Abstract
A sulfur solution with different metabisulfite concentrations (100, 400, 700, 1,000 and 2,000 ppm) was used to extract anthocyanins from saffron tepals. The extraction process was compared with acidified ethanol solution at similar extraction times of 20, 40, 60, 120, and 180 min at 40 °C. The recovery of anthocyanins with sulfur solution was higher than ethanol extraction and reached to 700 mg anthocyanins/100 g, when the sulfur concentration and extraction time were 700 ppm and 60 min, respectively. HPLC analysis showed that anthocyanins extracted with sulfur solution followed by partial desulfurization and reducing sulfur content (to less than 250 ppm) had around 100 % more cyanidin 3 glucosides and 100 % less pelargonidin 3,5 glucosides in comparison with ethanol extraction. Additionally, the color of low-sulfured anthocyanins had more saturation (chroma), less lightness, and more stability than the one extracted with ethanol solution. While monomeric and polymeric anthocyanins extracted with sulfur solution had less than 1 % changes after 3 h extraction time, they had more than 12 % changes when they extracted with alcoholic solution at similar conditions. Overall, the sulfur method had a potential to extract stable anthocyanins from waste and discarded saffron tepals in aqueous solvent, and with higher quantity and quality (more attractive color) than conventional ethanol extraction method.
Keywords: Saffron tepals, Metabisulfite, Anthocyanin stability, Monomeric and polymeric, Degradation index, Color parameters
Introduction
Owing to the high consumption of synthetic colorants in food processing and risks associated with it, intensive research has been focused on extraction, purification, and stabilization of natural pigments. For over 40 years, government-regulating agencies have attempted to prevent the use of artificial food colorant additives (Francis 1989; Hong and Wrolstad 1990). This is the main reason for food industry to focus on substituting artificial dyes, such as FD&C red 40 and the banned FD&C red 2, with natural plant pigments.
Saffron (Crocus sativus) is used as a food additive for its pleasant smell and antioxidants activity. However, in most cases the tepals of this spice with attractive colors (mainly pink and violate) are discarded after removing their stigmas (real saffron) from harvested blooms. The tepals of saffron are defined as the free segment of saffron flower (perianth) that is not clearly differentiated into calyx (sepals) and corolla (petals). While the delicate colors (mainly gold, red, and orange), flavor, and aroma of saffron are due to crocin, picrocrocin, and safranal compounds respectively (Rios et al. 1996; Abdullaev 2002), its tepals have the attractive color of anthocyanins (Hosseini and Shariatmadar 1994; Hemati-Kakhki 2010). Garrido and Diez De Bethencourt (1987) identified some antocyanidins after hydrolyzing tepals of saffron flower by using spectrophotometric and high-performance liquid chromatography (HPLC) methods. They also isolated three flavonol aglycones in this part of flower including myricetin, quercetin and kaempferol.
In the past, different solvents and acidifying solutions have been used to extract anthocyanins from wine pomace (Metivier et al. 1980), radish (Giusti and Wrolstad 1996), black carrot (Stintzing et al. 2002), Jaboticaba (Montes et al. 2005), and grape seeds (Nawaz et al. 2006). The type of solvent used for anthocyanins extraction depends on chemical composition of the plant tissue and ratio of solid–liquid extraction (Gao and Mazza 1996; Pifferi and Vaccari 1983). Gao and Mazza (1996) used aqueous SO2 solution (as a solvent) and successfully extracted anthocyanins from sunflower hulls with a considerably reduced cost. The temperature, pH, extraction time, and concentration of SO2 in the aqueous solvent of plant tissues have pronounced effects on dissolving of the cell walls and transferring their pigments into the liquid phase until the driving force of pigment from plant tissue to solvent becomes zero (Pifferi and Vaccari 1983; Bakker et al. 1998; Cacace and Mazza 2002).
The aim of this project was to study the effects of SO2 aqueous solution on pigment of saffron tepals by measuring quantity and quality criteria of its extracted anthocyanins and comparing with the one’s obtained by ethanol solution.
Materials and methods
Chemicals and sample preparation
All chemicals and reagents used in this study were of analytical grade and obtained from Merck Chemical Co (Germany). After detaching stigmas (real saffron) from saffron flowers (harvested from a farm located in the northeast of Iran), the remaining part (mainly tepals) were sorted (carefully), collected and transferred quickly to a cold storage at less than 5 °C. Then they were frozen in a kitchen freezer at less than −10 °C. Next, the frozen tepals were defrosted at room temperature and dried in a thermostatic vacuum oven (Townson & Mercer, England) at 40 °C for 36 h to around 3 % moisture. Dried tepals were crushed in a Moulinex Coffee Grinde (Moulinex International Corp., France) and passed through a 40 mesh sieve. The resulting fine powder was placed in brown vials and stored at −20 °C.
Extraction of anthocyanins by acidified ethanol (AE) solution
AE solution (with pH ~ 1) was prepared by mixing hydrochloric acid (1.5 mol/L) with ethanol (95 %) in ratio of 15:85. The tepal powder was mixed with this solution at solid–liquid ratio of 1:10 and held in a thermostatic chamber at 40 °C for various duration times (5, 10, 20, 60, 120, and 180 min). After washing the sediment of each sample with ethanol, a Hettich Universal 320 Centrifuge (Buckinghamshire, England) at 4,000 rpm for 15 min was used to separate the sediment phase of the mixture. The supernatant was collected and transferred into a 25 mL volumetric flask. A rotary evaporator (Laboratorium-Technic AG, Swiss) used to separate the ethanol portion of crude tepals at 40 °C and negative pressure of 0.1 MPa. The stock of alcohol free solution was stored at 0–5 °C for the anthocyanins determination.
