas | 0.28–1.60 | Ravindran et al. 1994 |
| Lentils | 0.27–1.51 | Ravindran et al. 1994 |
| Oilseeds | ||
| Soybeans | 1.0–2.22 | Lolas et al. 1976 |
| Linseed | 2.15–3.69 | Wise 1983 |
| Sesame seed | 1.44–5.36 | Harland and Oberleas 1986 |
| Sunflower meal | 3.9–4.3 | Kasim and Edwards 1998 |
| Nuts | ||
| Peanuts | 0.17–4.47 | Venktachalam and Sathe 2006 |
| Almonds | 0.35–9.42 | Harland and Oberleas 1986 |
| Walnuts | 0.20–6.69 | Chen 2004 |
| Cashew nuts | 0.19–4.98 | Chen 2004 |
The phytic acid content varies from approx. 1.0–5.4 % (dw) in oilseeds such as soybeans, sesame seeds, sunflower kernels, linseeds and rape seeds (Lolas et al. 1976). A maximum phytic acid content of 10.7 % is reported in soy concentrates (Lehrfeld 1994). The next group of phytate rich food is nuts such as walnuts, almond, cashew nuts etc. in which phytic acid content ranged from approx.0.1–9.4 % (Chen 2004; Venktachalam and Sathe 2006; Schlemmer et al. 2009). Zhang and Bai (2011) extracted phytic acid from rice bran and its content was 2.15 %.
Dephytinization and nutrition
Phytic acid binds to minerals and makes them unavailable due to its chelating property. It has been reported that phytic acid inhibits absorption of iron, zinc calcium, magnesium and manganese (Hallberg et al. 1989; Reddy et al. 1996; Bohn et al. 2004; Phillippy 2006). Removal of phytic acid increases bioavailability of many cations and thus nutritional value of meal. There are several methods which are developed for removal of phytic acid from grains (Nout 1993).
Milling and soaking
Milling is the most commonly used method to remove phytic acid from grains. This technique removes the phytic acid but also has major disadvantages as it also removes major parts of minerals and dietary fibers. Soaking is widely applied and most important method in germination and fermentation process of cereals. Soaking of cereal such as pearl millet with endogenous or exogenous phytase increased in vitro solubility of iron and zinc by 2–23 % (Lestienne et al. 2005). Soaking of sorghum flour for 24 h at room temperature reduces phytic acid level by 16–21 % (Mahgoub and Elhag 1998). Together soaking and cooking has shown much more effective to reduce phytic acid than only soaking for a short duration (Vidal-Valverde et al. 1994). In case of grains and beans soaking to be quite effective for reduction of phytic acid as well as consequent increase in mineral bioavailability (Perlas and Gibson 2002; Coulibaly et al. 2011). This method involves the complete submergence of grains in water for certain amount of time period which results in the activation of endogenous phytases. Soaking at temperature between 45 and 65 °C and pH value between 5 and 6 a considerable percentage of phytate was hydrolysed (Greiner and Konietzny 2006). These phytases are present in grains so by activation of these enzymes it has been reported that significant amount of phytic acid content in grains have been removed. This treatment also has certain disadvantages as during this treatment there occurs loss of minerals and water extractable proteins. As soaking time increased from 2 to 12 h phytic acid content in chick pea decreased by 47.4 to 55.71 % (Ertas and Turker 2012).
Fermentation
Fermentation is a metabolic process in which carbohydrates are oxidized to release energy in absence of external electron acceptor. Fermentation of food grains improves bioavailability of minerals. Phytic acid is present in cereals in the form of complexes with metal cations viz. iron, zinc, calcium and proteins. The enzymatic degradation of phytic acid requires an optimum pH which can be provided by natural fermentation. Such a degradation of phytic acid can increase the amount of soluble iron, zinc and calcium a number of folds (Haard et al. 1989). It have been reported that fermentation of millet grain for 12 and 24 h could reduce the food inhibitors, phytic acid and tannins (Coulibaly et al. 2011). Natural fermentation can achieve a large reduction in phytic acid in rice flour by the action of microbial as well as grain phytases. Phytases reduces the hexa form of phytic acid (IP6, myo-inositol 1,2,3,4,5,6-hexakisphosphate) into lower forms, such as IP5,IP4,IP3,IP2,IP1 and myo-inositol (Ragon et al. 2008). The lower forms of phytic acid have a lower binding capacity for metals like iron and zinc (Agte et al. 1997). There are 88.3 % reduction in phytate content was recorded when germinated pearl millet sprouts were fermented with mix pure cultures of Saccharomyces diasticus, S. cerevisiae, Lacto-bacillus brevis and L. fermentum at 30 °C for 72 h (Kaur et al. 2011).
Germination
This method reduces phytic acid content by up to 40 % (Masud et al. 2007). In non-germinated cereal and legume grains a little endogenous activity is found but during germination a marked increase in phytate degrading activity was observed (Greiner and Konietzny 2006). It is reported that malting of millet reduces 23.9 % phytic acid after 72 h and 45.3 % after 96 h (Makokha et al. 2002; Coulibaly et al. 2011). The greatest reduction of phytic acid phosphorus has been found in rye while smallest decrease was found in maize (Poiana et al. 2009). Marshall et al. (2011) screened cereal grains for phytic acid content and found that germination for 10 days resulted in a significant reduction (P < 0.05) in the phytate contents of all cereal grains screened. Autoclave and Microwave treatments decreased phytic acid content as they also increased total mineral content and HCl-extractability of minerals in whole wheat bread (Mustafa and Adem 2011).
Phytases and its classification
The hydrolysis of phytate to orthophosphate and lower substituted inositol phosphates is achieved enzymatically with phytase. This is the most beneficial method for reducing phytic acid content in grains as it can remove maximum amount of phytic acid without reducing mineral content of grains.
Phytases are myo-inositol hexakisphosphate phosphohydrolase that catalyses the hydrolysis of phytic acid to inorganic phosphate and myo inositol phosphate derivative (Mullaney et al. 2002). It is an acid phosphohydrolase that hydrolyzes phosphomonoester bonds from phytate thereby liberating inorganic phosphate. Phytases can be used as additives in many food products which strengthen the interest of isolation of new and efficient phytase producing microbes, obtaining efficient phytases that able to release adequate food phosphate in digestive tract and selecting thermostable phytases which are stable during processing with lowest production cost (Lei and Stahl 2001; Greiner and Konietzny 2006).
