Abstract
Cooking process is one of the most energy and time consuming steps in the edible oil extraction factories. The main goal of this study was cottonseed oil extraction by microwave radiation and elimination of any heat treatment of cottonseeds before extraction. The effect of cooking process on the physicochemical properties of extracted oil from two varieties of cottonseed (Pak and Sahel) was evaluated by free fatty acid content, melting point, smoke point and refractive index. Our results didn’t show any significant differences between cooked and uncooked samples (P > 0.05) regarding physicochemical characteristics. From GC analysis of extracted oils, it was found there is no significant difference in fatty acid composition of cooked, uncooked and control (conventional extraction) samples. The thermal stability (Rancimat) analysis of oil samples showed the cooking process could cause a slight increase in the stability of oils for both varieties (about 40 min). The cooking process also increased total extracted phenolic compounds and considerably decreased total gossypol content of the cottonseed oil; but the extraction efficiency didn’t change considerably after elimination of the cooking process. It can be concluded that microwave rays can destroy the structure of oil cells during process and facilitate the oil extraction without any heat treatment before extraction.
Keywords: Microwave, Cooking, Edible oil, Cottonseed, Gossypol
Introduction
The cooking process is one of the most energy and time consuming steps in the edible oil extraction factories. This process is performed after flaking of oilseeds and just before the extraction. The purpose of heat treatment of oilseeds before extraction is denaturizing oil cell’s proteins in order to facilitate extraction of oil within cells. It also helps coalescence of oil into droplets which otherwise would be present as emulsions. Cooking tends to reduce the extraction of impurities into the crude oil and therefore, can influence oil loss during refining and in cottonseed processing, it helps to bind gossypol, which lowers the compound’s uptake into the crude oil thereby, reducing problems and costs associated with oil refining and bleaching (Shahidi 2005; Akoh and Min 2008).
Microwave-assisted extraction (MAE) is a novel technology which has been studied extensively in recent years mainly in order to reduce the extraction time and solvent consumption (Camel 2000; Amarni and Kadi 2010). Microwave energy is a non-ionizing radiation by a frequency between 300 MHz and 300,000 MHz that causes dielectric heating due to molecular motion by migration of ions and rotation of dipoles (Camel 2000). The efficiency of the MAE process depends on the time and temperature of extraction, sample ratio and nature of both the solvent and the solid matrix (Terigar et al. 2010). It has been reported that microwave rays destroy the biological cell structure at the plant tissues like oil seeds (Chemat et al. 2005; Jun and Chun 1998). Microwave treatment of oilseeds during solvent extraction, leads to the protein denaturation of cells resulting in improved extraction. Azadmard et al. (2010) studied the effect of microwave pretreatment of rapeseeds on cold press extraction efficiency to investigate the possibility of enhancing oil extraction yield. They concluded that microwave pretreatment of rapeseed can increase the oil extraction yield by 10 %. In another study, Amarni and Kadi (2010) extracted oil from olive cake by microwave technology using hexane as solvent. It has been concluded that MAE of oil from olive cake gives better yields within shorter times and consumes less solvent.
The other advantage of MAE could be higher extraction of polyphenols which play an important role in preventing the oxidation of the edible oils during storage and use. For example, Azadmard et al. (2010) found that microwave pretreatment of rapeseed can increase the oil phytosterols (by 15 %) and tocopherols (by 55 %) and as a result, the oxidative stability of rapeseed oil (analyzed by Rancimat) was increased from 1 h (which was for untreated rapeseed) to 8 h after MAE.
Gossypol (1,1′,6,6′,7,7′-hexahydroxy-5,5′-diisopropyl-3,3′-dimethyl-[2,2′]-binaphthalenyl-[8,8′]-dicarbaldehyde) is a phenolic compound with antifertility and antioxidant effects that exists in crude cottonseed oil. Natural antioxidants like gossypol due to their phenolic structure are more exposed to microwave. This is because of the polarity of phenolic compounds. The more polarity a compound has, the more it moves during microwave radiation. This leads to a better extraction of such compounds (Camel 2000). Many researchers have been made applying microwave radiation in order to extract phenolic compounds such as isoflavones (Terigar et al. 2010), polycyclic aromatic hydrocarbons (Camel 2000), carvone and limonene (Chemat et al. 2005), gallic acid, vannilic acid, catechin, p-coumaric acid, ferulic acid and may others phenolic compounds (Proestos and Komaitis 2008), pigments (Jun and Chun 1998), tea polyphenols and caffeine (Pan et al. 2003), tocopherols and tocotrienol (Zigoneanu et al. 2008) and many others from various plant resources. Most of them concluded that MAE decreased the extraction time, solvent usage and increased the amount of extracted phenolic compounds. Therefore, microwave-assisted extracted oil could be rich in natural phenolic compounds with an improved shelf life.