Extraction of anthocyanins by sulfur water solution (SW)
Buffer solution was prepared by adding 200 mL NaOH (1 N) to 21.01 g of citric acid. Then 20 mL of the resulting solution was mixed with 80 mL of HCl (0.1 N) to make a solution with pH of 3.5. To prepare SW with pH of 3.5, different concentrations of 100, 400, 700, 1,000, and 2,000 ppm of sodium metabisulfite were added to aqueous sodium citrate. Next 1 g of pulverized tepals were mixed with 10 mL of each prepared solution and maintained at 40 °C. The anthocyanins recovery of each sulfur solution was measured after 1 h extraction time and based on the highest yield, the best sulfur concentration treatment was chosen. At this selected concentration, different extraction times of 20, 40, 60, 120, 180 min were tested to determine the optimum extraction time. The highest rate of anthocyanins recovery was at 40 °C. Each sample solution was centrifuged at similar conditions, and its supernatant was collected and transferred into a 25 mL volumetric flask. After washing the residue and centrifugation of the supernatant, it was stored at 0–5 °C for further analysis.
Anthocyanins determination
One mL of each extracted solution was diluted with two separate buffer solutions of pH 1.0 and 4.5 to make two independent volumes of 25 mL. The optical densities (O.D) of diluted samples were measured at 520 nm after their temperatures equilibrated with the surrounding environment. Distilled water was used as a blank. The Fuleki and Francis formula (1968b) and its later form Abdel-Aal and Hucl (1999) along with standard curves were then used to calculate the total anthocyanins content of each sample.
Degradation index and color properties
The DI (degradation index) is used to evaluate anthocyanin stability and it is the ratio of A420nm/A520nm in which A420nm and A520nm are the absorbance readings of anthocyanins at 420 and 520 nm (Tsai et al. 2004; Tsai and Huang 2004). Since this equation has some similarity with the basic equation (DI = T.O.DpH 1.0/ΔD.O) described by Fuleki and Francis (1968a), thus it was used to calculate DI. In this formula T.O.D (total optical density) is the absorbency of each anthocyanins sample at pH of 1, and ΔD.O is the difference between two T.O.D’s measured and calculated at pH’s of 4.5 and 1. In order to measure monomeric, polymeric, and color properties of the resulting anthocyanins stock solution, almost two-third of original sulfur was removed. The vacuum evaporation at 40 °C and negative pressure of 0.1 MPa was continued until its remaining sulfur reached to less than 250 ppm. A spectrophotometer (DR/4000U, HACK, USA) was used to measure color characteristics (L, a, and b) of low-sulfured Acy solutions. Each sample was placed in a 1 cm path length of optical glass cell and in a total transmission mode (using illuminant C and 2° observer angle) and it’s L, a, and b values were read and noted in duplicate. The formula of C = (a + b)1/2 was applied to determine the related chromas.
Monomeric and polymeric anthocyanins
To measure monomeric and polymeric anthocyanins in the extracted solutions of tepals, the bisulfate bleaching method (Giusti and Worsltad 2001) was used. The color density and the polymeric and monomeric colors were determined. Briefly in this method, bleached and unbleached trials were made by adding of 0.2 mL of 20%K2O5S2 and 0.2 mL water to 2.8 mL of each sample (de-sulfured or alcoholic) of anthocyanins solutions, respectively. The absorbance reading of two (bleached and unbleached) trials were obtained at 420 nm (λmax of anthocyanins) and at 700 nm (for correction of turbidity) and recorded. Polymeric and monomeric anthocyanins were determined using the following equations (Kalbasi and Cisneros-Zevallos 2007):
Anthocyanidins identification in tepals flowers of saffron
A Knauer HPLC system (Berlin, Germany) equipped with a Triathlon auto-sampler, a K-1001 pump, a UV–vis detector (K-2600) and Gil et al. (2000) method used to recognize and measure different anthocyanins in the tepals of saffron flowers by chromatographic analysis. Each sample of anthocyanins centrifuged in an Eppendorf tube (4 min at 5,000 rpm) and its supernatant was passed through a 0.45 μm PTFE filter (Chromafil CA-45/25 S, Duren, Germany) before 50 μL injection. To separate the components of each sample, an RP C18 Nucleosil 100 (12.5 cm _ 5.0 mm _ 5.0 μm) column was used. The mobile phase of the HPLC system had two solvents of A (2.5 % v/v, solution of acetic acid in water) and B (2.5 % v/v, solution of acetic acid in methanol). The gradient profiles of solvent A were 100 %, 90 %, 50 %, and 100 % respectively at 0–5, 15, 45, and 55 min. Furthermore, flow rate was 1.0 mL/min, and chromatograms recorded at 510 nm because it shows better sensitivity compared to other wavelengths (Gil et al. 2000). Chrom Gate software (Version3.1, build 3.1.0.2384, Knauer, Berlin, Germany) was used to record and process the data. Five different anthocyanidins including 3,5 cyanidin-diglucoside (Cy3,5), pelargonidin 3,5-diglucoside (Pg3,5), delphinidin 3-glucoside (Dp3), pelargonidin 3-glucoside (Pg3), and petunidin with different concentrations (10–40 μg/100 μL) were injected and their standard curves obtained. To quantify the amount of different anthocyanins, the peak area of each anthocyanidin was compared with its standard curve.
Measuring phenolic content in tepals of saffron flower
Five grams of dried tepal powder was added to 20 mL methanol, the resulting mixture was homogenized for 1 min with Ultra-Turrax homogenizer and stored at 4 °C for 12–24 h. Then it was centrifuged for 15 min at 14,500 RPM to prepare the methanol extract of saffron tepals. The UV spectrophotometry (based on a colorimetric oxidation/reduction reaction) method was used to measure its total phenolic content (as described by Picerno et al. 2003, and Cioffi et al. 2010). To 0.5 ml of diluted extract and 0.5 ml of distilled water (or control) samples, 2.5 ml of Folin–Ciocalteu reagent or oxidizing agent was added separately and few minutes later 10 ml of Na2CO3 (1 M) was added to each sample. After letting them react for 1 h, the absorbency of solutions were read at 750 nm. The standard curve of chlorogenic acid was obtained and the results were expressed in mg of chlorogenic acid per gram of raw material. According to Escarpa and González (2001), the total phenolic compounds of plant extracts measured by spectrophotometry can be expressed by different phenolic acids including chlorogenic, or galic acids if there is no protein and sugar in raw materials.
