Abstract
In the present work, Qingzhuan tea, a unique dark tea produced by post-fermentation technology, was selected to investigate its antioxidant and pancreatic α-amylase inhibiting activities. Water extract of Qingzhuan tea was successively isolated by solvent partitioning procedures to obtain chloroform, ethyl acetate, n-butanol, sediment and residual aqua fractions. Of different fractions, the ethyl acetate fraction (QEF) had the highest total polyphenols and catechins contents, demonstrated the strongest DPPH radical scavenging activity and exhibited the greatest inhibitory effect on porcine pancreatic α-amylase activity in vitro. Further separation of QEF by a Sephadex LH-20 column generated eight subfractions (QEF1–QEF8), with QEF8 being the most active subfraction based on the assays above mentioned. The major active components in QEF8 were identified as catechins EGCG and ECG by LC-MS analysis, with contents of 22.29 % and 11.11 % respectively. Inhibitory effects of catechin standards EGCG and ECG on porcine pancreatic α-amylase activity were also observed. In conclusion, Qingzhuan tea or its water extract could be potentially used as complementary therapy ingredients for diabetes treatment through lowering postprandial blood glucose, and catechins EGCG and ECG may be the most efficient components in the water extract.
Keywords: Qingzhuan tea, Tea extract, Antioxidant, α-amylase, Polyphenol, Column chromatography
Introduction
Global diabetes cases are increasing rapidly and cost vast amounts of resources around the world. Excessive consumption of carbohydrates is revealed to play causative roles in development of various chronic diseases such as obesity, type 2 diabetes and cardiovascular diseases (CVD) (Ludwig 2002). As the major dietary energy source, starch is mainly digested in the gastrointestinal tract by pancreatic α-amylase, which is synthesized and secreted by the pancreatic. Slowing the digestion or breakdown of starch may have beneficial effects on insulin resistance and glycemic index control in diabetes patients (Notkins 2002). Introduction of α-amylase inhibitor into diets has been demonstrated to be effective in retarding carbohydrate digestion (Golay et al. 1991). Amylase inhibition has gastrointestinal and metabolic effects that may aid in the treatment of diabetes (Layer et al. 1986). Synthetic hypoglycaemic chemicals can produce serious side effects and are not suitable for use during pregnancy (Gilman et al. 1985). Therefore, isolating more effective and safer hypoglycaemic compounds from natural plants has become research trend. Natural α-amylase inhibitor has been identified in various plant sources such as cumin seeds, amaranthus caudatus seeds and mangosteen pericarp (Ani and Naidu 2008; Eng Kiat Loo and Huang 2007; Conforti et al. 2005).
Recent evidence suggests that oxidative stress is the underlying mechanism for diabetes development and diabetic complications (Halliwell and Gutteridge 1989). Oxidative stress results from the imbalance between pro-oxidant and antioxidant chemicals and leads to cell and tissue damages. Implications of oxidative stress in the pathogenesis of diabetes are comprehensive, involving oxygen free-radical generation, alteration in antioxidant enzymes and lipid peroxides formation etc. (Moussa 2008). Dietary antioxidants have been proposed to slow the progression and ameliorate diabetes. Grapes and tea, which contain many kinds of phenolic compounds, have been verified to induce an anti-hyperglycemic effect in diabetes animal models (Zunino 2009; Hosoda et al. 2003).
Tea is one of the most widely consumed beverage in the world. Recent studies have suggested that it has numerous beneficial health effects in preventing various chronic diseases such as cancer, diabetes, CVD and obesity (Zhu et al. 2006). Furthermore, the radical scavenging and antioxidant properties of tea polyphenols are frequently cited as important contributors to its health improving mechanisms (Higdon and Frei 2003). Aside from their antioxidant bioactivity, tea polyphenols are also shown to be inhibitors of a-amylase (Hara and Honda 1990; He et al. 2007), which provided support to the finding that consumption of tea decreased utilization of dietary carbohydrates (Zhong et al. 2006).
Qingzhuan tea is categorized as a compressed dark tea as Pu-erh tea with over 100 years of production history. It is made with primary tea, which was classified into two kinds of tea, superface tea and inner tea. Superface tea was prepared with fresh leaves with green stem through the processing of green removing, primary rolling, primary sunning, the second parching, the second rolling, pile-fermenting and sun drying. Inner tea was processed with more old fresh leaves with red stem by green removing, rolling, pile-fermenting and sun drying. After cutting and sieving, superface tea and inner tea were steamed for 1.5 min at 120–130 °C, and then poured into a mold in a certain proportion and order to harden into shape. After cooling, the formed tea were removed to the barn to dry for 15 days at 36 °C. When the content of water declined to 8.5–9 %, Qingzhuan tea product was obtained. As an indispensable beverage for people living in Sinkiang and Tibet areas where vegetables and fruits are often in shortage, Qingzhuan tea’s disease prevention and general health care effects were long believed by people living there. Current publications have reported the in vivo pharmacological efficacy of Qingzhuan tea in obesity control (Chen et al. 2008) and lipid clearance (Chen et al. 2010). However, the in vitro antioxidant activity and inhibitory effect on pancreatic α-amylase activity of Qingzhuan tea have not been reported yet.
The objectives of this work were to evaluate the in vitro DPPH radical-scavenging and pancreatic α-amylase inhibitory activities of different Qingzhuan tea extracts and chromatographical isolations and tentatively identify bioactive components contributing to their bioactivities.