The cottonseed oil which has around 30 % of saturated fatty acids (palmitic and stearic acid), has a suitable stability against oxidation and is mainly consumed in frying oils formulations (Shahidi 2005), hence it is subjected to high temperatures and moisture levels (which is occurring during frying process). The presence of food moisture, atmospheric oxygen and high temperatures could cause various chemical changes and loss of antioxidants such as steam distillation of antioxidants, oxidation of phenolic compounds, and reduction of their pro-oxidative activity due to reaction with fried materials and polymerisation (Pokorny et al. 2000). Synthetic antioxidants such as BHA and BHT are more susceptible to steam distillation (Warner et al. 1986). Losses of natural antioxidants could be comparatively small, since their volatility is much lower than that of common synthetic antioxidants (Pokorny et al. 2000; Taghvaei and Jafari 2013).
The two main objectives of this study were: a) elimination of the cooking process from the process of cottonseed oil extraction with the help of microwave treatment. b) Comparison of physical and chemical properties and the oxidative stability of the resulted oil with or without cooking process.
Materials and methods
Sample
Two varieties of cottonseed (Pak and Sahel) were obtained from Cotton Research Institute of Iran (crop year 2011). The Sahel is the most cultivated cotton plant variety in Iran and Pak is a variety of cotton which has trace amounts of gossypol and it has been selected in order to eliminate the effect of gossypol on stability and total phenolic content of the final oil.
Cottonseed powder preparation was carried out according to Bhattacharjee et al. (2007) with a little modification. Briefly, the seeds got delinted and decorticated and the whole kernels were then removed from the hulls by screening and were ground with a laboratory mill (Sunny, SFP-820) and sieved through a 30–60 mesh (clear openings 0.6–0.25 mm).
Solvents and chemicals were obtained from Merck (Darmstadt, Germany). Gallic acid, gossypol standards and Folin-Ciocalteu reagent were purchased from Sigma–Aldrich Co. (St. Louis, MO, USA).
The cooking process
The cottonseed powder was placed as a uniform layer on an aluminum tray with 5 mm diameter. The cooking process was consisted of two steps of wet and dry cooking. The wet cooking was carried out by placing the aluminum trays in an autoclave (Zirbus, LTA400, Germany) at 121 °C for 30 min. After that, dry cooking process was performed by an oven (Memmert, ULM 400, Germany) at 110 °C for 1 h. The initial moisture content of cottonseed powder was 7 % (dry basis) which reached to 14 % after wet cooking and adjusted to 6 % after dry cooking.
Microwave assisted extraction
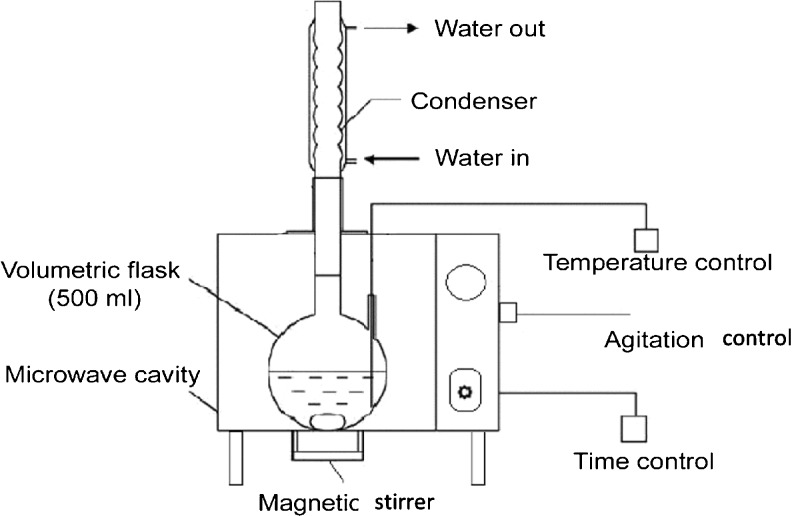
A modified microwave oven (Samsung, model: CF3110N-5, Korea) was used for oil extraction based on the work of Rafiee et al. (2011, 2012). The modified MAE system consisted of a volumetric flask (500 ml) coupled with a condenser at the top and a magnetic stirrer beneath as illustrated in Fig. 1. The microwave output was 900 W with 2,450 MHz frequency and its inner cavity dimensions were 400 mm × 300 mm × 250 mm. For each extraction, 100 g cottonseed powder (6 % moisture content, dry basis), 200 ml of solvent (n-hexane) and a magnet were placed in the microwave oven. After 2 min of radiation and simultaneous magnetic stirring, the extraction process was stopped. Then, the cottonseed meal removed from miscella by means of filtration followed by centrifugation (30,000 rpm, 5 min). Also, the solvent removed under reduced pressure at 50 °C by a rotary evaporator (IKA RV 10 basic, Japan).