Phytases have been classified as 3-phytases (EC 3.1.3.8), and 6-phytases (EC 3.1.3.26) based on the position of first phosphate hydrolyzed. The 3-phytases initiates dephosphorylation of phytic acid at the 3 position of phytic acid and 6-phytases at position 6. The 3-phytases are the largest group of phytases which are generally found in bacteria and fungi. The 6-phytases acts basically on the carbon atom next to C5 of the inositol ring. Plant phytases acts preferentially at the C6 carbon and are 6-phytase. Phytases can be categorized into acid phytases and alkaline phytases on the basis of pH optimum (Milko et al. 2008). On the basis of catalytic property phytases have also been classified as Histidine acid phosphatase (HAP), b-Propeller phytase (BPP), cysteine phosphatase (CP) and purple acid phosphatase(PAP) (Vats and Banerjee 2004; Mullaney and Ullah, 2003; Singh et al. 2011). The 3-phytases are structurally homologous with beta-propeller phosphates and histidine acid phosphatases. It has been suggested that end product by action of 3-phytases on phytic acid is Ins (2, 4, 6) P3. Most bacterial, fungal and plant phytases belong to the HAPs. HAPs can initiate hydrolysis of phytic acid on either the C3 or the C6 position of the inositol ring and produce myo-inositol monophosphate as the final product.
Sources of phytase
Phytases are commonly found in nature and can be obtained from a number of sources including plant, animal and microorganisms (Konietzny and Greiner 2002; Vohra and Satyanarayana 2003; Milko et al. 2008). Phytase occurs very frequently in the plant kingdom. Its activity was detected in many plants species such as wheat, rye, barley, pea, bean, soybean, maize, rice, lettuce, spinach, grass, lily pollen, etc. Generally, it is assumed that during seed germination phytate, after decomposition by phytase, is utilized in the form of phosphate and inositol (Asada et al. 1969). The first report on animal phytase in calf liver and blood was given by McCollum and Hart (1908). However, phytase was reported in the blood of lower vertebrates, birds, reptiles, fishes, batrachians, sea turtle (Rapoport et al. 1941).
The major problem in production of plant phytases is that a cost-effective and efficient production of the enzymes is yet to be developed. The higher pH and thermal stability of microbial phytases compared to plant phytases have made the microbial phytases more investigated for industrial purposes (Bohn et al. 2008). The phytase production from plant is not economical since preliminary treatment is necessary and production procedure becomes time consuming, troublesome and expensive. So, the production of phytase from microbial origin is of greater potential in development. Various strains of microbial origin for phytase production were discovered which are highly responsible for the production of the phytase. Several screening programs have been carried out aiming at the isolation of different groups of bacteria, yeast and fungi having extra cellular phytase activity. Singh et al. (2013) isolated phytase producing bacteria from different soil sample and screened using phytase screening medium (PSM). Bacterium isolated from poultry farm soil (Bacillus sp.) shows 39 mm clear zone on PSM and having better phytase activity.
Over 200 fungal isolates belonging to genera Aspergillus, Mucor, Penicillium and Rhizopus have been tested for Phytase production. All isolates produces active extra cellular phytase. A. niger was identified as the most active fungal phytase producer. A survey of fungi for production of extra cellular phytase has been reported (Shieh and Ware 1968). More than 58 strains of fungi exhibited the ability of hydrolyzing phytate when grown in rape seed meal. Of them, most efficient producer of active phytase was A. ficuum. Extra cellular phytase has also been found in other Aspergillus species such as A. oryzae, A. amstelodami, A. candidus, A. flavus and A. repen (Hawson and Davis 1983). Bacillus, Klebsiella, E.coli, Pseudomonas sp. are some examples of phytase producing bacteria (Greiner and Carlsson 2006). There are few studies on the phytase of yeast such as Saccharomyces cerevisiae and Schwanniomyces castellii.
Properties of phytases
Properties of enzymes are important in determining their potential use in industrial application. The different molecular forms of phytases obtained from different sources exhibited differences in properties such as thermostability, optimum pH etc. Temperature optimum for phytases ranged from 25 to 80 °C. Thermomyces lanuginosus, a thermophilic fungus, has optimum phytase activity at 65 °C. Mesophilic fungal sps. A. fumigatus and A. niger NRRL 3135 have optimum activity at 37 °C and 55 °C respectively. Temperature optimum of phytase produced by Thermoascus auranticus is 55 °C. When temperature increases to 70 °C its 80 % activity still remained. A highly thermostable phytase from A. fumigatus can withstand temperature upto 100 °C over a period of 20 min with loss of only 10 % of initial enzyme activity, was reported by Pasamonts et al. (1997). Bacterial phytase from B. subtilis war. Notto remains active at temperature 60 °C (Shimizu 1992). Phytases active within pH range 4.5–6.0 and stability decrease dramatically when pH value is less than 3.0 and greater than 7.5. pH optimum for fungal origin phytases is between 4.5 to 5.5 and 6.5 to 7.5 for bacterial origin. A. niger NRRL3135 produces two different types of phytase-Phy A and Phy B. Phy A has optimum pH of 5.5 whereas optimum pH for Phy B is 2.0. Plant seeds phytases have been described to have usually pH optimum between 4.0 and 5.6. Recently, alkaline phytases having a pH optimum at eight were extracted by a non-ionic detergent from legume seeds (Scott 1991). Another alkaline phytase with a pH optimum at 8 was found in mature lily pollen (Hara et al. 1985; Scott and Loewus 1986). Phytases show broad substrate specificity with highest affinity for phytic acid. Phytases are high molecular weight protein ranging from 40 to 500 kDa. A. ficuum phytase contained 594 amino acids. The phytase gene (phy) of A. niger is cloned and characterized whose translated product resulted in peptide sequence containing 10 potential glycosylation sites.