Statistical analysis
One-way analysis of variance and Minitab Statistical Software was used to evaluate the effects of sulfur concentration and extraction time on the total anthocyanins content of SW and AE extracts. The results announced in this study were the means of three replicates (n = 3) each with two measurements of the same solution. The data shown in figures and tables correspond to average values ± standard deviation. A statistical package of Student’s t test (available in Microsoft Office Software) including ANOVA was used to evaluate significance levels at probability of 0.05.
Results and discussion
Sulfur concentration
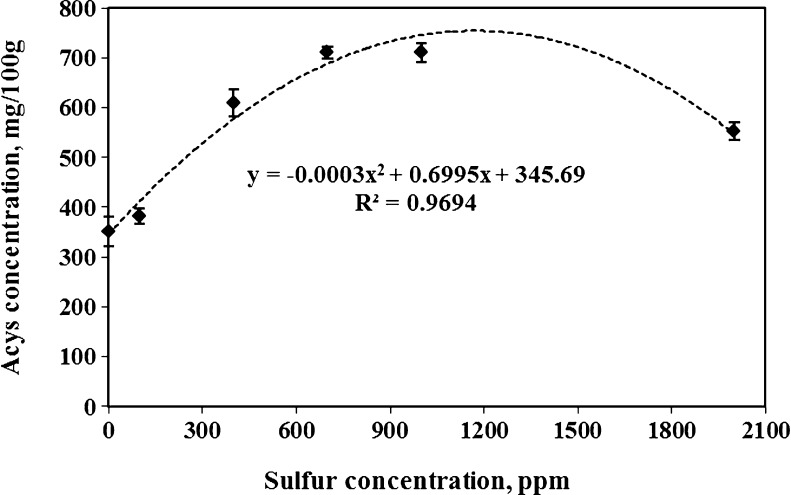
Higher yield of anthocyanins was recovered when sulfur concentration in tepals solution increased during 60 min extraction time (Fig. 1). Although part of anthocyanins was extracted in aqueous solution with zero sulfur concentration due to its high acidic environment (pH = 3.5), adding sulfur to the extracting solution drastically increased pigment recovery (30 % more in comparison with check sample or zero sulfur concentration) and became more than 500 mg/100 g. Researchers such as Pifferi and Vaccari (1983) reported that anthocyanins of plant materials are released to some extent when they are placed in acidic environment. The highest anthocyanins (close to 650 mg/100 g) from saffron tepals were obtained at a 700 ppm metabisulfite concentration. However, increasing sulfur concentration more than this amount did not enhance the amount of extracted anthocyanins. Although the trend of anthocyanin extraction for the complete range of sulfur concentration was a quadratic function (see Fig. 1), it’s very low coefficient of x2 proved this trend was very close to linear trend especially when the sulfur concentration increased from 0 to 1,000 ppm. Cacace and Mazza (2002) obtained a nearly linear increased trend when they used sulfur solutions in range of 300 to 1,100 ppm for anthocyanins extraction of black current. Increasing of SO2 concentration (up to 1,100 ppm) reduces the dielectric constant of the solvent and diminishes the required separation energy. Thus it facilitates the movement of solute particles between the solvent molecules (Cacace and Mazza 2002). Because hot (temperatures >100 °C) and subcritical water (passing water in a very short time through the extraction cell) has a lower dielectric constant than tap water. Ju and Howard (2005) used pressurized neutral hot water to speed up the extraction and diffusion rate of complex compounds. However, aqueous and acidic solutions with higher temperatures (beyond 35–40 °C) tend to degrade the polymer and complex compounds such as cellulose and anthocyanins (Jackman et al. 1987; Ju and Howard 2005). Additionally, low pH of the extracting solution makes anthocyanins molecules generally more stable (Gao and Mazza 1996). These are the main reason that each sample of sulfured-water with pH of 3.5 was heated up to 40 °C and this temperature was maintained during extraction time.
Fig. 1.
Effects of sulfur concentration on anthocyanins recovery of saffron tepals after 1 h extraction time (averages of 3 replicates)
Extraction time
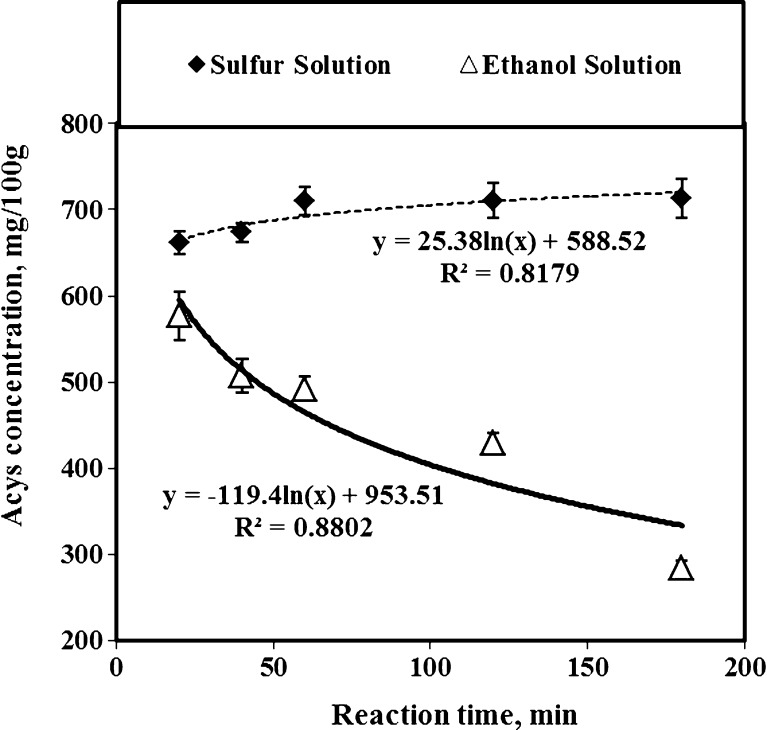
The extraction time affected the total anthocyanins recovery of tepals in sulfur (at concentration of 700 ppm) and ethanol (Fig. 2) solutions. While the SW recovered an anthocyanins yield of 650 mg/100 g after 20 min of extraction time, the anthocyanins yield extracted by AE with the same extraction time was about 580 mg/100 g. When the extraction time was extended from 20 to 60 min, the total recovered anthocyanins of sulfur method changed from 650 to 710 mg/100 g. However, when the extraction time increased more than one hour, the change in anthocyanins recovery was insignificant. Hence, it was not useful to extend the extraction time for more than 60 min (Table 1). The negative coefficient of trendline equation for anthocyanin extracted by AE method showed clearly that the increasing of extraction time did not improve the anthocyanins removal of tepal powder, and diminished the recovered anthocyanins considerably due to degradation (Fig. 2). Although the role of sulfur for anthocyanins of different plant material has not been fully elucidated, Cacace and Mazza (2002) theorized that the extraction time helps sulfured-water to diffuse more easily through the cell walls of plant materials and increases solubility and therefore improve the transport of pigment compounds. A one-way analysis of variances (ANOVA) showed significant differences (p < 0.05) between the extracted anthocyanins and their color properties at the extraction time of more than 60 min. While increasing the extraction time of anthocyanins with AE method changed the chorma, lightness, and hue up to 4 levels of significances, the same criteria changed much less with SW solution when the extraction time increased at similar conditions. However, the chroma (or color saturation) of anthocyanins extracted by SW method was about 40 % stronger than the ones recovered by AE method. Additionally, the calculated coefficients of determination (CD) between the extraction time and color properties showed that the R2 values of SW method were much lower than AE method. As a result, the chroma, lightness and hue of anthocyanins extracted by SW method were stable and remained relatively constant with the extraction time (Table 1).