Fig. 1.
Schematic diagram of microwave assisted solvent extraction
Conventional extraction
In most cottonseed oil extraction factories, the cooking process consists of a 30 min wet cooking by steam followed by 1 h dry cooking at 110 °C. Regarding the oil extraction, the extraction time, temperature and the amount of solvent usage is highly dependent on the type of extractor and extraction procedure (Shahidi 2005). Generally, extraction parameters were chosen in such a manner that be close to conventional batch solvent extraction in industry. After performing the cooking process (as it has been explained in The Cooking Process), the control (blank) oil was extracted by soaking of 100 g cottonseed powder in 200 ml of n-hexane at 50 °C for 30 min without any microwave treatment for comparison.
Total oil extraction
The total oil content of cottonseeds was determined by Soxhlet apparatus for 16 h using n-hexane (Whitaker 2003). This was done only for determination of total oil content of cottonseeds.
Oil physicochemical properties
In order to compare the effect of microwave and cooking process on physicochemical properties of final oils, the following experiments were carried out: Free fatty acid content (AOCS Ca 5a-40), melting point (AOCS Cc 3–25), smoke point (AOCS Cc 9a-48), refractive index (AOCS Cc 7–25) and the moisture content of cottonseed powder (AOCS Aa 3–38) (AOCS 2007). The color was evaluated by transferring cottonseed oil samples into a micro-plate cell (4 mm diameter) and analyzing using a Lovibond Tintometer model cam-system 500 in the L, a, b, mode of CIE (L, a, b, indicate lightness, redness/greenness, and yellowness/blueness, respectively).
Oil oxidative stability
For the determination of oil stability, the oxidative stability index was analyzed by Rancimat (Metrohm, 743, Switzerland) apparatus according to AOCS Cd 12b-92 method (AOCS 2007). The induction period was evaluated until the end stability point of oil samples at the temperature of 110 °C. The phenolic compounds of oils were extracted by methanol–water as described by Bail et al. (2008) and total phenolic content was evaluated according to Folin-Ciocalteu method (Whitaker 2003).
Gossypol analysis
Total gossypol content of cottonseed oil was analyzed spectrophotometrically (by spectrophotometer WPA, S2000, UK) according to AOCS Ca 13–56 (AOCS 2007).
GC analysis
Preparation of methyl esters of fatty acids for GC analysis was performed by AOCS Ce 2–66 and GC analysis was carried out by AOCS Ce 1–62 (AOCS 2007). A fused-silica capillary column with inner dimension of 0.25 mm, outer dimension of 0.39 mm and film thickness of 0.2 μm was connected to a Chrompack CP 3800 gas chromatograph (Middleburg, The Netherlands) equipped with a FID detector. GC conditions were: injector temperature 250 °C, detector temperature 270 °C, column temperature 130 °C and nitrogen as the carrier gas with a flow rate of 25 ml/min.
Statistical analysis
The statistical analyses were carried out by Minitab 16 (Mini-tab, Inc., State College, PA, USA) using full factorial design with two independent factors of variety (Pak and Sahel) and cooking (blank, cooked and uncooked). All experiments were performed in triplicate and the mean values were reported. All graphs were drawn by Microsoft Excel (2010).
Results and discussion
The oil extraction efficiency
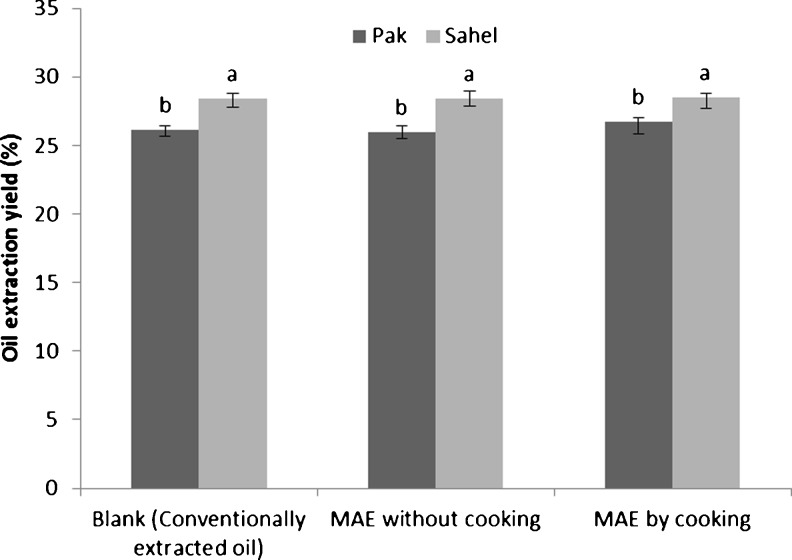
Firstly, we were looking for the effect of cooking process on the cottonseed oil yield from microwave-assisted extraction samples compared with conventionally extracted blank oil (through soaking). The total amount of extracted oil by soxhlet apparatus was 34.8 % and 38.7 % for Pak and Sahel cottonseed varieties, respectively. Our data revealed that there was no significant difference (P > 0.05) between conventional extraction oil yield (30 min soaking) and MAE oil yield which was only a 2 min process (Fig. 2). This proved that we can highly reduce the extraction time by applying MAE. As it is shown in Fig. 2, the cooking process had no significant influence (P > 0.05) on microwave-assisted extraction efficiency which could have a potential benefit for the industry. It could be explained by the fact that the main goal of implementing cooking process is coagulation of cellular proteins, aggregation and coalescence of internal oil droplets and destruction of cellular walls. The microwave radiation could be capable of inducing similar effects as cooking process, so there is no need to the cooking process. In other words, by elimination of the cooking process through MAE, there would be a decrease in the energy usage and the process time, making it economically profitable for the edible oil industries.