Materials and methods
Plant materials
Qingzhuan tea (produced in 1997 and stored for 12 years) supplied by Zhaoliqiao Tea Factory, Xianning city, Hubei province, China., and stored at −20 °C before extracting.
Chemicals and reagents
α-Amylase from porcine pancreatic and 2-Diphenyl-1-picryhydrazyl (DPPH) radical were purchased from Sigma Chemical Co. (St. Louis, MO). The standards of (-)-epicatechin (EC), (-)-epigallocate-chin (EGC), (+)-catechin (C), (-)-epicatechin gallate (ECG) and (-)-epigallocatechin gallate (EGCG) were purchased from Fisher Chemical Reagent Co. Ltd., USA. Methanol (chromatographic grade) was purchased from Fisher ChemAlert Guide, New Jersey, USA. Sephadex LH-20 was purchased from Pharmacia Biotech, Sweden. All other chemicals used were of analytical grades.
Preparation of extracts from Qingzhuan Tea
Qingzhuan tea (50 g) was minced adequately and then extracted with 1,000 mL of distilled water at 100 °C for 7 min while stirring continuously. After cooling, the slurries were filtered through medium speed filter papers under vacuum condition and the supernatant was collected. The residue was extracted once more under the same conditions, and the supernatants were combined. The solution was concentrated to 200 mL by a rotary evaporator at 55 °C under reduced pressure. The concentrated water extract was then successively extracted with 600 mL of chloroform, ethyl acetate, and n-butanol, 3 times per organic solvent. After removal of the organic solvents by vacuum concentration, four fractions were obtained: chloroform, ethyl acetate, n-butanol and aqua fractions. The aqua fraction was precipitated with 3 times volume of 95 % ethanol (ethanol/water, 95:5 v/v) for 12 h at room temperature to get the sediment fraction and the residual aqua fraction.
Chromatography on Sephadex LH-20
The ethyl acetate fraction of Qingzhuan tea water extract (QEF) was selected for further isolating according to the method described by Jie et al. (Jie et al. 2006) with minor modification. In brief, the Sephadex LH-20 column (50 cm × 1.6 cm i.d., GE Healthcare Bio-Sciences AB, Sweden) was previously equilibrated with water. Then the ethyl acetate fraction was dissolved in methanol and loaded to the column. The solution was eluted with gradient elution (began with water, followed by an increase of 10 % per degree, 500 mL per degree, and finally with 500 mL of 50 % acetone at a flow rate of 1.0 mL/min). The eluent was sequentially collected, using a fraction collector, and the absorbance was detected at 280 nm, using a UV spectrophotometer. The QEF was fractionated into eight subfractions assigned as QEF1-8, which were evaluated by in vitro DPPH radical-scavenging activity and inhibitory effect on pancreatic α-amylase activity.
Determination of total polyphenols and catechins contents
The measurement of total polyphenol was followed by the China National standard method (The first research institute of China Standards Publisher 2003). In brief, 1 mL of sample solution was added to 4 ml of ferrous tartrate, then phosphate buffer (pH = 7.5) was added to the mixture to make the volumn up to 25 mL. After mixing well, absorbance was measured with 10 mm cuvette at 540 nm. Distilled water was used instead of the sample solution as the control. Catechins were determined by high performance liquid chromatography (HPLC) method as described by Zhou et al. (2009). An VARIAN HPLC (Model PROSEAR230,USA) equipped with a photo-diode-array detector was employed in the present assay. The analytical column is Agilent TC-C18 column (150 × 4.6 mm inner diameter, with a particle size of 5 μm, USA). Mobile phases consisted of methanol (with 0.1 % formic acid, mobile phase A) and water (with 0.1 % formic acid). A gradient elution was adopted as follows: 0–1 min, 75 % A; 1–2 min, 75–80 % A; 2–5 min, 80 % A; 5–10 min, 80–75 % A; 10–17 min, 75 % A. The flow rate and column temperature were maintained at 1.0 mL/min and 30 °C, respectively. Samples were dissolved, filtered and injected to the column. The injection volume was set at 20 μL and detection wavelength was 280 nm.
DPPH radical scavenging assay
The DPPH radical-scavenging assay was determined by using a previously reported method with a slight modification (Kondo et al. 2002). The reaction mixture contained 1.0 mL of 0.15 mM DPPH radical solution dissolved in methanol and 1.0 mL of various concentrations of samples. The absorbance at 516 nm was measured after the reaction was kept at room temperature in the dark for 30 min. Reagent solution without test samples was used as the control. The scavenging ability was expressed as EC50, represented the effective concentration providing a 50 % of scavenging rate. A lower EC50 value means higher DPPH radical-scavenging activity.
Pancreatic α-amylase inhibition assay
The inhibitory activity of samples against pancreatic α-amylase was assayed with an iodine-starch kit following the method of AI-Dabbas et al. (Al-Dabbas et al. 2006). Briefly, 50 μL of aqueous solution of the isolated compounds of different concentrations was mixed with 1.0 mL of starch substrate (0.4 g/L) in phosphate buffer (pH 7.0). After 5 min of incubation at 37 °C, 50 μL of α-amylase solution (1 mg/mL) was added to the mixture. After the mixture was further incubated for 7.5 min, 1.0 mL of iodine diluent (0.01 mol/L) was added to end the reaction and 3.0 mL of deionized water was added to dilute the solution to an appropriate concentration for measuring the absorbance at 660 nm. Each sample was analyzed in triplicates. The inhibition of α-amylase activity in the presence of samples is calculated by the following equations:
Inhibitory abilities of Qingzhuan tea fractions on α-amylase activity were expressed as their 50 % inhibition concentration (IC50). The lower the IC50 value, the higher the activity for inhibiting effect the Qingzhuan tea fractions possessed on α-amylase.