Fig. 2.
Effects of extraction time on anthocyanins recovery of saffron tepals by using SW (with sulfur concentration of 700 ppm) and AE methods
Table 1.
Effect of extraction time on instrumental color properties of saffron tepals extracted with acidified ethanol and sulfur (after partial desulfurization (less than 250 ppm)) water solutions (the numbers in this table are averaged of three replicates and values with different superscripts in each column are significantly (α = 0.05) different)
| Extraction time | Chroma = (a2 + b2)0.5 | Lightness | Hue | |||
|---|---|---|---|---|---|---|
| (min) | Ethanol | Sulfur | Ethanol | Sulfur | Ethanol | Sulfur |
| 20 | 39.2 ± 0.4a | 63.0 ± 0.5a | 12.8 ± 0.7a | 16.9 ± 0.6a | 38.4 ± 0.8a | 38.5 ± 0.7a |
| 40 | 39.8 ± 0.8b | 63.8 ± 0.4a | 12.7 ± 0.7a | 16.5 ± 0.5a | 37.4 ± 0.6a | 38.7 ± 0.6a |
| 60 | 40.4 ± 1.1c | 65.8 ± 0.2b | 16.2 ± 0.9b | 16.2 ± 0.1b | 40.3 ± 1.3b | 38.8 ± 0.8a |
| 120 | 41.3 ± 1.2c | 65.6 ± 0.2b | 19.6 ± 1.1c | 16.2 ± 0.2b | 44.5 ± 1.6c | 38.9 ± 0.9a |
| 180 | 41.1 ± 1.1c | 64.6 ± 0.1b | 22.6 ± 1.2c | 16.2 ± 0.2b | 48.2 ± 1.9d | 38.9 ± 0.7a |
| CDa or (R2) | 0.78 | 0.22 | 0.96 | 0.52 | 0.96 | 0.71 |
aCoefficient of determination between extraction time and instrumental color properties
The effects of sodium metabisulfite concentration and extraction time on anthocyanins stability and color parameters
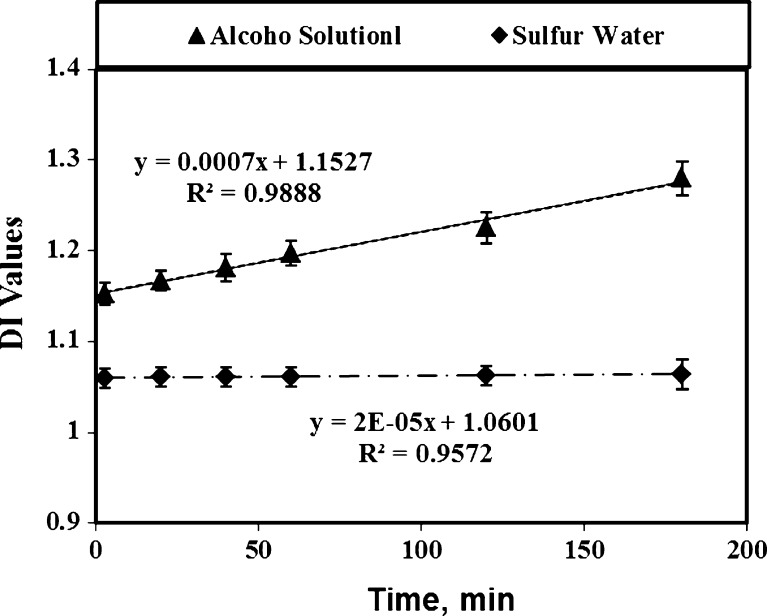
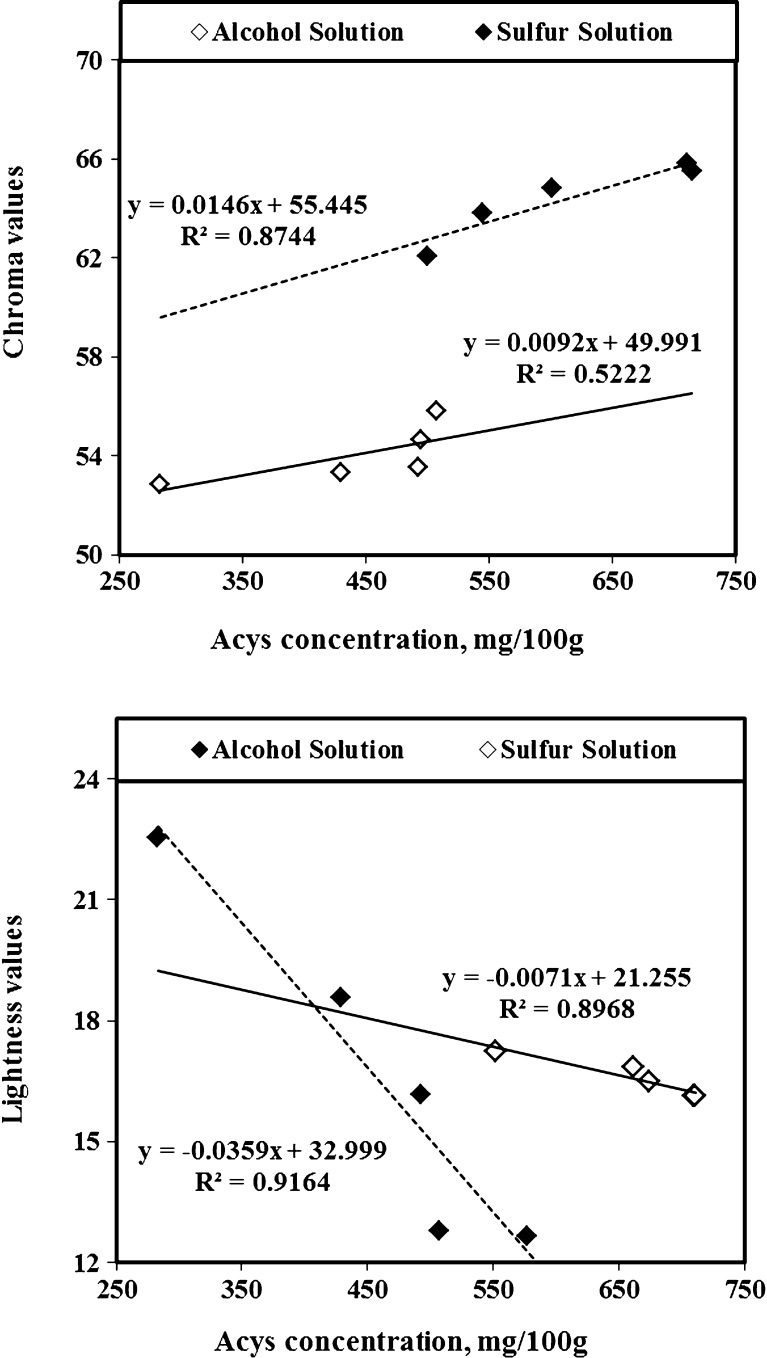
While the DI values of anthocyanins recovered by aqueous sulfur solution (with less than 250 ppm concentration) remained constant, the DI values of anthocyanins recovered by AE method increased significantly (P < 0.05) during 200 min storage time (Fig. 3). Furthermore, the remaining of sulfur compound up to 250 ppm shielded the anthocyanins compounds against degradation. Ju and Howard (2005) used other plant materials containing anthocyanins and found the same results for extracted anthocyanins. According to Gomez-Plaza et al. (2002), additional of meta-bisulfide to plant tissues results in the formation of a SO2-anthocyanin complex. This compound retards oxidation reactions and prevents anthocyanins degradation. In the degradation experiment, the DI values of anthocyanins in SW was less than in AE solvent and remained constant during extraction time up to 3 h because of the sulfur’s protecting effects against destabilization of anthocyanins pigments (Fig. 3). Tsai and Huang (2004) reported that degradation of anthocyanins in tepals extraction of Roselle (a kind of Hibiscus) without using sulfur compound caused an increase of brown color (polymeric anthocyanins) formation and losses of color (monomeric anthocyanins) along with the reduction of antioxidant power. Hence, the stabilizing effect of metabisulfite protecting anthocyanins pigments avoided browning and decomposition. Three color parameters of anthocyanins including L (lightness), a (redness), b (yellowness) and hue (dominant wavelength) were measured and the a and b values were converted to chroma (saturation) by using the equation of C = (a + b)1/2 as an index of food color property (Yang et al. 2008). The color values of extracted and de-sulfured anthocyanins changed considerably with extraction time and a more reddish color developed with time (Table 1). The highest red color of tepal’s anthocyanins (with low sulfur content) was obtained at a 60 min extraction time. While the pigment chroma extracted by SW method had a correlation (R2 = 0.87) with anthocyanins content, the similar chroma extracted by AE method had a lower correlation (R2 = 0.87). Byamukama et al. (2006) extracted Acy from flowers of Hippeastrum cultivars and found a good correlation between anthocyanins quality and color values. The F test analysis of anthocyanin content, DI, chroma, (shown in Table 2) confirmed that the SW method extracted more anthocyanin from saffron tepals with much lower degradation rate and higher chroma than the ones extracted by AE solution. As Fig. 4 (top) shows, the pigment chroma extracted by SW method had a higher correlation with anthocyanins concentration (R2 = 0.87) than the ones extracted by AE method which was (R2 = 0.52). This is again due to a higher degradation of anthocyanins extracted with AE method. Furthermore, the sulfur had a strong stabilizing effect on the anthocyanins and chroma sulfur of saffron tepals as it is used to fix the anthocyanins and chroma of red wine. The Fig. 4 (bottom) shows that L values of low-sulfured anthocyanins solution was much less than the same color parameter in anthocyanins extracted by AE solution. When anthocyanins concentration increased, the lightness reduction of SW was much less than AE solvent, due to the significant (P < 0.05) differences between their linear coefficients. Since higher concentration of anthocyanins extraction was proportional to lower L values of light transmissions in both anthocyanins extraction (SW and AE) methods, the pigment with low L values is more desirable. Additionally, as the opaqueness of extracted samples increased, their brightness (L) decreased.
Fig. 3.
Effects of storage time on degradation indexes of anthocyanins extracted by SW and AE methods (averages of 3 replicates)
Table 2.
The effects of 3 h extraction time on concentration and color properties of Acys extracted by separate SW and AE solutions using F test statistical analysis
| Sources | DF dominator | F value | F from table | Probability |
|---|---|---|---|---|
| Acys mg/100 g | 8 | 25.17a | 5.31 | P < 0.05 |
| Degradation Index | 8 | 96.3b | 5.31 | P < 0.05 |
| Chroma | 8 | 293.0b | 5.31 | P < 0.05 |
aThe aqueous sulfur could recover significantly higher Acys than ethanol solutions
bThe Acys recovered by sulfur solution had significantly lower degradation index and higher chroma than the similar Acys extracted by ethanol solution
Fig. 4.