Fig. 2.
The effect of cooking process on the oil extraction efficiency. Similar superscripts on a parameter indicate no significant difference (P > 0.05)
Gossypol and phenolic content
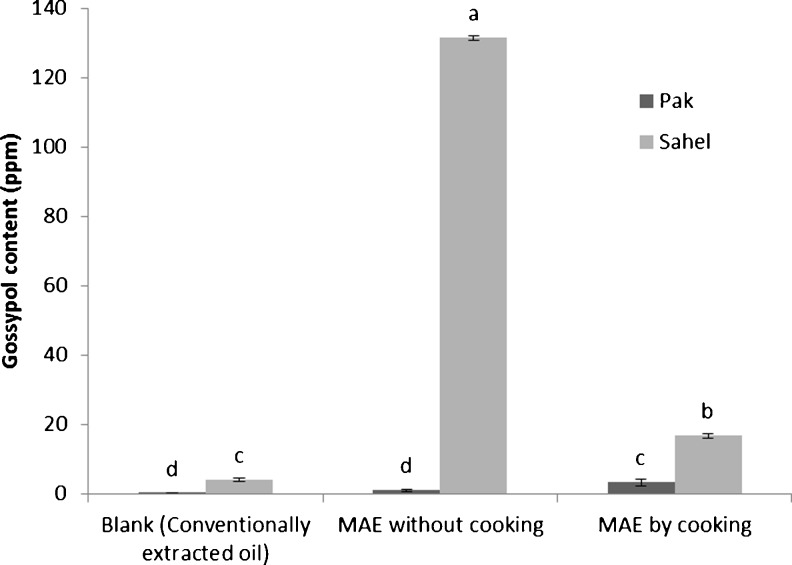
The analysis of total gossypol content of cottonseed oil revealed that the cooking process had a significant influence (P < 0.05) on gossypol extraction. Total gossypol in the oil extracted from Sahel cottonseed variety by MAE (without cooking) was 131 ppm, while it was dramatically decreased to 17 ppm after precooking the same variety before MAE. The amount of gossypol in Pak cottonseed oil was inconsiderable (Fig. 3). Heat treatments such as cooking could cause gossypol to react with cell proteins and remain bounded in cottonseed meal (Shahidi 2005; Bhattacharjee et al. 2007). Also, we found that total gossypol content of the oil samples obtained from MAE was significantly (P < 0.05) higher than control sample (conventionally extracted one). This could be because of the higher influence of microwave on polar compounds like gossypol (Camel 2000).
Fig. 3.