Enzyme assay
Phytase activity has been detected by several assay procedures developed by Fiske and Subbarao (1925), Ames (1966), Harland and Harland (1980), Heinonen and Lahti (1981). The most common method to detect phytase activity is by measuring the phosphate liberated by action of enzyme. The hydrolyzed inorganic phosphate is measured by the method based on colorimetric measurement of phosphomolybdate. Assay developed by Harland and Harland (1980) is most commonly employed.
Genetic modification of phytase source
Genetic modification technique can be used efficiently to reduce phytic acid content in cereals by cloning the genes of phytase enzyme and by creating the transgenic plant with modified genome encoding for phytase enzyme. In this scenario transgenic rice has been developed to over-express genes encoding for phytase from Aspergillus fumigatus, ferritin from Phaseolus vulgaris and a cysteine-rich metallothionein-like protein to improve rice iron bioavailability to humans. The plant has been crossed with a recently developed β-carotene producing rice line (Lucca et al. 2001; Lie and Porres 2003). Genetic modification of crop plants for production of heterologous phytase reduces phosphate load on agricultural ecosystems as well as improving phosphate bioavalability (Brinch-Pedersen et al. 2002; Vats and Banerjee 2004). After expression of A. niger NRRL3135 phyA gene in soybean it was found that recombinant phytase exhibits similar temperature and pH optima as of native enzyme except the molecular weight of enzyme (Vats and Banerjee 2004). It has been reported that biofarming of phytase is cost effective approach for phytase production. Strain improvement studies of A. niger NRRL 3135 by UV radiation, has been showed that phytase catalytic mutant producing 3.3-fold higher phytase (phyA) than the wild type strain (Chelius and Wodzinski 1994; Vats and Banerjee 2004).
Protein modification
An ‘ideal phytase’ that has the desirable characteristics for application in animal feed industry should be active in the stomach, catalytically efficient, thermostable during animal feed processing and storage, proteolysis-resistant and cheap (Lei and Stahl 2001). It should be easily processed by the feed manufacturer for its suitability as an animal feed additive (Singh et al. 2011). Genetic manipulation techniques such as site directed mutagenesis could be employed for further amelioration of the properties. There is a need to improve phytase enzyme structure by genetic manipulations in coding sequence of genes (Kostrewa et al. 1997; Lim et al.2000). But before designing an ideal phytase and its genetic manipulation, its structure is important. The specific activity of the heat-stable A. fumigatus phytase (Tomschy et al. 2000), pH stabilility of A. niger PhyA phytase (Mullaney et al. 2002) and thermostability of E. coli AppA phytase has been improved using this approach (Rodriguez et al. 2000; Lie and Porres 2003).
Application of phytase
There are many areas where phytase can be used such as phytate elimination in feed and food industries, in fighting phosphorus pollution or environmental protection, plant growth promotion and the preparation of special myo-inositol phosphates as tools for biochemical investigation (Idriss et al. 2002; Greiner and Carlsson 2006; Singh et al. 2011). Phytase, releasing phosphate from phytate, reduces the need of feed supplementation by phosphorus. There are two basic ways how to use phytase in feeds. The first possibility is replacement of inorganic phosphorus supplementation with phytase. However, the pH, temperature etc. condition in the animal stomach or intestine are not optimal, the second method of phytase use, feed pretreatment, becomes more attractive. Canola meal used as a feedstuff for livestock and fowl was successfully dephytinitated by A. niger NRRL 3135 in a solid-state fermentation (Nair and Duvnjak 1990; Ebune et al. 1995) aiming to increase phytate phosphorus bioavailability and the nutritive value of the feed. Segueilha et al. (1993) removed phytic acid in wheat bran using phytase from the yeast S. castelii. Increased dietary consumption of cereal fibers, legumes and soy protein isolates results in an increased intake of phytate. Some food processing methods such as cooking, germination, hydrothermal treatment, fermentation and soaking are shown to reduce or remove considerable amounts of phytate in legumes (Nout and Rambouts 1990; Rehms and Barz 1995). Phytase is incorporated into commercial poultry, swine, and fish diets to improve the availability of phosphorus, minerals, amino acids, and energy. The phytate molecule and thus the nutrients bound to it cannot be absorbed in the digestive tract without enzymatic degradation by phytases. It is well known for many years that degradation of phytic acid during bread making effect mineral bioavailability (Mollgaard 1946). Therefore, several bread making procedures designed to decrease the phytate content have been reported. These include the addition of commercial phytase from wheat to whole wheat flour (Knorr et al. 1981) and the activation of the naturally occurring phytase by soaking and malting the grain. Feed treatment with phytase increases the bioavailability of inorganic phosphorus thus improving the nutritional value of food and help in fighting phosphorus pollution. Pollution caused by excess of phosphorus accumulation in soil and water can be decreased by phytases (Nahm 2002). From Bacillus subtilis, a beta-propeller phytase was constitutively expressed in tobacco and Arabidopsis. Phytase activities in leaf and root extracts in transgenic tobacco, were 7 to 9-fold higher than those in wild-type and 4 to 6-fold higher extracellular phytase activity had been recorded in transgenic plants (Lung et al. 2005; Singh et al. 2011).
Screening of micronutrient dense germplasm
Minerals and vitamins in food staples eaten widely by the poor may be increased either through conventional plant breeding or through use of transgenic technique, a process known as biofortification. Genetic variation needed for germplasm collection is a new technique such as TILLING (Till et al. 2007) proved to be very beneficial in achieving the required goal of creation of genetically variant population. The transgenic approaches can supplement ongoing breeding efforts and provide the urgently needed biofortified crop to feed the world population with nutritious food. These include recent work on tomato, which increased folate accumulation 15 fold by targeting a highly compartmentalized pathway (Diaz et al. 2007). Also iron content in rice grain was doubled by over expression of bean ferritin (Lucca et al. 2001).