Effects of anthocyanins concentration on chroma (top) and lightness (bottom) of saffron tepals extracted by SW and AE methods (averages of 3 replicates)
Polymeric and monomeric color
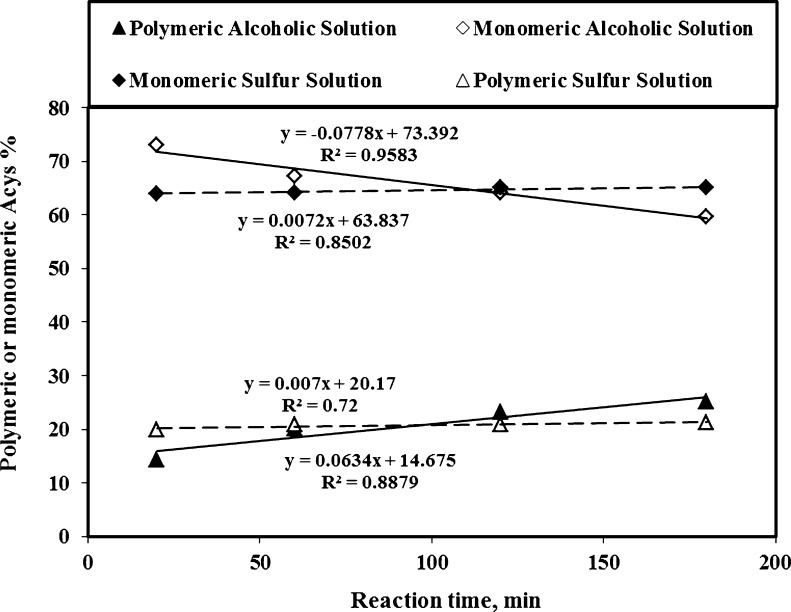
The storage time of the extracted saffron tepal pigment affected the amount of polymeric and monomeric anthocyanins (Fig. 5). Extending anthocyanins extraction of saffron tepals in the acidic solution for up to 3 h (20 to 180 min) did not show any alteration on monomeric (65 to 64) and polymeric (20 to 21) anthocyanins, whereas with AE solution there were changes in both the monomeric (72 to 60) and polymeric (14 to 29) anthocyanins. Since monomeric anthocyanins in sulfur solution are attached with metabisulfite at the free C4 position, they cannot combine with complex compounds (such as phenolics, tannins and/or proteins), and polymerization process does not occur (Bakker et al. 1998; Wrolstad et al. 2005). According to Gomez-Plaza et al. (2002), the anthocyanins stability in sulfur solution is due to formation of a SO2-anthocyanin complex, which retards or even avoids the oxidation reactions of pigment compounds. Conversely, monomeric anthocyanins extracted with alcoholic solution are not attached at this carbon position and they are easily polymerized. The presence of branched and complex groups of 3-methylbutyl and 2-methylpropyl, makes a good shield for pigment compound against bleaching sulfur-attack on the nucleophilic part of the anthocyanins (Freitas and Mateus 2006). They reported that some of the anthocyanin compounds (such as vinylpyranoMv-3-catechin) become more resistant to discoloration when the SO2 is added to their aqueous solutions. Additionally, the stabilized color of tepals was probably due to its chelating activity. Shanchez-Vioque et al. (2012) reported the color rate reduction of tepals was much lower than leaf and corm of saffron due to its very low Fe2+-chelating activity. Furthermore, the higher linear coefficients (absolute values) of polymeric and monomeric anthocyanins recovered by AE solvent in comparison with the similar values in SW method indicates that SW method had a good potential to extract anthocyanins with more color stability (Fig. 5). Most probably this was due the stronger browning reaction and higher instability of anthocyanins pigment extracted by AE solvent in contrast with the ones obtained by SW solution.
Fig. 5.
Effects of extraction time on polymeric and monomeric Acys recovered from saffron tepals by SS and AE methods (averages of 3 replicates)
HPLC analysis and total phenolic
Both solvents had 3,5 cyanidin-diglucosides (Cy3,5), pelargonidin 3 and 5 glucosides (Pg3,5), delphinidin-diglucosides (Dp3), pelargonidin 3 glucosides (Pg3) and petunidin along with traces of other anthocyanins (see Table 3 and Chromatograms in Fig. 6). The major colors of saffron tepals were pink and violet. According to Holton et al. (1993), the flowers with violet and blue colors have derivatives of delphinidin; and the ones with red and pink colors contain spinoffs of cyanidin or pelargonidin. Garrido and Diez De Bethencourt (1987) identified delphinidin and petunidin in tepals of flower in saffron. Although the method used for HPLC analysis in this study method was different from the method used by Nørbæk et al. (2002), it confirmed that petunidin and delphinidin are the major anthocyanidins of saffron tepals. The tepals and perianth of Crocus species have different color characteristics; however, Nørbæk et al. (2002) reported that different forms of dephinidin and petunidin are the major anthocyanin in perianth (the green outer plus fused petals) segment of C. sativus. While the cyanidin 3 and 5 glucosides of anthocyanins extracted by SW method was more than AE method, its pelargonidin 3 and 5 glucosides was lesser. Most probably the high difference of pelargonidin was due to the transformation of pelargonidin into other compounds during anthocyanins extraction with AE solvent. Some researchers believe that some of anthcyanidins of saffron tepals such as pelargonidin are converted to kaempferol through its hydrolysis or during pigment extraction (Hadizadeh et al. 2003). Since sulfur extraction was done in acidic conditions, most probably, 3,5 cyanidin-diglucosides is the one that makes color pigment more stable. According to Oliveira et al. (2006), the color strength of anthocyanin at high acidic values becomes higher when cyanidin-3-O-glucoside is available in pigment of plant tissue. According to Hadizadeh et al. (2003), the cyanidin and delphinidin are the main components in tepals of the saffron (C. sativus). The total phenolic content (15.24 mg Chlorogenic acid equivalent/g) was three times that of the anthocyanins content which gives tepal extracts good antioxidant properties as previously reported by Shanchez-Vioque et al. (2012).