Total gossypol content of the conventionally extracted oil (CEO) and MAE oil from two varieties of cottonseed. Similar superscripts on a parameter indicate no significant difference (P > 0.05)
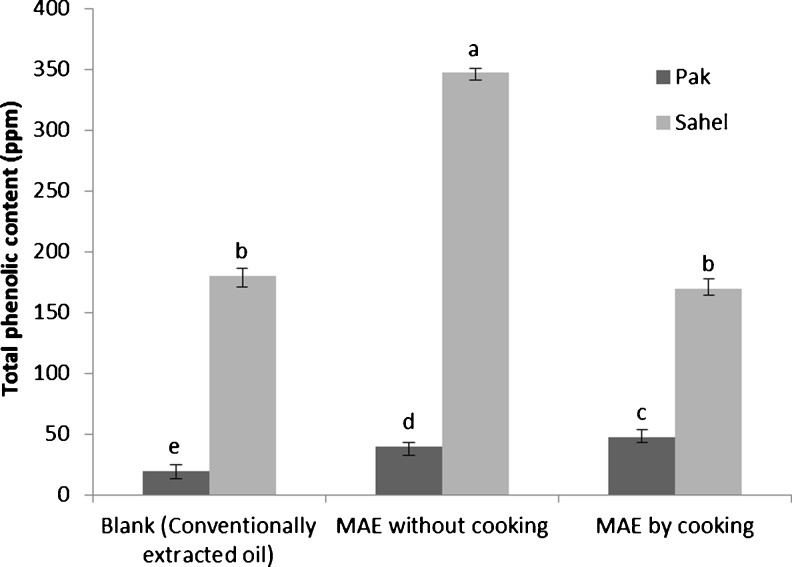
The results of total phenolics analysis have been showed in Fig. 4. It was revealed that phenolic compounds like gossypol can be extracted easier through MAE than conventional extraction. Our results are in agreement with previously published results (Camel 2000; Terigar et al. 2010; Zigoneanu et al. 2008; Rafiee et al. 2011; Rafiee et al. 2012) indicating that MAE decreased the extraction time, solvent usage and increased the amount of extracted phenolic compounds.
Fig. 4.
Total phenolic content of conventionally extracted oil (CEO) and MAE oil from two varieties of cottonseed samples. Similar superscripts on a parameter indicate no significant difference (P > 0.05)
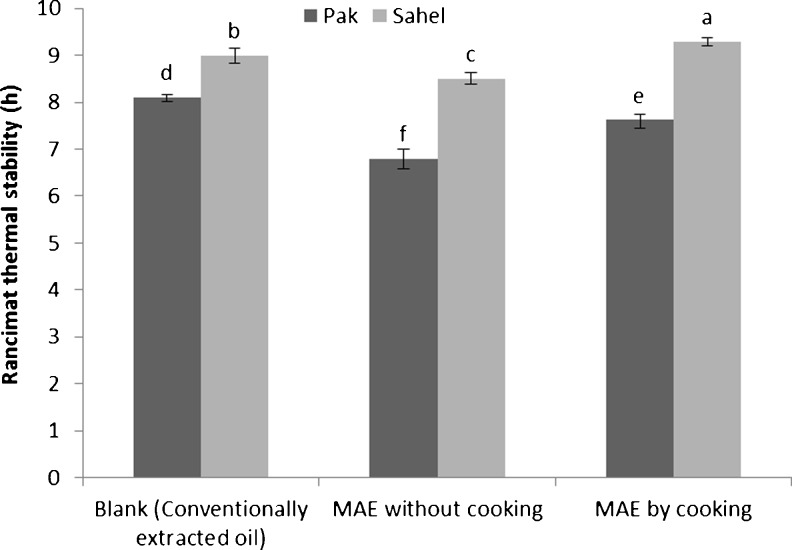
Oil stability
The stability analysis of extracted oil samples are shown in Fig. 5 and results of ANOVA are presented in Table 1. In an overall view, the stability of Sahel oil in Rancimat test was higher (about 1 to 2 h) than Pak samples. The reason could be attributed to the total phenolic content of final oils. As it has been illustrated in Fig. 4, total phenolic content of Sahel variety was higher than Pak variety. Antioxidants which enhance the thermal stability of oils are phenolic compounds. Higher the phenolic content, higher the thermal stability of extracted oil. These results agree with data obtained by Azadmard et al. (2010) which examined the effect of microwave pretreatment on oxidative stability and nutraceuticals content of rapeseed oil. They concluded that higher stability of extracted oils through MAE may arise from their high natural phenolic antioxidant content such as tocopherols. There was a small significant difference among cooked, uncooked and blank samples of both varieties which couldn’t be explained by total phenolic content. The cooking process caused a slight increase in the stability of MAE oils for both varieties (about 40 min). The stability of blank samples which also were cooked before extraction was similarly higher than uncooked samples. According to Shahidi (2005), the cooking process decreases the amount of impurities in extracted oil. The slightly lower stability of uncooked samples could be related to their higher impurities like metal ions which act as prooxidant.
Fig. 5.
Thermal stability of conventionally extracted oil (CEO) and MAE oil from two varieties of cottonseed samples evaluated by Rancimat. Different superscripts on parameters indicate significant difference (P < 0.05)
Table 1.