Liquid chromatography–mass spectrometry (LC-MS) analysis
Because of its best activity in pancreatic α-amylase inhibition and DPPH radical scavenging, QEF8 was further analyzed by LC-MS. In brief, the sample was applied to a C18 column (5 μm,150 mm*4.6 mm i.d., Agilent TC) maintained at 35 °C, and eluted using a gradient of 3 % acetonitrile (0.5 % formic acid) to 30 % acetonitrile (0.5 % formic acid) over 45 min at a flow rate of 1.0 mL/min. The injection volume was 10 μL. The UV spectra were scanned from 190 to 400 nm. Peaks were determined at 275 nm. The MS parameters were set as follows: negative mode; flow rate of dry gas, 40 L/min; dry temperature, 250 °C; m/z, 100–900; capillary voltage, 3500v; ESI voltage, 10kv; discharge voltage, 124.8v.
Statistical analysis
All the data were expressed as means ± standard deviation (SD) of three replicates. Significant differences at p < 0.05 among means were determined using one-way analysis of variance (one-way ANOVA) in SAS system for Windows V8.
Results and discussion
Total polyphenols and catechins contents of five fractions from Qingzhuan tea crude water extract
Qingzhuan tea is a speciality tea produced with postfermentation technology and characterized by a period of fungal growth during its manufacturing process. Previous study by the present authors indicated that levels of total polyphenols and catechins in Qingzhuan tea after fermentation with microorganisms were decreased due to oxidation and degradation under the catalytic effects of endogenous and exogenous enzymes (Chen et al. 2009). In the present study, crude water extract of Qingzhuan tea was divided into five fractions by polarity and the total polyphenols and catechins contents of these fractions were detected (Table 1). Among the five fractions, the ethyl acetate fraction had the highest total polyphenols content, followed by the n-butanol fraction and residual aqua fraction, while the sediment fraction and chloroform fraction had the lowest values (p < 0.05). Fu et al. applied the liquid–liquid partition to Fuzhuan tea and obtained the same trend (Fu et al. 2008). HPLC analysis indicated different catechin profiles for various fractions. Total catechins include C, EC, EGC, ECG and EGCG. The total catechins content of the ethyl acetate fraction was significantly greater than that in the n-butanol fraction (p < 0.05), while there was negligible amount of catechin in the chloroform fraction and residual aqua fraction and no detection in the sediment fraction. In the ethyl acetate fraction, the content of ester catechins was 1.53-fold higher than the non-ester catechins. These results showed that extracts of different solvents from Qingzhuan tea had substantially different chemical compositions and structures and tea polyphenols and catechins were much more soluble in ethyl acetate than in other organic solvents and water.
Table 1.
The contents of tea polyphenols and catechins of different fractions from Qingzhuan tea water extract (mg/g)
| Total polyphenols | Total catechins | Individual catechin | |||||
|---|---|---|---|---|---|---|---|
| C | EC | EGC | ECG | EGCG | |||
| Chloroform fraction | 9.8 ± 0.57e | 3.6 ± 0.59c | 3.6 ± 0.59 | ND | ND | ND | ND |
| Ethyl acetate fraction | 787. 2 ± 26.32a | 331.4 ± 9.54a | 86.5 ± 0.89 | 21.2 ± 0.21 | 23.1 ± 0.89 | 53.7 ± 0.38 | 146.9 ± 8.60 |
| n-Butanol fraction | 310.9 ± 7.25b | 28.1 ± 0.67b | 19.8 ± 1.07 | ND | 6.6 ± 0.20 | 1.7 ± 0.07 | ND |
| Sediment fraction | 91.6 ± 3.54d | ND | ND | ND | ND | ND | ND |
| Residial aqua fraction | 111.5 ± 3.13c | 4.9 ± 0.29c | 4.9 ± 0.29 | ND | ND | ND | ND |
n = 3, Mean ± SD
Values with no letter in common are significantly different (p < 0.05)
C, (+)-catechin; EC, (-)-epicatechin; EGC, (-)-epigallocate-chin; ECG, (-)-epicatechin gallate; EGCG, (-)-epigallocatechin gallate
ND means not detected
Compared with green tea, Qingzhuan tea had lower level of polyphenols but higher levels of polysaccharides and thearubigins (Chen et al. 2009). In the fermentation process, ester catechins are decomposed by hydrolysis with the production of non-ester catechins and gallic acid (Zhang et al. 2011). Therefore, Qingzhuan tea may contain higher amount of gallic acid than green tea. In addition, catechins react with each other to generate theaflavins by polymerization, and then theaflavins continue to converge into thearubigins, finally the small molecular polyphenols polymerize into theabrownins with higher molecular weight. Thus, Qingzhuan tea has a high concentration of theabrownins, the main cause of its color. Due to variations of environmental conditons, composition of fermented tea was significantly changed and some new compounds were identified for the first time in Fuzhuan tea and Pu-erh tea (Ling et al. 2010; Zhang et al. 2009). Qingzhuan tea may also contain other unknown chemicals.