A small content of Fe and Zn present in wheat (21–32 mg kg−1 and 15–22 mg kg−1) respectively (Rawat et al. 2009) and a very small portion of existing amount is retained during processing, hence low bioavailable due to presence of phytic acid food inhibitors. Using genetic biofortification approach to reduce the amount of Zn deficiency is cost-effective, easily applicable and affordable in the target populations. For a breeding program to develop new genotypes with high Zn concentration first requires existence of useful genetic variation for Zn accumulation in grain. Compared to cultivated wheat, wild and primitive wheat is a better genetic resource for high Zn concentrations. Among wild wheat tested so far, the collections of wild emmer wheat, Triticum turgidum ssp. dicoccoides, showed prominent genetic variation and the highest concentrations of Zn ranging from 14 to 190 mg Zinc kg −1. New wild emmer wheat accession have been identified recently showing very high concentrations of Zn up to 139 mg kg −1, Iron up to 88 mg kg −1 and protein up to 380 g kg −1 in seed . It has also high tolerance to drought stress and Zn deficiency in soil (Cantrell and Joppa 1991; Cakmak et al.2010)
Reducing phytic acid content through low phytic acid (lpa) mutants
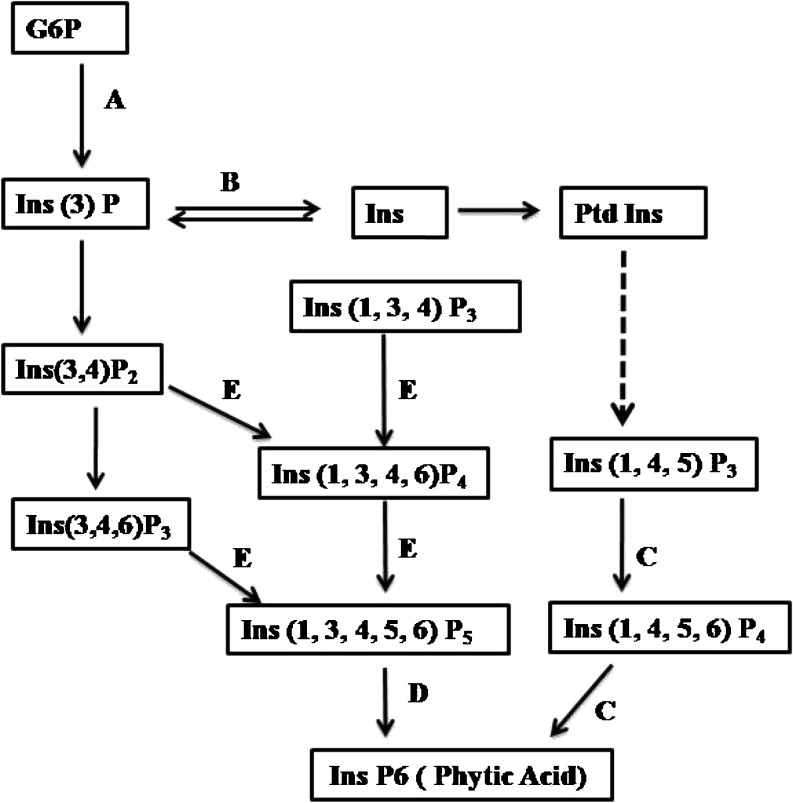
This involves the creation of gene knockout mutants by knocking out genes involved in phytic acid biosynthesis pathway (Fig. 1).
Fig. 1.
The phytic acid biosynthetic pathway in plants where A, B, C, D and E are the genes involved in phytic acid biosynthesis pathway. A.MIPS(myo-inositol 3-phosphate synthase) B. IMP(inositol monophosphatase) C. IPK2(inositol1,4,5-tris phosphate kinase) D. IPK1(inositol 1,3,4,5,6-pentakis phosphate 2-kinase) E. ITP5/6 K(inositol 1,3,4-trisphophate 5/6-kinase). G6P:glucose 6-phosphate, Ins:myo-inositol, PtdIns:phophatidyl inositol (Suzuki et al. 2007)
The TILLING population was developed by Random mutations using Ethyl methanesulfonate (EMS) chemical mutagen agents for generation of low phytic acid content as well as high endogenous phytase activity showing mutants in Pusa Basmati rice (Shukla and Singh 2012). RNAi technology has been used to reduce maize phytic acid by silencing MRP4 ATP- binding cassette (ABC) transporter (Shi et al. 2007; Gupta et al. 2011).
Conclusion and perspective
Abolition of micronutrient malnutrition remained a widespread global health problem in developing countries. Increasing micronutrient intake in food through food based approaches is a sustainable method of prevention of micronutrient malnutrition which should be achieved through food diversification. Biofortification offers a long-term, sustainable, food-based solution for a world population (Jorge et al. 2008). Breeding programs designed to improve grain Zn and Fe concentrations. In low-income countries breeding for mineral solidity may remain the only agricultural involvement to improve the nutritional content of staple crops (Cakmak et al. 2010). Genetic improvement as well as several pre-treatment methods such as fermentation, soaking, germination also improves nutritional quality. The ability of any given phytase to hydrolyze the anti nutrient phytic acid in the digestive tract is determined by its enzymatic property such as catalytic efficiency, substrate specificity, temperature stability, pH optima and resistance to proteolysis. Research is needed to discover new phytases and to engineer them to develop desired characteristic for specific purpose. Cost effective process for commercial production should be developed. The antinutritive properties and its values as a possible phosphorus source have encouraged researchers to develop a safe method to remove phytic acid. Future research is needed to determine the optimal dose and appropriate delivery of phytase to human foods.