Table 3.
Anthocyanidins (%) in saffron tepals extracted by SW and AE methods (n = 2)
| Extracting solvent | Pelargonidin 3 glycosides | Pelargonidin 3,5 glycosides | Petunidin | 3,5 cyanidin-diglycosides | Delphinidin 3 glycosides |
|---|---|---|---|---|---|
| AE method | 3.3 ± 0.3a | 58.1 ± 2.9a | 16.5 ± 0.8a | 17.9 ± 1.1a | 4.2 ± 0.6a |
| SW method | 4.1 ± 0.2a | 29.5 ± 1.6b | 16.7 ± 0.9a | 41.8 ± 2.2b | 7.9 ± 1.7b |
The numbers in this table are averaged of two replicates and values with different superscripts in each column are significantly (α = 0.05) different
Fig. 6.
Chromatograms of different anthocyanidins (from left to right pelargonidin 3 glycoside, pelargonidine 3,5 glycosides, petunidin, 3,5 cyanidin-diglycosides, and delphinidin 3 glycosides) in two samples of saffron tepals anthocyanins extracted by SW (top) and AE (bottom) solutions
Conclusions
Although 700 ppm sodium metabisulfite was used in aqueous solution of saffron tepals to dissociate its cells and release anthocyanins up to 7 mg/g (significantly higher than acidified ethanol solution), only part of sulfur compound was necessary to shield this pigment from degradation or gradual decomposition. While ethanol extraction caused considerable changes in monomeric and polymeric anthocyanins of saffron tepals, the sulfur solution with the remaining sulfur (less than 250 ppm) was able to stabilize the monomeric and polymeric anthocyanins, and also stabilize the color values (hue and chroma) of recovered pigment most probably because it had a higher amount of 3,5 cyanidin diglucosides. Overall, sulfur solution is a valuable and promising tool to rapidly extract a high quantity and quality anthocyanins and also phenolic pigments from the freshly harvested and discarded tepals of saffron and avoid using expensive and sometimes toxic organic solvents.
Acknowledgments
The authors wish to thank University of Tehran (Campus of Agricultural and Natural Sciences) and Iranian National Science Foundation for their assistances and supports that they made for this project.
References
- Abdel-Aal ESM, Hucl P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999;76:350–354. doi: 10.1094/CCHEM.1999.76.3.350. [DOI] [Google Scholar]
- Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L) Exp Biol Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- Bakker J, Bridle P, Bellworthy SJ, García-Viguera C, Reader HP, Watkins SJ. Effect of sulphur dioxide and must extraction on colour, phenolic composition and sensory quality of red table wine. J Sci Food Agric. 1998;78:297–307. doi: 10.1002/(SICI)1097-0010(199811)78:3<297::AID-JSFA117>3.0.CO;2-G. [DOI] [Google Scholar]
- Byamukama R, Jordheim M, Kiremire B, Namukobe J, Andersen ØM. Anthocyanins from flowers of Hippeastrum cultivars. Sci Hortic. 2006;109:262–266. doi: 10.1016/j.scienta.2006.05.007. [DOI] [Google Scholar]
- Cacace JE, Mazza G. Extraction of anthocyanins and other phenolics from black currants with sulfured water. J Agric Food Chem. 2002;50:5939–5946. doi: 10.1021/jf025614x. [DOI] [PubMed] [Google Scholar]
- Cioffi G, Sabina-Pesca M, De Caprariis P, Braca A, Severino L, De Tommasi N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010;121:105–111. doi: 10.1016/j.foodchem.2009.12.013. [DOI] [Google Scholar]
- Escarpa A, González MC. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal Chim Acta. 2001;427:119–127. doi: 10.1016/S0003-2670(00)01188-0. [DOI] [Google Scholar]
- Francis FJ. Food colorants: anthocyanins. Crit Rev Food Sci Nutr. 1989;28:273–314. doi: 10.1080/10408398909527503. [DOI] [PubMed] [Google Scholar]
- Freitas V, Mateus N. Chemical transformations of anthocyanins yielding a variety of colours (review) Environ Chem Lett. 2006;4:175–183. doi: 10.1007/s10311-006-0060-3. [DOI] [Google Scholar]
- Fuleki T, Francis FJ. Quantitative methods for anthocyanins II. Determination of total anthocyanins and degradation index for cranberry. J Food Sci. 1968;33:78–83. doi: 10.1111/j.1365-2621.1968.tb00888.x. [DOI] [Google Scholar]
- Fuleki T, Francis FJ. Quantitative methods for anthocyanins I. Extraction and determination of total anthocyanins in cranberry. J Food Sci. 1968;33:72–76. doi: 10.1111/j.1365-2621.1968.tb00887.x. [DOI] [Google Scholar]
- Gao L, Mazza G. Extraction of anthocyanin pigments from purple sunflower hulls. J Food Sci. 1996;61:600–603. doi: 10.1111/j.1365-2621.1996.tb13167.x. [DOI] [Google Scholar]
- Garrido JL, Diez De Bethencourt C (1987) Estudio de los flavonoides contenidos en extractors hidrolizados de tepalos de Crocus Sativus L. Anal Bromatol XXXIX or 39(1):69–80
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48(10):4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Worsltad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Current protocols in food analytical chemistry. New York: Wiley; 2001. [Google Scholar]
- Giusti MM, Wrolstad RE. Radish anthocyanin extract as a natural red colorant for maraschino cherries. J Food Sci. 1996;61(4):688–694. doi: 10.1111/j.1365-2621.1996.tb12182.x. [DOI] [Google Scholar]