DPPH radical-scavenging activity of five fractions from Qingzhuan tea water extract
In a previous study of the inhibitory effect of Qingzhuan tea on free radicals, the present authors used the in vitro xanthine oxidase and D-deoxyribose-iron system methods and observed that Qingzhuan tea water extract had strong scavenging effects on superoxide anions and hydroxyl radicals (Chen et al. 2009). In the present study, DPPH radical-scavenging assay, which is widely used in determining the hydrogen donating ability of various natural products (Miliauskas et al. 2004; Chen et al. 1999), was employed to measure the antioxidant capacity of different fractions of Qingzhuan tea water extract. Linear regression analysis revealed that all the fractions displayed a good linear relationship between the scavenging rate and the sample concentration. The regression equations, correlation coefficients and EC50 values were listed in Table 2. Compared with the ethyl acetate fraction, the EC50 value of the n-butanol fraction was 2.63-fold higher while the residual aqua fraction, the sediment fraction and the chloroform fraction were 7.40-fold, 8.25-fold and 37.11-fold higher, respectively. The ethyl acetate fraction had the strongest DPPH radical-scavenging activity, followed by the n-butanol fraction, while the scavenging activity of the chloroform fraction was the weakest.
Table 2.
Scavenging effects on DPPH and inhibiting effects on pancreatic α-amylase activity of Qingzhuan Tea fractions
| Fractions | Scavenging effect | Inhibiting effect | ||||
|---|---|---|---|---|---|---|
| EC50(μg/mL) | Regression equation | R2 | IC50(mg/mL) | Regression equation | R2 | |
| Chloroform fraction | 244.6 ± 24.62 | y = 0.1049x + 24.3730 | 0.9667 | no activity | ||
| Ethyl acetate fraction | 6.6 ± 0.90 | y = 6.0419x + 10.2290 | 0.9668 | 4.6 ± 0.02 | y = 9.5624x + 5.6776 | 0.9832 |
| n-Butanol fraction | 17.3 ± 2.89 | y = 2.0741x + 14.0570 | 0.9680 | 11.3 ± 0.32 | y = 4.7757x–3.8321 | 0.9497 |
| Sediment fraction | 54.5 ± 8.16 | y = 0.6736x + 13.4080 | 0.9879 | no activity | ||
| Residial aqua fraction | 48.8 ± 6.66 | y = 0.7486x + 13.4940 | 0.9835 | no activity | ||
n = 3, Mean ± SD
The above results showed that total polyphenols, total catechins and antioxidant activities were significantly different among different isolations. Tea polyphenols and catechins were verified to have antioxidant activities (Higdon and Frei 2003). Correlation analysis between the levels of total polyphenols and catechins and EC50 values were undertaken and both were greatly correlated with DPPH assays (r2 = 0.607 and r2 = 0.654, respectively). Previous studies reported similar correlations between these parameters for green tea and other types of tea products (Fukushima et al. 2009; Anesini et al. 2008; Karori et al. 2010). It was inferred that DPPH radical-scavenging activity was likely due to polyphenols and catechins existed in the Qingzhuan tea extracts.
Inhibitory effect of five fractions from Qingzhuan tea water extract on in vitro pancreatic α-amylase activity
α-Amylases catalyze the hydrolysis of α-1, 4-glucosidic linkage of starch to initiate starch digestion and promote glucose absorption. α- Amylase inhibitors were considered to be effective in diabetes control (Ponnusamy et al. 2011). In this study, an in vitro α-amylase inhibition model was used to screen the extracts of Qingzhuan tea to evaluate their potential hypoglycaemic effects.
The α-amylase inhibitory activity of Qingzhuan tea fractions was assayed by the method of iodine-starch reaction. As shown in Table 2, no α-amylase inhibition was observed in the chloroform fraction, sediment fraction and residual aqua fraction of Qingzhuan tea. The ethyl acetate and n-butanol fraction exhibited a dose-dependent inhibitory effect on α-amylase activity. The IC50 value of n-butanol fraction (11.27 mg/mL) was 2.43-fold higher than the ethyl acetate fraction (4.64 mg/mL), suggesting that the ethyl acetate fraction exhibited greater inhibitory activity than n-butanol fraction. A similar situation was found in a study performed on black tea (Kusano et al. 2008).
Based on the above result, we found that the ethyl acetate fraction contained the highest amount of tea polyphenols and had the greatest α-amylase inhibitory activity. It’s obvious that increasing polyphenols concentration would increase α-amylase inhibition. Thompson and Yoon (1984) reached a similar conclusion in their study on starch digestibility affected by polyphenols. Other studies suggested that tea polyphenols had the capacity to inhibit α-amylase activity (Hara and Honda 1990; He et al. 2007). It was concluded that tea polyphenols might be an important factor in contributing to the α-amylase inhibitory activity of Qingzhuan tea extract.
DPPH radical-scavenging activity of eight subfractions from the ethyl acetate fraction
As the ethyl acetate fraction had the highest tea polyphenols and catechins contents, the strongest DPPH radical-scavenging activity and the greatest inhibitory effect on α-amylase activity in vitro, further separation with a Sephadex LH-20 column by different concentrations of methanol as mobile phase was performed and generated eight subfractions. Table 3 showed that their IC50 values ranged from 4.55 to 360.27 μg/mL, and were in the following order: QEF1 > QEF2 > QEF3 > QEF4 > QEF5 > QEF6 > QEF7 > QEF8. It was concluded that QEF8 exhibited the strongest scavenging effect on DPPH radical, which was consistent with the report of Jie et al.. Eight fractions was also obtained using liquid-liquid partition and column chromatography in Pu-erh tea, and fraction 8 from the ethyl acetate extract was found to display the greatest hydroxyl radical scavenging activity as well (Jie et al. 2006). According to the normal-phase and size-exclusion chromatographic separation mechanism, QEF8 may have relatively stronger polarity and higher molecular weight than the other fractions.