References
- Agte VV, Gokhale MK, Chiplonkar SA. Effect of natural fermentation on in vitro zinc bioavailability in cereal-legume mixture. Int J Food Sci Tech. 1997;31:29–32. doi: 10.1046/j.1365-2621.1997.00372.x. [DOI] [Google Scholar]
- Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118
- Asada K, Tanaka K, Kasai Z. Formation of phytic acid in cereal grains. Ann NY Acad Sci. 1969;165:801–814. [PubMed] [Google Scholar]
- Boesch DF, Brinsfield RB, Magnien RE. Chesapeake bay eutrophication: scientific understanding, ecosystem restoration, and challenges for agriculture. J Environ Qua. 2001;30:303–320. doi: 10.2134/jeq2001.302303x. [DOI] [PubMed] [Google Scholar]
- Bohn T, Davidsson L, Walczyk T, Hurrell RF. Phytic acid added to white-wheat bread inhibits fractional apparent magnesium absorption in humans. Am J Clin Nutr. 2004;79:418–423. doi: 10.1093/ajcn/79.3.418. [DOI] [PubMed] [Google Scholar]
- Bohn L, Meyer AS, Rasmussen SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B. 2008;9:165–191. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling SD, Douglas MW, Johnson ML, Wang X, Parsons CM, Koelkebeck KW. The effects of dietary available phosphorus levels and phytase performance of young and older laying hens. Poult Sci. 2000;79:224–230. doi: 10.1093/ps/79.2.224. [DOI] [PubMed] [Google Scholar]
- Brinch-Pedersen H, Sorensen LD, Holm PB. Engineering crop plants: getting a handle on phosphate. Trends Plant Sci. 2002;7:118–125. doi: 10.1016/S1360-1385(01)02222-1. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Wolfgang HP, Bonnie M (2010) Biofortification of durum wheat with zinc and iron cereal chem 87:10–20
- Cantrell RG, Joppa LR. Genetic analysis of quantitative traits in wild emmer (Triticum turgidum L. var. dicoccoides) Crop Sci. 1991;31:645–649. doi: 10.2135/cropsci1991.0011183X003100030020x. [DOI] [Google Scholar]
- Chelius MK, Wodzinski RJ. Strain improvement of Aspergillus niger for phytase production. Appl Micro Biotech. 1994;41:79–83. doi: 10.1007/BF00166085. [DOI] [Google Scholar]
- Chen QC. Determination of phytic acid and inositol pentakis phosphate in foods by HPLC. Agric Food Chem. 2004;52:4604–4613. doi: 10.1021/jf035294x. [DOI] [PubMed] [Google Scholar]
- Coulibaly A, Kouakou B, Chen J. Phytic acid in cereal grains: Healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am J plant Nutr Fert Technol. 2011;1:1–22. doi: 10.3923/ajpnft.2011.1.22. [DOI] [Google Scholar]
- Das A, Raychaudhuri U, Chakraborty R. Cereal based functional food of Indian subcontinent: a review. J Food Sci Tech. 2011 doi: 10.1007/s13197-011-0474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RI, Gregory JF, III, Hanson AD. Folate biofortification of tomato fruit. Proc Natl Acad Sci USA. 2007;104:4218–4222. doi: 10.1073/pnas.0700409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebune A, Al-Asheh S, Duvnjak Z. Production of phytase during solid-state fermentation using Aspergillus ficuum NRRL 3135 in canola meal. Biores Technol. 1995;53:7–12. doi: 10.1016/0960-8524(95)00041-C. [DOI] [Google Scholar]
- Erdman JW., Jr Oilseeds phytate: nutritional implications. JAOCS. 1979;56:736–741. [Google Scholar]
- Ertas N, Turker S. Bulgur processes increase nutrition value: possible role in in-vitro protein digestability, phytic acid, trypsin inhibitor activity and mineral bioavailability. J Food Sci Tech. 2012 doi: 10.1007/s13197-012-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil B. Phytic acid. J New Seeds. 2001;3:1–35. doi: 10.1300/J153v03n03_01. [DOI] [Google Scholar]
- Fiske CH, Subbarao Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
- Greiner R, Carlsson NG. Myo-Inositol phosphate isomers generated by the action of a phytate-degrading enzyme from Klebsiella terrigena on phytate. Can J Microbiol. 2006;52:759–768. doi: 10.1139/w06-028. [DOI] [PubMed] [Google Scholar]
- Greiner R, Konietzny U. Phytase for food application. Food Technol Biotechnol. 2006;44:125–140. [Google Scholar]
- Gupta RK, Singh NK, Sharma S, Shukla KP, Singh V. Role of MicroRNA in crop plant improvement. OIJB. 2011;1:14–24. [Google Scholar]
- Guttieri MJ, Bowen D, Dorsch JA, Raboy V, Souza E. Identification and characterization of low phytic acid wheat. Crop Sci. 2004;44:418–424. doi: 10.2135/cropsci2004.4180. [DOI] [Google Scholar]
- Haard NF, Odunfa SA, Lee CH, Quintero-Ramirez A, Lorence-Quinones A, Wacher-Radarte C (1989) Fermented cereals.: a global perspective. FAO, Agricultural Service Bulletin 138
- Haefner S, Knietsch A, Scholten E, Braun J, Lohscheidt M, Zelder O. Biotechnological production and applications of phytases. Appl Microbiol Biotechnol. 2005;68:588–597. doi: 10.1007/s00253-005-0005-y. [DOI] [PubMed] [Google Scholar]
- Hallberg L, Brune M, Rossander L. Iron-absorption in man—ascorbic-acid and dose-dependent inhibition by phytate. Am J Clin Nutr. 1989;49:140–144. doi: 10.1093/ajcn/49.1.140. [DOI] [PubMed] [Google Scholar]
- Hara A, Ebina S, Kondo A, Funaguma T. A new type of phytase from pollen of Typha latifolia Agric. Biol Chem. 1985;49:3539–3544. [Google Scholar]
- Harland BF, Harland J (1980) Fermentative reduction of phytate in rye, white and whole wheat breads. Cereal Chem 57:226–229
- Harland BF, Oberleas D. Anion-exchange method for determination of phytate in foods-collaborative study. J Assoc Off Anal Chem. 1986;69:667–670. [PubMed] [Google Scholar]
- Harland BF, Prosky LD. Development of dietary fibre values for foods. Cereal Foods World. 1979;24:387–394. [Google Scholar]
- Hawson SJ, Davis RP. Production of phytate hydrolyzing enzyme by some fungi. Enzyme Microb Technol. 1983;5:377–382. doi: 10.1016/0141-0229(83)90012-1. [DOI] [Google Scholar]