- Gomez-Plaza E, Gil-Muñoz R, Lopez-Roca JM, Martinez-Cutillas A, Fernandez-Fernandez JI. Maintenance of color composition of a red wine during storage. Influence of prefermentative practices, maceration time and storage. Lebensm Wiss Technol. 2002;35:46–53. doi: 10.1006/fstl.2001.0809. [DOI] [Google Scholar]
- Hadizadeh F, Naaman Khalili N, Hossein Hosseinzadeh H, Khair-Aldine R. Kaempferol from saffron petals. Iran J Pharm Res. 2003;2:251–252. [Google Scholar]
- Hemati-Kakhki A (2010) Stability of anthocyanin extracted from saffron (crocus sativus l.) petals in a model beverage. In: Tsimidou MZ et al (eds) Proc. 3rd IS on Saffron. Acta Hort. 850, ISHS
- Holton TA, Brugliera F, Lester DR, Tanaka Y, Hyland CD, Menting JGT, Lu CY, Farcy E, Stevenson TW, Cornish EC. Cloning and expression of cytochrome P450 genes controlling flower color. Lett Nat. 1993;366:276–279. doi: 10.1038/366276a0. [DOI] [PubMed] [Google Scholar]
- Hong V, Wrolstad RE. Use of HPLC separation/photodiode array detection for characterization of anthocyanins. J Agric Food Chem. 1990;38(3):708–715. doi: 10.1021/jf00093a026. [DOI] [Google Scholar]
- Hosseini DK, Shariatmadar S (1994) Identification of anthocyanins of Crocus sativus petals. Iranian Inst. of Sci. and Tech. Rep. Khorasan Center, Iran
- Jackman RL, Yada RY, Tung MA, Speers RA. Anthocyanins as food colorants—a review. J Food Biochem. 1987;11(3):201–247. doi: 10.1111/j.1745-4514.1987.tb00123.x. [DOI] [Google Scholar]
- Ju ZY, Howard RL. Subcritical water and sulfured water extraction of anthocyanins and other phenolic from dried red grape skin. J Food Sci. 2005;70(4):270–276. doi: 10.1111/j.1365-2621.2005.tb07202.x. [DOI] [Google Scholar]
- Kalbasi A, Cisneros-Zevallos L. Fractionation of monomeric and polymeric anthocyanins from Cocord grape (Vitis Labrusca L.) juice by membrane ultrafiltration. J Agric Food Chem. 2007;55(17):7036–7042. doi: 10.1021/jf0706068. [DOI] [PubMed] [Google Scholar]
- Metivier RP, Francis FJ, Clydesdale FM. Solvent extraction of anthocyanins from wine pomace. J Food Sci. 1980;45(4):1099–1100. doi: 10.1111/j.1365-2621.1980.tb07534.x. [DOI] [Google Scholar]
- Montes C, Vicario IM, Raymundo M, Fett R, Heredia FJ. Application of tristimulus colorimetry to optimize the extraction of anthocyanins from jaboticaba (Myricia Jaboticaba Berg.) Food Res Int. 2005;38(8–9):983–988. doi: 10.1016/j.foodres.2005.01.016. [DOI] [Google Scholar]
- Nawaz H, Shi J, Mittal GS, Kakuda Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep Puriff Technol. 2006;48(2):176–181. doi: 10.1016/j.seppur.2005.07.006. [DOI] [Google Scholar]
- Nørbæk R, Brandt K, Nielsen JK, Ørgaard M, Jacobsen N. Flower pigment composition of crocus species and cultivars used for a chemotaxonomic investigation. Biochem Syst Ecol. 2002;30:763–791. doi: 10.1016/S0305-1978(02)00020-0. [DOI] [Google Scholar]
- Oliveira J, Fernandes V, Miranda C, Santos-Buelga C, Silva A, Freitas VD, Mateus N. Color properties of four cyanidin-pyruvic acid adducts. J Agric Food Chem. 2006;54(18):6894–6903. doi: 10.1021/jf061085b. [DOI] [PubMed] [Google Scholar]
- Picerno P, Mencherini T, Rosaria-Laurom M, Barbato F, Aquino R. Phenolic constituents and antioxidant properties of Xanthosoma violaceum leaves. J Agric Food Chem. 2003;51(22):6423–6428. doi: 10.1021/jf030284h. [DOI] [PubMed] [Google Scholar]
- Pifferi PG, Vaccari A. The anthocyanins of sunflower. II. A study of the extraction process. Int J Food Sci Technol. 1983;18(5):629–638. doi: 10.1111/j.1365-2621.1983.tb00302.x. [DOI] [Google Scholar]
- Rios JL, Recio MC, Giner RM, Manez S. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–193. doi: 10.1002/(SICI)1099-1573(199605)10:3<189::AID-PTR754>3.0.CO;2-C. [DOI] [Google Scholar]
- Shanchez-Vioque R, Rodríguez-Conde MF, Reina-Ureña JV, Escolano-Tercero MA, Herraiz-Peñalver D, Santana-Méridas O. In vitro antioxidant and metal chelating properties of corm, tepal and leaf from saffron (Crocus sativus L.) Ind Crop Prod. 2012;39:149–153. doi: 10.1016/j.indcrop.2012.02.028. [DOI] [Google Scholar]
- Stintzing FC, Stintzing AS, Carle R, Frei B, Wrolstad RE. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J Agric Food Chem. 2002;50(21):6172–6181. doi: 10.1021/jf0204811. [DOI] [PubMed] [Google Scholar]
- Tsai P-J, Huang T-C. Effect of polymerization on the antioxidant capacity of anthocyanins in roselle. Food Res Int. 2004;37:313–318. doi: 10.1016/j.foodres.2003.12.007. [DOI] [Google Scholar]
- Tsai P-J, Hsieh Y-Y, Huang T-C. Effect of sugar on anthocyanin degradation and water mobility in a roselle anthocyanin model system using 17O NMR. J Agric Food Chem. 2004;52(10):3097–3099. doi: 10.1021/jf0306587. [DOI] [PubMed] [Google Scholar]
- Wrolstad RE, Durst RW, Lee J. Tracking color and pigment changes in anthocyanin products. Food Sci Technol. 2005;16:423–428. doi: 10.1016/j.tifs.2005.03.019. [DOI] [Google Scholar]
- Yang Z, Han Y, Gu Z, Fan G. Thermal degradation kinetics of aqueous anthocyanins and visual color of purple corn (Zea mays L.) cob. Innov Food Sci Emerg Technol. 2008;9:341–347. doi: 10.1016/j.ifset.2007.09.001. [DOI] [Google Scholar]