Table 3.
Scavenging effects on DPPH and inhibiting effects on pancreatic α-amylase activityof purification products from the ethyl acetate fraction
| Subfractions | Scavenging effect | Inhibiting effect | ||||
|---|---|---|---|---|---|---|
| EC50(μg/mL) | Regression equation | R2 | IC50(mg/mL) | Regression equation | R2 | |
| QEF1 | 360.3 ± 0.12 | y = 1.1760x + 7.3522 | 0.9956 | >16 | ||
| QEF2 | 270.1 ± 10.53 | y = 7.0445x–0.1542 | 0.9984 | 10.9 ± 0.40 | y = 5.1760x–6.3324 | 0.9974 |
| QEF3 | 235.2 ± 9.64 | y = 0.1610x + 12.1430 | 0.9587 | 9.4 ± 0.31 | y = 6.2048x–8.0609 | 0.9594 |
| QEF4 | 109.7 ±8.73 | y = 0.4350x + 6.6394 | 0.9762 | 8.2 ± 0.25 | y = 6.3727x–2.2339 | 0.9509 |
| QEF5 | 105.4 ± 0.97 | y = 0.4274x + 4.9564 | 0.9926 | 7.9 ± 0.23 | y = 4.9771x + 10.5100 | 0.9932 |
| QEF6 | 81.9 ± 1.75 | y = 0.5252x + 7.7326 | 0.9820 | 2.4 ± 0.10 | y = 14.2170x + 16.3680 | 0.9144 |
| QEF7 | 77.9 ± 1.32 | y = 0.5195x + 9.5402 | 0.9918 | 1.6 ± 0.04 | y = 16.6360x + 24.1290 | 0.9164 |
| QEF8 | 4.6 ± 0.43 | y = 10.1880x + 3.6992 | 0.9821 | 0.4 ± 0.01 | y = 163.6900x–10.1260 | 0.9717 |
n = 3, Mean ± SD
QEF1-QEF8, the first to eighth subfraction of the ethyl acetate fraction through Sephadex LH-20 column
Inhibitory effect of eight subfractions from ethyl acetate fraction on in vitro pancreatic α-amylase activity
The inhibitory potency of Qingzhuan Tea subfractions against α-amylase was determined, and their IC50 values were displayed in Table 3. All subfractions showed α-amylase inhibition capabilities. The order of their IC50 values was the same with that of DPPH assays, and QEF8 had the highest inhibitory activity against α-amylase and the lowest IC50 value of 0.37 mg/mL.
LC-MS analysis of QEF8
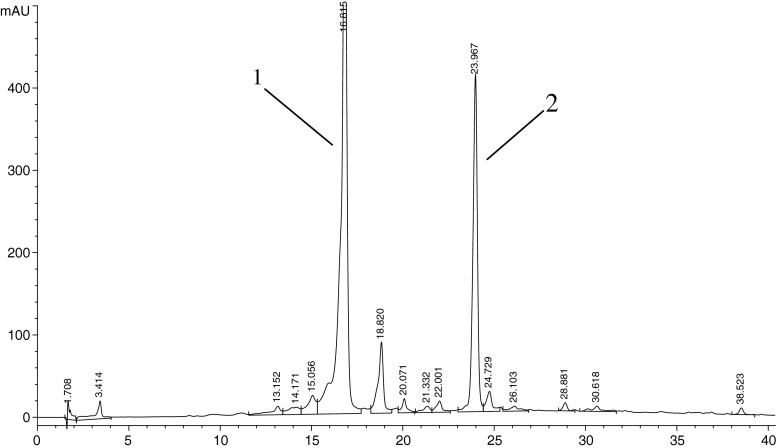
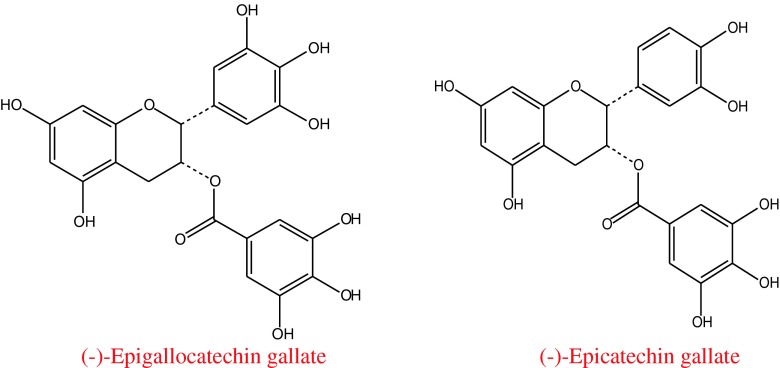
The compounds responsible for antioxidant activity and α-amylase inhibition in QEF8 were further analyzed by HPLC and LC-MS method, as this component showed the in vitro greatest DPPH radical scavenging activity and inhibitory effect against α-amylase. Figure 1 revealed that HPLC chromatogram of QEF8 had two major peaks, peak 1 and peak 2 with the retention time of 16.815 min and 23.967 min respectively. The tentatively identified compounds were depicted in Table 4. The EI spectrum of Peak 1 contains the molecular [M−H]+ ion at m/z = 457 Da, with prominent fragments at m/z = 331, 305 and 169 Da. The EI spectrum of Peaks 2 contains the molecular [M−H]+ ion at m/z = 441 Da, with prominent fragments at m/z = 289 Da. The mass ion and the fragment ions of the two peaks were essentially identical to the pattern obtained by Gondoin et al. (Gondoin et al. 2010). Thereby, it is proposed that they were EGCG and ECG accordingly (Fig. 2). Based on the comparison of peak areas with the authentic samples, EGCG and ECG were quantified to account for 22.29 % and 11.11 % respectively in QEF8.