- Heinonen JK, Lahti RJA (1981) New and convenient calorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Analytic Biochem 113:313–317 [DOI] [PubMed]
- Idriss E, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow HT, Richter T, Borriss R. Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology. 2002;148:2097–2109. doi: 10.1099/00221287-148-7-2097. [DOI] [PubMed] [Google Scholar]
- Iskander FY, Morad MM. Multielement determination in wheat and bran. J R N C. 1986;105:151–156. [Google Scholar]
- Joanna S, Zbigniew K. Evaluation of the content and bioaccessibility of iron, zinc, calcium and magnesium from groats, rice, leguminous grains and nuts. J Food Sci Tech. 2011 doi: 10.1007/s13197-011-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge EM, Wolfgang HP, Peter B. Biofortified crops to alleviate micronutrient malnutrition. Curr Opin Plant Biol. 2008;11:166–170. doi: 10.1016/j.pbi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Kasim AB, Edwards HMJ. The analysis of inositol phosphate forms in feed ingredients. Sci Food Agric. 1998;76:1–9. doi: 10.1002/(SICI)1097-0010(199801)76:1<1::AID-JSFA922>3.0.CO;2-9. [DOI] [Google Scholar]
- Knorr D, Watkins TR, Carlson BL. Enzymatic reduction of phytate in whole wheat breads. J Food Science. 1981;46:1866–1869. doi: 10.1111/j.1365-2621.1981.tb04506.x. [DOI] [Google Scholar]
- Konietzny U, Greiner R. Molecular and catalytic properties of phytate-degrading enzymes (phytases) Int J Food Sci Technol. 2002;37:791–812. doi: 10.1046/j.1365-2621.2002.00617.x. [DOI] [Google Scholar]
- Kostrewa D, Gruninger-Leitch F, Darcy A, Broger C, Mitchell D, Van Loon AP. Crystal structure of phytase from Aspergillus ficuum at 2.5 Å resolution. Nat Struc Biol. 1997;4:185–190. doi: 10.1038/nsb0397-185. [DOI] [PubMed] [Google Scholar]
- Kaur KD, Jha A, Sabikhi L, Singh AK. Significance of coarse cereals in health and nutrition: a review. J Food Sci Tech. 2011 doi: 10.1007/s13197-011-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrfeld J. HPLC separation and quantitation of phytic acid and some inositol phosphates in foods: problems and solutions. J Agric Food Chem. 1994;42:2726–2731. doi: 10.1021/jf00048a015. [DOI] [Google Scholar]
- Lei XG, Stahl CH. Biotechnological development of effective phytases for mineral nutrition and environmental protection. Appl Microbiol Biotechnol. 2001;57:474–481. doi: 10.1007/s002530100795. [DOI] [PubMed] [Google Scholar]
- Lestienne I, Caporiccio B, Besancon P, Rochette I, Treche S. Relative contribution of phytates, fibers and tannins to low iron and zinc in vitro solubility in pearl millet (Pennisetum glaucum) flour and grain fractions. J Agric Food Chem. 2005;53:8342–8348. doi: 10.1021/jf050741p. [DOI] [PubMed] [Google Scholar]
- Lie XG, Porres JM. Phytase enzymology, applications and biotechnology. Biotechnol Lett. 2003;25:1787–1794. doi: 10.1023/A:1026224101580. [DOI] [PubMed] [Google Scholar]
- Lim D, Golovan S, Forsberg C, Jia Z. Crystal structures of Escherichia coli phytase and its complex with phytase. Nat Struct Biol. 2000;7:108–113. doi: 10.1038/72371. [DOI] [PubMed] [Google Scholar]
- Lolas GM, Palamidids N, Markakis P. The phytic acid—total phosphorus relationship in barley, oats, soybeans and wheat. Cereal Chem. 1976;53:867–871. [Google Scholar]
- Lucca P, Hurrel R, Potrykus I. Approaches to improving the bioavailability and level of iron in rice seeds. Theor Appl Genet. 2001;102:392–397. doi: 10.1007/s001220051659. [DOI] [Google Scholar]
- Lung SC, Chan WL, Yip W, Wang L, Yeung EC, Lim BL. Secretion of beta-propeller phytase from tobacco and Arabidopsis roots enhances phosphorus utilization. Plant Sci. 2005;169:341–349. doi: 10.1016/j.plantsci.2005.03.006. [DOI] [Google Scholar]
- Maenz DD, Classen HL. Phytase activity in the small intestinal brush-border membrane of the chicken. Poult Sci. 1998;77:557–563. doi: 10.1093/ps/77.4.557. [DOI] [PubMed] [Google Scholar]
- Mahgoub SEO, Elhag SA. Effect of milling, soaking, malting, heat-treatment and fermentation on phytate level of four Sudanese sorghum cultivars. Food Chem. 1998;61:77–80. doi: 10.1016/S0308-8146(97)00109-X. [DOI] [Google Scholar]
- Makokha AO, Oniango RK, Njoroge SM, Kamar OK. Effect of traditional fermentation and malting on phytic acid and mineral availability from sorghum (Sorghum bicolor) and funger millet (Eleusine caracana) grain varieties grown in Kenya. Food Nutr Bull. 2002;23:241–245. [PubMed] [Google Scholar]
- Mallin MA. Impacts of industrial animal production on rivers and estuaries. Am Sci. 2000;88:26–37. doi: 10.1511/2000.1.26. [DOI] [Google Scholar]
- Marshall AA, Samuel JE, Mary UE, Inegbenose GI. Effect of germination on the phytase activity, phytate and total phosphorus contents of rice, maize, millet, sorghum and wheat. J Food Sci Tech. 2011;48:724–729. doi: 10.1007/s13197-010-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masud T, Mahmood T, Latif A, Sammi S, Hameed T. Influence of processing and cooking methodologies for reduction of phytic acid content in wheat (Triticum aestivum) varieties. J Food Process Pres. 2007;31:583–594. doi: 10.1111/j.1745-4549.2007.00147.x. [DOI] [Google Scholar]
- McCollum EV, Hart EB. On the occurrence of a phytin-splitting enzyme in animal tissue. J Biol Chem. 1908;4:497–500. [Google Scholar]
- Milko J, Oscar M, Fumito M, Petra M, De La Maria LM. Current and future biotechnological applications of bacterial phytases and phytase-producing bacteria. Microbes Enron. 2008;23:182–191. doi: 10.1264/jsme2.23.182. [DOI] [PubMed] [Google Scholar]