Summary of ANOVA model statistics for Rancimat experiments
| Source | d.f. | Sum of squares | Mean squares | F-ratio | p-Value |
|---|---|---|---|---|---|
| Variety (Pak and Sahel) | 1 | 9.3312 | 9.3312 | 473.40 | 0.000 |
| Cooking (cooked, uncooked and blank) | 2 | 2.8412 | 1.4206 | 72.07 | 0.000 |
| Variety*Cooking | 2 | 0.6480 | 0.3240 | 16.44 | 0.000 |
| Error | 12 | 0.2365 | 0.0197 | ||
| Total | 17 | 13.0570 |
Azadmard et al. (2010) implied that 2 min pretreatment with microwave caused 2.5 h increase in the Rancimat stability test of extracted oil by solvent which regarding Pak variety, it wasn’t concurred with our results and in Sahel variety, the increasing in Rancimat stability wasn’t that much (about 30 min). This could be due to repelling effect of solvent. N-hexane is a non-polar solvent which reflects a great portion of radiated microwave. In our study, microwave treatment was applied during extraction by solvent, whereas it was radiated directly on seeds as a pretreatment by Azadmard et al. (2010). It could cause a higher effect of microwave on seeds.
On the other hand, Jittrepotch et al. (2010) implied that increasing in microwave radiation time from 0 to 6 min causes an enhancement of oxidation for peanut oil samples. The reason can be related with the long duration of radiation which probably have adverse effects on final oil when the oilseed is processed with microwave directly (without solvent). According to Jittrepotch et al. (2010), under 3.5 min of radiation, the oxidation didn’t increase significantly whereas in this study, 2 min of microwave radiation has been applied.
Physicochemical properties
As it has been shown in Table 2, there is no significant difference (P > 0.05) between free fatty acid content, melting point, smoke point, refractive index and color of cooked and uncooked MAE oil samples. The differences were mostly between two varieties which could be due to the differences in oil structures since the cultivation year, storage conditions and extraction parameters such as moisture content, temperature, etc. was the same for both varieties. The physicochemical properties of oils and fats are mostly dependent on their fatty acid and triglyceride composition (Gunstone 2011). In our study, the fatty acid composition was significantly (P > 0.05) different between two varieties (Table 2).
Table 2.
The effect of cooking process on physicochemical properties of cottonseed oil extracted by conventional soaking (blank) and MAE (uncooked and cooked)
| Blank | Uncooked | Cooked | ||||
|---|---|---|---|---|---|---|
| Sahel | Pak | Sahel | Pak | Sahel | Pak | |
| FFA (%)* | 0.8 ± 0.07a | 0.8 ± 0.04a | 0.9 ± 0.07a | 0.9 ± 0.1a | 0.8 ± 0.05a | 0.7 ± 0.08a |
| Melting p. (°C)* | 9 ± 0.7c | 10 ± 1.4bc | 16 ± 0.9a | 11 ± 1b | 16 ± 0.8a | 11 ± 1.2b |
| Smoke p. (°C)* | 141 ± 2.1d | 177 ± 1.9b | 159 ± 1.6c | 196 ± 2.2a | 160 ± 2.3c | 195 ± 1.9a |
| Refractive index* | 1.4688 ± 0.002a | 1.4684 ± 0.002a | 1.4712 ± 0.003a | 1.4690 ± 0.002a | 1.4690 ± 0.002a | 1.4687 ± 0.003a |
| Color* | L: 23.9 ± 1.3b | L: 31.4 ± 0.9a | L: 24.7 ± 1.1b | L: 31.4 ± 1.2a | L: 25.1 ± 1.3b | L: 31.8 ± 1.1a |
| a: 9 ± 0.3c | a: 6.7 ± 0.9d | a: 12.2 ± 0.5a | a: 5.9 ± 0.8d | a: 9.8 ± 0.4b | a: 5.9 ± 0.6d | |
| b: −0.4 ± 1.6a | b: −1.2 ± 1.5ab | b: 0.4 ± 1.6a | b: −1.2 ± 1.2ab | b: 1.2 ± 1.4a | b: −2.7 ± 1.4b | |
*Average of three replicates
The smoke point of MAE samples was significantly higher (P > 0.05) than blank samples for both varieties. Cottonseed oil’s smoke points, like other fats and oils, are almost entirely dependent on the free fatty acid content (Shahidi 2005). Since there wasn’t any significant difference between the free fatty acid content of all oil samples, the reason could attributed to the higher natural phenolic antioxidants of MAE oils. According to Yen et al. (1997), both natural phenolics and synthetic antioxidants like TBHQ, BHA and BHT could decrease several degrees the smoke point of soybean oil.
There was also a significant difference between the melting point of MAE oils and blank sample in Sahel variety. The melting points of oils and fats are dependent not only on the structures and position of the fatty acids and triglycerides, but also on presence of some unsaponifiable compounds such as phospholipids and waxes (Shahidi 2005). In Sahel variety, the higher melting point of MAE oils could be the result of higher extraction of phospholipids and waxes (which have a considerable high melting point) through MAE.