Fig. 1.
Chromatogram of QEF8 from the ethyl acetate fraction by HPLC. QEF8, the eighth subfraction of ethyl acetate fraction through Sephadex LH-20 column
Table 4.
LC-MS Data for QEF8
| Peak No. | Retention time (min) | PDA | Ratio (100 %) | M/Z (M–H) | MS2 | Tentative identification |
|---|---|---|---|---|---|---|
| 1 | 16.815 | 275 | 22.29 | 457, 305, 169 | 331, 305, 169 | EGCG |
| 2 | 23.967 | 275 | 11.11 | 441, 289 | 289 | ECG |
QEF8: the eighth subfraction of ethyl acetate fraction through Sephadex LH-20 column; EGCG, (-)-epigallocatechin gallate; ECG, (-)-epicatechin gallate
Fig. 2.
The basic structures of catechins considered in the present study
Inhibitory effect of catechin standards (EGCG, ECG) on in vitro pancreatic α-amylase activity
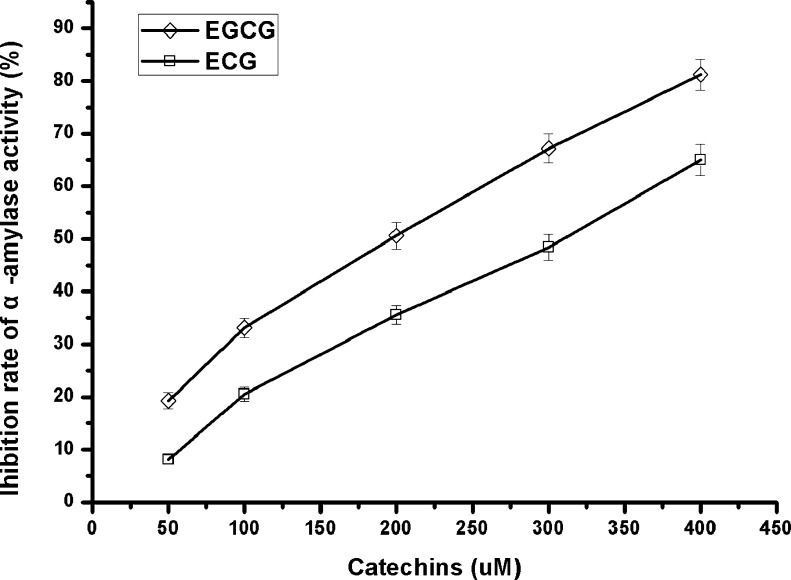
Based on the above result, we found that EGCG and ECG were the major active compounds in QEF8. Apart from their antioxidant bioactivity, catechins were reported to inhibit intestinal α-amylase or sucrase, deter the digestion of certain amounts of starch or sucrose and eventually reduce the plasma glucose levels in vivo (Matsumoto et al. 1991). To further confirm components in QEF8 that are the most effective as inhibitors, EGCG and ECG were tested on the inhibition of porcine pancreatic α-amylase. As shown in Fig. 3, EGCG showed greater inhibitory effect than ECG, which was inconsistent with the result reported by Hara and Honda (1990). The discrepancy may be due to the difference of the α-amylase source or the test conditions. The essence of enzyme inhibition herein was protein precipitation through forming various complexes with polyphenols (Siebert et al. 1996) or calcium (required as a cofactor for amylase enzyme activity) binding (Yoon et al. 1983). EGCG had more galloylated groups and higher molecular weight, which may account for its better inhibiting activity.
Fig. 3.
Inhibitory effects of EGCG and ECG on pancreatic a-amylase activity. EGCG, (-)-epigallocatechin gallate; ECG, (-)-epicatechin gallate. Values are expressed as mean ± SD (n = 3).
Earlier studies showed that there are synergistic effects between different catechins. Chung et al. reported that the effect of green tea extract on inhibition of lung tumor genesis was better than that of EGCG alone (Chung et al. 2003). Shi and Kakuda found that their antioxidant activity was enhanced by the synergistic action between catechins, e.g. EGCG, EGC, ECG, EC, pheophytins a and b, and other components in tea leaves (Shi and Kakuda 2006). Yang also discovered that free radical-scavenging was affected by different proportions of catechin monomers, and the best proportion of EGCG:ECG:EGC:EC would be 5:2:2:1 (Yang 2003). Thereby, the inhibitory effect against α-amylase of QEF8 may be due to the synergistic action of catechins and other compounds, which demands further research.
In conclusion, Qingzhuan tea exhibited good antioxidant activity and inhibiting effect against pancreatic α-amylase, and may be used as oral antidiabetic diet. EGCG and ECG were responsible for the inhibitory activity in Qingzhuan tea extracts.