- Mollgaard H. On phytic acid, its importance in metabolism and its enzymic cleavage in bread supplemented with calcium. Biochem J. 1946;40:589–603. doi: 10.1042/bj0400589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney EJ, Daly CB, Kim T, Porres JM, Lei XG, Sethumadhavan K, Ullah AH. Site-directed mutagenesis of Aspergillus niger NRRL 3135 phytase at residue 300 to enhance catalysis at pH 4.0. Biochem Biophys Res Commun. 2002;297:1016–1020. doi: 10.1016/S0006-291X(02)02325-2. [DOI] [PubMed] [Google Scholar]
- Mullaney EJ, Ullah AH. The term phytase comprises several different classes of enzymes. Biochem Biophys Res Commun. 2003;312:179–184. doi: 10.1016/j.bbrc.2003.09.176. [DOI] [PubMed] [Google Scholar]
- Mustafa KD, Adem E. Comparison of autoclave, microwave, IR and UV-stabilization of whole wheat flour branny fractions upon the nutritional properties of whole wheat bread. J Food Sci Tech. 2011 doi: 10.1007/s13197-011-0475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm KH. Efficient feed nutrient utilization to reduce pollutants in poultry and swine manure. Crit Rev Environ Sci Technol. 2002;32:1–16. doi: 10.1080/10643380290813435. [DOI] [Google Scholar]
- Nair VC, Duvnjak Z. Reduction of phytic acid content in canola meal by Aspergillus ficuum in solid-state fermentation process. Appl Micro Biotech. 1990;34:183–188. doi: 10.1007/BF00166777. [DOI] [PubMed] [Google Scholar]
- Naqvi SWA, Jayakumar DA, Narvekar PV, Naik H, Sarma VS, Souza DW. Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature. 2000;408:346–349. doi: 10.1038/35042551. [DOI] [PubMed] [Google Scholar]
- Nout MJR, Rambouts FM. Recent developments in tempere search: a review. J Appl Bacteriol. 1990;69:609–633. doi: 10.1111/j.1365-2672.1990.tb01555.x. [DOI] [Google Scholar]
- Nout MJR. Processed weaning foods for tropical climates. Int J Food Sci Nutr. 1993;43:213–221. doi: 10.3109/09637489309027545. [DOI] [Google Scholar]
- O’Dell BL, Boland AR, Koirtyohann SR. Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J Agric Food Chem. 1972;20:18–724. [Google Scholar]
- Pasamonts L, Haiker M, Wyss M, Van Loon AP. Gene cloning, purification, and characterization of a heat stable phytase from the fungus Aspergillus fumigatus. Appl Environ Microbiol. 1997;63:1696–1700. doi: 10.1128/aem.63.5.1696-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlas LA, Gibson RS. Use of soaking to enhance the bioavailability of iron and zinc from rice-based complementary foods used in the Philippines. J Sci Food Agric. 2002;82:1115–1121. doi: 10.1002/jsfa.1156. [DOI] [Google Scholar]
- Phillippy BQ. Transport of calcium across Caco-2 cells in the presence of inositol hexakisphosphate. Nutr Res. 2006;26:146–149. doi: 10.1016/j.nutres.2006.02.008. [DOI] [Google Scholar]
- Poiana MA, Alexa E, Bragea M. Studies concerning the phosphorus bioavailability improvement of some cereals used in nourishment. Roumanian Biotechnol Lett. 2009;14:4467–4473. [Google Scholar]
- Ragon M, Aumelas A, Chemardin P, Santiago S, Moulin G, Boze H. Complete hydrolysis of myo-inositol hexakisphosphate by a novel phytase from Debaryomyces castellii CBS 2923. Appl Microbiol Biotechnol. 2008;78:47–53. doi: 10.1007/s00253-007-1275-3. [DOI] [PubMed] [Google Scholar]
- Rapoport S, Leva E, Guest GM. Phytase in plasma and erythrocytes of vertebrates. Biol Chem. 1941;139:621–632. [Google Scholar]
- Ravindran V, Ravindran G, Sivalogan S. Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem. 1994;50:133–136. doi: 10.1016/0308-8146(94)90109-0. [DOI] [Google Scholar]
- Ravindran V, Bryden WL, Kornegay ET. Phytates: occurrence, bioavailability and implications in poultry nutrition. Poult Avian Biol Rev. 1995;6:125–143. [Google Scholar]
- Rawat N, Tiwari VK, Singh N, Randhawa GS, Singh K, Chhuneja P, Dhaliwal HS. Evaluation and utilization of Aegilops and wild Triticum species for enhancing iron and zinc content in wheat. Genet Resour Crop Evol. 2009;56:53–64. doi: 10.1007/s10722-008-9344-8. [DOI] [Google Scholar]
- Reddy NR, Sathe SK, Salunkhe DK. Phytases in legumes and cereals. Adv Food Res. 1982;82:1–92. doi: 10.1016/S0065-2628(08)60110-X. [DOI] [PubMed] [Google Scholar]
- Reddy MB, Hurrell RF, Juillerat MA, Cook JD. The influence of different protein sources on phytate inhibition of nonheme-iron absorption in humans. A J Clin Nutr. 1996;63:203–207. doi: 10.1093/ajcn/63.2.203. [DOI] [PubMed] [Google Scholar]
- Rehms H, Barz W. Degradation of stachyose, raffinose, melibiose and sucrose by different tempe-producing Rhizopus fungi. Appl Microbiol Biotechnol. 1995;44:47–52. doi: 10.1007/BF00164479. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Wood ZA, Karplus PA, Lei XG. Site-directed mutagenesis improves catalytic efficiency and thermostability of Escherichia coli pH 2.5 acid phosphatase/phytase expressed in Pichia pastoris. Arch Biochem Biophys. 2000;382:105–112. doi: 10.1006/abbi.2000.2021. [DOI] [PubMed] [Google Scholar]
- Sandsted HH. Is Zinc deficiency a public health problem? Nutrition. 1995;11:87–92. [PubMed] [Google Scholar]
- Schlemmer U, Frolich W, Prieto RM, Grases F. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res. 2009;53:S330–S375. doi: 10.1002/mnfr.200900099. [DOI] [PubMed] [Google Scholar]
- Schroder B, Breve G, Rodehutscord M. Mechanisms of intestinal phosphorus absorption and availability of dietary phosphorus in pigs. Dtsch Tieraerztl Wochenschr. 1996;103:209–214. [PubMed] [Google Scholar]