The color of oil is also one of the important physical properties which influence the acceptance of final product. A significant difference was observed between the factor “a” (redness/greenness) of Sahel samples, where as it was not the same for Pak variety (Table 2). Generally, the color of oil from Sahel variety was more reddish than Pak which is probably because of the much higher gossypol content of Sahel. Gossypol is a red pigment which is responsible for crude cottonseed oil’s dark reddish-brown color (Shahidi 2005). Comparison of gossypol content of Sahel oil samples (Fig. 3) and “a” factor revealed that higher the gossypol content, higher the “a” factor. There wasn’t any significant difference in lightness (L factor) and the “b” factor of cooked, uncooked and blank samples. Based on these results and no significant difference in “a” factor of Pak variety samples, it could be concluded that MAE and cooking process had no effect on the color of Pak variety samples and it could change the color of Sahel oil by influencing the gossypol content.
The results for refractive index showed that cooking process had no significant effect (P > 0.05) on triglyceride structure of MAE cottonseed oil.
GC analysis for fatty acid composition
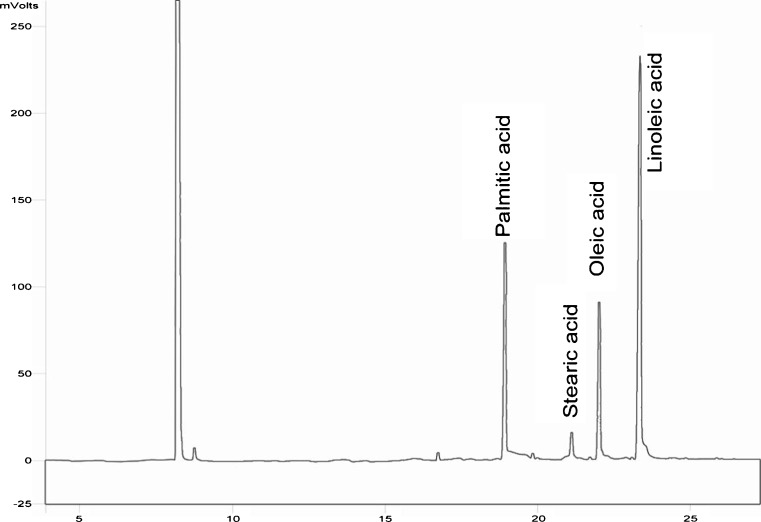
The fatty acid composition of cottonseed oil samples are given in Fig. 6. The main fatty acids were as palmitic acid (23 % for Pak and 24.2 % for Sahel variety), stearic acid (2.7 % for Pak and 2.9 % for Sahel variety), oleic acid (17 % for Pak and 15.1 % for Sahel variety), and linoleic acid (55 % for Pak and 55.8 % for Sahel variety).
Fig. 6.
GC analysis of fatty acid composition of cotton seed oil using fused-silica capillary column with inner dimension of 0.25 mm, outer dimension of 0.39 mm and film thickness of 0.2 μm connected with a FID detector. GC conditions were: injector temperature 250 °C, detector temperature 270 °C, column temperature 130 °C and nitrogen as the carrier gas with a flow rate of 25 ml/min. There was no significant difference (P > 0.05) between control, cooked and uncooked MAE oil samples in terms of fatty acid composition
We found that there was no significant difference (P > 0.05) between control, cooked and uncooked MAE oil samples in terms of fatty acid composition. In other words, GC results showed that cooking process and microwave radiation had no influence on fatty acid composition of cottonseed oil samples which could prove the MAE process as efficient as conventional extraction process and the edible oil industry can apply it efficiently without any problems. The GC analysis results concurred with the study of Azadmard et al. (2010) which also analyzed the effect of microwave on fatty acid composition of rapeseed oil and indicated that 2 and 4 min of microwave pretreatment before extraction had no significant effect (p > 0.05) on rapeseed fatty acid composition.
Conclusion
The results of this study confirmed that through microwave-assisted extraction of edible oils from cottonseed, there is no need to apply heat treatment (cooking process) in order to destruct oil cells and coagulate proteins. We found that the oil yield of a 30 min conventional extraction was equal to a 2 min MAE with or without cooking of the cottonseeds. The GC analysis and physicochemical properties of cottonseed oil samples revealed that elimination of cooking process causes no significant changes in fatty acid and triglyceride composition of final oil. On the other hand, it was observed that the cooking process slightly increased the stability of cotton seed oil which was consisted with a considerable reduction of total gossypol content. Further studies need to be carried out in order to assess the quality of resulting cottonseed meal after MAE without cooking process.
Contributor Information
Mostafa Taghvaei, Phone: +98-171-4426432, FAX: +98-171-4426432, Email: m.taghvaii@gmail.com.
Seid Mahdi Jafari, Phone: +98-171-4426432, FAX: +98-171-4426432, Email: smjafari@gau.ac.ir.