Contributor Information
Baoping Ji, Phone: +86-10-62736628, FAX: +86-10-62347334, Email: jbp332@gmail.com.
Yuqiong Chen, Phone: +86-27-87281741, FAX: +86-27-87280781, Email: chenyq@mail.hzau.edu.cn.
References
- Al-Dabbas MM, Kitahara K, Suganuma T, Hashimoto F, Tadera K. Antioxidant and alpha-amylase inhibitory compounds from aerial parts of Varthemia iphionoides Boiss. Biosci Biotechnol Biochem. 2006;70(9):2178–2184. doi: 10.1271/bbb.60132. [DOI] [PubMed] [Google Scholar]
- Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agric Food Chem. 2008;56(19):9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- Ani V, Naidu KA. Antihyperglycemic activity of polyphenolic components of black/bitter cumin Centratherum anthelminticum (L.) Kuntze seeds. Eur Food Res Technol. 2008;226(4):897–903. doi: 10.1007/s00217-007-0612-1. [DOI] [Google Scholar]
- Chen Y, Wang M, Rosen RT, Ho CT. 2, 2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum Thunb. J Agric Food Chem. 1999;47(6):2226–2228. doi: 10.1021/jf990092f. [DOI] [PubMed] [Google Scholar]
- Chen YQ, Zhang W, Cheng Q, Dong JF, Liu XH, Gan DP, Qian YP. The anti-obesity effects of Hubei Qingzhuan tea on rats (Chinese) J Tea Sci. 2008;28(5):363–369. [Google Scholar]
- Chen YQ, Ni Dj, Cheng Q, Ma R, Kong LQ (2009) Study on the main components and capacities of scavenging free radicals of Qingzhuan tea. Proceedings of the Symposium on technological innovation and industrial development of Tea 427–432
- Chen YQ, Zhang W, Ni DJ, Cheng Q, Liu XH, Gan DP. Study on the hypolipidemic effect and antioxidative activity of Hubei Qingzhuan tea (Chinese) J Tea Sci. 2010;30(2):124–128. [Google Scholar]
- Chung FL, Schwartz J, Herzog CR, Yang YM. Tea and cancer prevention: studies in animals and humans. J Nutr. 2003;133(10):3268–3274. doi: 10.1093/jn/133.10.3268S. [DOI] [PubMed] [Google Scholar]
- Conforti F, Statti G, Loizzo MR, Sacchetti G, Poli F, Menichini F. In vitro antioxidant effect and inhibition of α-amylase of two varieties of Amaranthus caudatus seeds. Biol Pharm Bull. 2005;28(6):1098–1102. doi: 10.1248/bpb.28.1098. [DOI] [PubMed] [Google Scholar]
- Fu DH, Liu ZH, Huang JA, Chen HH. The effect of different extracts of Fuzhuan tea on the activities of digesting enzyme (Chinese) J Tea Sci. 2008;28(1):62–66. [Google Scholar]
- Fukushima Y, Ohie T, Yonekawa Y, Yonemoto K, Aizawa H, Mori Y, Watanabe M, Takeuchi M, Hasegawa M, Taguchi C. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J Agric Food Chem. 2009;57(4):1253–1259. doi: 10.1021/jf802418j. [DOI] [PubMed] [Google Scholar]
- Gilman AG, Goodman LS, Rail TW, Murad F. The pharmacological basis of therapeutics. New York: Macmillan; 1985. [Google Scholar]
- Golay A, Schneider H, Temler E, Felber JP. Effect of trestatin, an amylase inhibitor, incorporated into bread, on glycemic responses in normal and diabetic patients. Am J Clin Nutr. 1991;53(1):61–65. doi: 10.1093/ajcn/53.1.61. [DOI] [PubMed] [Google Scholar]
- Gondoin A, Grussu D, Stewart D, McDougall GJ. White and green tea polyphenols inhibit pancreatic lipase in vitro. Food Res Int. 2010;43(5):1537–1544. doi: 10.1016/j.foodres.2010.04.029. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Clarendon; 1989. [Google Scholar]
- Hara Y, Honda M. The inhibition of α-Amylase by tea polyphenols. Agric Biol Chem. 1990;54(8):1939–1945. doi: 10.1271/bbb1961.54.1939. [DOI] [Google Scholar]
- He Q, Lv Y, Yao K. Effects of tea polyphenols on the activities of alpha-amylase, pepsin, trypsin and lipase. Food Chem. 2007;101(3):1178–1182. doi: 10.1016/j.foodchem.2006.03.020. [DOI] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci. 2003;43(1):89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Wang MF, Liao ML, Chuang CK, Iha M, Clevidence B, Yamamoto S. Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care. 2003;26(6):1714–1718. doi: 10.2337/diacare.26.6.1714. [DOI] [PubMed] [Google Scholar]
- Jie G, Lin Z, Zhang L, Lv H, He P, Zhao B. Free radical scavenging effect of Pu-erh tea extracts and their protective effect on oxidative damage in human fibroblast cells. J Agric Food Chem. 2006;54(21):8058–8064. doi: 10.1021/jf061663o. [DOI] [PubMed] [Google Scholar]
- Karori S, Wachira F, Wanyoko J, Ngure R. Antioxidant capacity of different types of tea products. Afr J Biotechnol. 2010;6(19):2287–2296. [Google Scholar]