- Scott JJ. Alkaline phytase activity in nonionic detergent extracts of legume seeds. Plant Physiol. 1991;95:1298–1301. doi: 10.1104/pp.95.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JJ, Loewus FA. A calcium activated phytasr from pollen of Lilium longiflorum. Plant Physiol. 1986;82:333–335. doi: 10.1104/pp.82.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segueilha L, Moulin G, Galzy P. Reduction of phytate content in wheat bran and glandless cotton flour by Schwan niomyces castelii. J Agric Food Chem. 1993;41:2451–2454. doi: 10.1021/jf00036a046. [DOI] [Google Scholar]
- Selle PH, Ravindran V. Microbial phytase in poultry nutrition. Animal Feed Sci Technol. 2007;135:1–41. doi: 10.1016/j.anifeedsci.2006.06.010. [DOI] [Google Scholar]
- Shimizu M. Purification and characterization of phytase from Bacillus subtilis (natto) N-77. Biosci Biotechnol Biochem. 1992;56:1266–1269. doi: 10.1271/bbb.56.1266. [DOI] [Google Scholar]
- Shi J, Wang H, Schellin K, Li B, Faller M, Stoop JM, Meeley RB, Ertl DS, Ranch JP, Glassman K. Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nat Biotechnol. 2007;25:930–937. doi: 10.1038/nbt1322. [DOI] [PubMed] [Google Scholar]
- Shieh TR, Ware JH. Survey of microorganism for the production of extracellular phytase. Appl Microbiol. 1968;16:1348–1351. doi: 10.1128/am.16.9.1348-1351.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Singh NK (2012) Development and characterization of Indian Indam rice TILLING population and identification of mutants having low phytic acid content by endogenous phytase activity determination. Proc World Congress Biotechnol, Hyderabad 4–6
- Singh B, Kunze G, Satyanarayana T. Developments in biochemical aspects and biotechnological applications of microbial phytases. Biotechnol Mol Bio Rev. 2011;6:69–87. [Google Scholar]
- Singh NK, Joshi DK, Gupta RK (2013) Isolation of phytase producing bacteria and optimization of phytase production parameters. J J Microbiol (in press)
- Steiner T, Mosenthin R, Zimmermann B, Greiner R, Roth S (2007) Distribution of phytase activity, total phosphorus and phytate phosphorus in legume seeds, cereals and cereal by-products as influenced by harvest year and cultivar. Anim Feed Sci Tech 133:320–334
- Suma PF, Urooj A. Nutrients, antinutrients and bioaccessible mineral content (invitro) of pearl millet as influenced by milling. J Food Sci Tech. 2011 doi: 10.1007/s13197-011-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Tanaka K, Kuwano M, Yoshida KT. Expression pattern of inositol phosphate related enzymes in rice (Oryza sativa L.): Implications for the phytic acid biosynthetic pathway. Gene. 2007;405:55–64. doi: 10.1016/j.gene.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Till BJ, Cooper J, Tai TH, Colowit P, Greene EA, Henikoff S, Comai L. Discovery of chemically induced mutation in rice by TILLING. BMC Plant Biol. 2007;7:19. doi: 10.1186/1471-2229-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomschy A, Tessier M, Wyss M, Brugger R, Broger C, Schnoebelen L, Van Loon APGM, Pasamontes M. Optimization of the catalytic properties of Aspergillus fumigatus phytase based on the three-dimensional structure. Protein Sci. 2000;9:1304–1311. doi: 10.1110/ps.9.7.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BL, Haygarth PM. Phosphorus forms and concentrations in leachate under four grassland soil types. Soil Sci Soc Am J. 2000;64:1090–1097. doi: 10.2136/sssaj2000.6431090x. [DOI] [Google Scholar]
- Urbano G, Lopez-Jurado M, Aranda P, Vidal-Valverde C, Tenorio E, Porres J. The role of phytic acid in legumes: antinutrient or beneficial function? J Physiol Biochem. 2000;56:283–294. doi: 10.1007/BF03179796. [DOI] [PubMed] [Google Scholar]
- Vats P, Banerjee UC. Production studies and catalytic properties of phytases (myo-inositol-hexakis-phosphate phosphohydrolases): an overview. Enzyme Microb Technol. 2004;35:3–14. doi: 10.1016/j.enzmictec.2004.03.010. [DOI] [Google Scholar]
- Vellingiri V, Hans KB. Effect of certain indigenous processing methods on the bioactive compounds of ten different wild type legume grains. J Food Sci Tech. 2010;49:673–684. doi: 10.1007/s13197-010-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venktachalam M, Sathe SK. Chemical composition of selected edible nut seeds. J Agric Food Chem. 2006;54:4705–4714. doi: 10.1021/jf0606959. [DOI] [PubMed] [Google Scholar]
- Vidal-Valverde C, Frias J, Estrella I, Gorospe MJ, Ruiz R, Bacon J. Effect of processing on some antinutritional factors of lentils. J Agric Food Chem. 1994;42:2291–2295. doi: 10.1021/jf00046a039. [DOI] [Google Scholar]
- Vohra A, Satyanarayana T. Phytases: microbial sources, production, purification, and potential biotechnological applications. Crit Rev Biotechnol. 2003;23:29–36. doi: 10.1080/713609297. [DOI] [PubMed] [Google Scholar]
- Wise A. Dietary factors determining the biological activities of phytase. Nutr Abstr Rev. 1983;53:791–806. [Google Scholar]
- Wodzinski RJ, Ullah AH. Phytase. Adv Appl Microbiol. 1996;42:263–301. doi: 10.1016/S0065-2164(08)70375-7. [DOI] [PubMed] [Google Scholar]
- Yano F, Nakajima T, Matsuda M. Reduction of nitrogen and phosphorus from livestock waste: a major priority for intensive animal production. Asian-Aust J Anim Sci. 1999;12:651–656. doi: 10.5713/ajas.1999.651. [DOI] [Google Scholar]
- Zhang HW, Bai XL. Optimization of extraction conditions for phytic acid from rice bran using response surface methodology and its antioxidant effects. J Food Sci Tech. 2011 doi: 10.1007/s13197-011-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JR, Erdman JW. Phytic acid in health and disease. Crit Rev Food Sci Nutr. 1995;35:495–508. doi: 10.1080/10408399509527712. [DOI] [PubMed] [Google Scholar]