References
- Akoh CC, Min DB. Food lipids chemistry, nutrition, and biotechnology. Boca Raton, London, New York: CRC Press, Taylor & Francis Group; 2008. [Google Scholar]
- Amarni F, Kadi H. Kinetics study of microwave-assisted solvent extraction of oil from olive cake using hexane: comparison with the conventional extraction. Innov Food Sci Emerg Technol. 2010;11:322–327. doi: 10.1016/j.ifset.2010.01.002. [DOI] [Google Scholar]
- AOCS (2007) Official methods and recommended practices of the American Oil Chemist’s Society. 5th edn. Champaign
- Azadmard DS, Habibi NF, Hesari J, Nemati M, Fathi AB. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010;121:1211–1215. doi: 10.1016/j.foodchem.2010.02.006. [DOI] [Google Scholar]
- Bail S, Stuebiger G, Krist S, Unterweger H, Buchbauer G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008;108:1122–1132. doi: 10.1016/j.foodchem.2007.11.063. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee P, Singhal RS, Tiwari SR. Supercritical carbon dioxide extraction of cottonseed oil. J Food Eng. 2007;79:892–898. doi: 10.1016/j.jfoodeng.2006.03.009. [DOI] [Google Scholar]
- Camel V. Microwave-assisted solvent extraction of environmental samples. TrAC Trends Anal Chem. 2000;19:229–248. doi: 10.1016/S0165-9936(99)00185-5. [DOI] [Google Scholar]
- Chemat S, Amar HA, Lagha A, Esveld DC. Microwave-assisted extraction kinetics of terpenes from caraway seeds. Chem Eng Process. 2005;44:1320–1326. doi: 10.1016/j.cep.2005.03.011. [DOI] [Google Scholar]
- Gunstone DF (2011) Vegetable oils in food technology: composition, properties and uses. 2nd edn. Wiley
- Jittrepotch N, Kongbangkerd T, Rojsuntornkitti K. Influence of microwave irradiation on lipid oxidation and acceptance in peanut (Arachis hypogaea L.) seeds. Int Food Res J. 2010;17:173–179. [Google Scholar]
- Jun SJ, Chun JK. Design of u-column microwave-assisted extraction system and its application to pigment extraction from food. Trans IChemE. 1998;76:231–236. [Google Scholar]
- Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process. 2003;42:129–133. doi: 10.1016/S0255-2701(02)00037-5. [DOI] [Google Scholar]
- Pokorny J, Yanishlieva N, Gordon M. Antioxidants in food practical applications. New York: CRC press; 2000. [Google Scholar]
- Proestos C, Komaitis M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT. 2008;41:652–659. doi: 10.1016/j.lwt.2007.04.013. [DOI] [Google Scholar]
- Rafiee Z, Jafari SM, Alami M, Khomeiri M. Microwave-assisted extraction of phenolic compounds from olive leaves: a comparison with maceration. J Anim Plant Sci. 2011;21:738–745. [Google Scholar]
- Rafiee Z, Jafari SM, Alami M, Khomeiri M (2012) Antioxidant effect of microwave-assisted extracts of olive leaves on sunflower oil. J Agric Sci Technol (Tehran, Islamic Repub. Iran) 14:1497–1509
- Shahidi F. Bailey’s industrial oil and fat products. New Jersey: Wiley; 2005. [Google Scholar]
- Taghvaei M, Jafari SM (2013) Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol. In press [DOI] [PMC free article] [PubMed]
- Terigar BG, Balasubramanian S, Boldor D, Xu Z, Lima M, Sabliov CM. Continuous microwave-assisted isoflavone extraction system: design and performance evaluation. Bioresour Technol. 2010;101:2466–2471. doi: 10.1016/j.biortech.2009.11.039. [DOI] [PubMed] [Google Scholar]
- Warner CR, Brumley WC, Daniels DH, Joe JFL, Fazio T. Reactions of antioxidants in foods. Food Chem Toxicol. 1986;24:10–15. doi: 10.1016/0278-6915(86)90282-6. [DOI] [PubMed] [Google Scholar]
- Whitaker J (2003) Current protocols in food analytical chemistry. Wiley
- Yen GC, Shao CH, Chen CJ, Duh PD. Effects of antioxidant and cholesterol on smoke point of oils. LWT Food Sci Technol. 1997;30:648–652. doi: 10.1006/fstl.1996.0236. [DOI] [Google Scholar]
- Zigoneanu IG, Williams L, Xu Z, Sabliov CM. Determination of antioxidant components in rice bran oil extracted by microwave-assisted method. Bioresour Technol. 2008;99:4910–4918. doi: 10.1016/j.biortech.2007.09.067. [DOI] [PubMed] [Google Scholar]