- Kondo S, Tsuda K, Muto N, Ueda J. Antioxidative activity of apple skin or flesh extracts associated with fruit development on selected apple cultivars. Sci Hortic-Amsterdam. 2002;96(1–4):177–185. doi: 10.1016/S0304-4238(02)00127-9. [DOI] [Google Scholar]
- Kusano R, Andou H, Fujieda M, Tanaka T, Matsuo Y, Kouno I. Polymer-like polyphenols of black tea and their lipase and amylase inhibitory activities. Chem Pharm Bull. 2008;56(3):266–272. doi: 10.1248/cpb.56.266. [DOI] [PubMed] [Google Scholar]
- Layer P, Rizza RA, Zinsmeister AR, Carlson GL, Dimagno EP. Effect of a purified amylase inhibitor on carbohydrate tolerance in normal subjects and patients with diabetes mellitus. Mayo Clin Proc. 1986;61(6):442–447. doi: 10.1016/S0025-6196(12)61978-8. [DOI] [PubMed] [Google Scholar]
- Ling TJ, Wan XC, Ling WW, Zhang ZZ, Xia T, Li DX, Hou RY. New triterpenoids and other constituents from a special microbial-fermented Tea-Fuzhuan brick tea. J Agric Food Chem. 2010;58(8):4945–4950. doi: 10.1021/jf9043524. [DOI] [PubMed] [Google Scholar]
- Loo AE, Huang D. Assay-guided fractionation study of α-amylase inhibitors from Garcinia mangostana pericarp. J Agric Food Chem. 2007;55(24):9805–9810. doi: 10.1021/jf071500f. [DOI] [PubMed] [Google Scholar]
- Ludwig DDS. The glycemic index - physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. Jama-J Am Med Assoc. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Tonooka F, Ishigaki A. Hara Y (1991) Reduction of blood-glucose level by catechins. Proceedings of the International Symposium on Tea Science 318–321
- Miliauskas G, Venskutonis P, Van Beek T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85(2):231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- Moussa SA. Oxidative stress in diabetes mellitus. Romanian J Biophys. 2008;18(3):225–236. [Google Scholar]
- Notkins AL. Immunologic and genetic factors in type 1 diabetes. J Biol Chem. 2002;277(46):43545–43548. doi: 10.1074/jbc.R200012200. [DOI] [PubMed] [Google Scholar]
- Ponnusamy S, Ravindran R, Zinjarde S, Bhargava S, Kumar AR. Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evid Based Complement Alternat. 2011;2011:1–10. doi: 10.1155/2011/515647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Kakuda Y. Herbs: challenges in chemistry and biolog. Oxford: Oxford University Press; 2006. [Google Scholar]
- Siebert KJ, Troukhanova NV, Lynn PY. Nature of polyphenol-protein interactions. J Agric Food Chem. 1996;44(1):80–85. doi: 10.1021/jf9502459. [DOI] [Google Scholar]
- The first research institute of China Standards Publisher . Standards collection for tea (Chinese) Beijing: China Standards Publisher; 2003. [Google Scholar]
- Thompson LU, Yoon JH. Starch digestibility as affected by polyphenols and phytic acid. J Food Sci. 1984;49(4):1228–1229. doi: 10.1111/j.1365-2621.1984.tb10443.x. [DOI] [Google Scholar]
- Yang XQ. Tea polyphenol chemistry (Chinese) Shanghai: Shanghai Science and Technology Press; 2003. [Google Scholar]
- Yoon JH, Thompson LU, Jenkins D. The effect of phytic acid on in vitro rate of starch digestibility and blood glucose response. Am J Clin Nutr. 1983;38(6):835–842. doi: 10.1093/ajcn/38.6.835. [DOI] [PubMed] [Google Scholar]
- Zhang DY, Shao WF, Liu ZH, Huang YW, Shi ZP. Research on the chemical constituents of Pu-erh tea and its inhibition effect on α-amylase (Chinese) Southwest China J Agric Sci. 2009;22(1):52–54. [Google Scholar]
- Zhang L, Li N, Ma ZZ, Tu PF. Comparison of the chemical constituents of aged Pu-erh tea, ripened Pu-erh tea, and other teas using HPLC-DAD-ESI-MS(n) J Agric Food Chem. 2011;59(16):8754–8760. doi: 10.1021/jf2015733. [DOI] [PubMed] [Google Scholar]
- Zhong L, Furne JK, Levitt MD. An extract of black, green, and mulberry teas causes malabsorption of carbohydrate but not of triacylglycerol in healthy volunteers. Am J Clin Nutr. 2006;84(3):551–555. doi: 10.1093/ajcn/84.3.551. [DOI] [PubMed] [Google Scholar]
- Zhou DR, Chen YQ, Ni DJ. Effect of water quality on the nutritional components and antioxidant activity of green tea extracts. Food Chem. 2009;113(1):110–114. doi: 10.1016/j.foodchem.2008.07.033. [DOI] [Google Scholar]
- Zhu Y, Huang H, Tu Y. A review of recent studies in China on the possible beneficial health effects of tea. Int J Food Sci Technol. 2006;41(4):333–340. doi: 10.1111/j.1365-2621.2005.01076.x. [DOI] [Google Scholar]
- Zunino SJ. Type 2 diabetes and glycemic response to grapes or grape products. J Nutr. 2009;139(9):1794–1800. doi: 10.3945/jn.109.107631. [DOI] [PubMed] [Google Scholar]