Abstract
The effects of 400 ppm ascorbyl palmitate (AP) on fatty acids composition, tocopherol, peroxide value (PV) and malonaldehyde (MAD) contents of refined cottonseed oil (CO) and virgin olive oil (OO) during chemical interesterification (CI), and storage at 60 °C for 28 days were investigated. CI significantly decreased (p < 0.05) the tocopherol contents of CO and OO. PVs and MAD contents of oil samples considerably increased up to 20 min of CI, followed by a reduction at 30 min. The unsaturated fatty acids/saturated fatty acids (UFA/SFA) ratios of the samples showed slight but significant (p < 0.05) reduction during accelerated oxidation process. Oils with added 400 ppm AP had higher tocopherol, and lower PVs and MAD contents than their counterparts without AP during CI, and storage at 60 °C. AP increased the oxidative stability of interesterified and non-interesterified CO and OO.
Keywords: Chemical interesterification, Cottonseed oil, Olive oil, Oxidative stability, Tocopherol
Introduction
The adverse effects of hydrogenation; the formation of saturated and trans-unsaturated fatty acids makes the use of interesterified oils an attractive alternative (Adhikari and Adhikari 1992). Chemical and enzymatic interesterification modify the physical properties of oils by rearranging the distribution of fatty acids on the glycerol backbone without changing their chemical composition (Norizzah et al. 2004). Recently, interesterification has been applied to improve the cold spreadability of butterfat-canola oil blends (Rousseau and Marangoni 1999), and the production of margarine (Alpaslan and Karaali 1998), frankfurter (Vural and Javidipour 2002; Vural et al. 2004), Turkish-type salami (Javidipour and Vural 2002; Javidipour et al. 2005) cheese (Javidipour and Tunçtürk 2007), cake (Dogan et al. 2007) and cookie (Öztürk et al. 2008; Ozturk et al. 2009; Dinç et al. 2011). However, interesterification can be successfully applied in different food products, there are a few reports related to the effects of interesterification on oxidative stability of fats and oils. Zalewski and Gaddis (1967) reported that the interesterification under vacuum produced an odorless and colorless randomized lard with natural stability in the range of the parent lard. Wada and Koizumi (1983) noted that the chemically randomized triacylglycerole mixture was more stable toward oxidation in 50 °C than its unrandomized counterparts, which were prepared by mixing the equivalent quantities of the same monoacid triacylglyceroles as used in the random interesterification. Kimoto et al. (1994) indicated that CI improved oxidative stability of cod liver and skip-jack oils, but did not for sardine oil. Tautorus and McCurdy (1990) reported that non-interesterified and chemically interesterified canola, corn, linseed, soybean and sunflower oils stored at 55 °C demonstrated little difference to lipid oxidation. Basturk et al. (2007) noted that based on PV, anisidin value (AV) and reaction rate constants, the oxidative stability of chemically interesterified cottonseed, palm and soybean oils were higher than their non-interesterified counterparts. In contrast; Park et al. (1983) found that the loss of tocopherols accelerated the autoxidation of randomized soybean oil. According to Lau et al. (1982), and Gavriilidou and Boskou (1993) randomized corn oil and corn oil methyl esters, and interesterified 80 % olive oil–20 % tristearin blend (respectively) were less stable than the non-treated controls. These discrepancies in the literature show that there is a need to investigate the effect of interesterification more thoroughly, especially in different oils and fats.
AP is a fat soluble synthetic ester of ascorbic acid which its Food Drug Administration status is “generally recognized as safe” (GRAS) with no limitation on levels used in food or cosmetics (Perricone et al. 1999). However, the mechanism action of AP is not well known, Lee et al. (1997) reported the effective singlet oxygen quenching ability of AP for the reduction of photosensitized oxidation of oils, Coppen (1994) noted the ability of AP to remove or sequester trace metals that catalyze peroxide formation.
Beddows et al. (2001) reported that AP (200 ppm) preserved α-tocopherol in sunflower oil at 95 °C and delayed the onset of rancidity. Karabulut (2010) indicated a strong synergistic effect for the binary mixtures of α-tocopherol and AP in the oxidation of butter oil triacylglycerols, and prooxidative effect for AP at the absence of α-tocopherol. Gordon and Kourimska (1995) found that the presence of a rosemary extract or AP in the frying oil caused a marked reduction in the rate of loss of the tocopherols. Bartee et al. (2007) reported that AP (300, 600, 900 or 1,200 ppm) had a significant effect on the oxidative stability of different oils containing different ratios of arachidonic, docosapentanoic and docosahexanoic acids, and they did not observe any prooxidative effect for AP in tested concentrations. Baştürk (2011) noted that 400 ppm AP considerably reduced the peroxide formation in different vegetable oils stored under accelerated oxidation condition.
The aim of this study was to evaluate the effects of 400 ppm AP on some chemical properties (fatty acid composition and tocopherol) and oxidative indices (PV and MAD) of CO and OO during 30 min CI, and storage at 60 °C for 28 days.
Materials and methods
Materials
CO (Kucukbay Oil Co. İzmir, Turkey) and OO (Komili Oil Co. Istanbul, Turkey) certificated by Turkish Standard Institute (Anonymous 2003, 2011) were obtained from local supermarkets in Van, Turkey. A mixture of 37 FAME (C4- C24) was purchased from Supelco (Bellefonte, PA, USA). Tocopherol and tocotrienol, AP and all other chemicals were obtained from Sigma-Aldrich Chem. Co. (St. Louis, MO, USA). The common chemicals were of analytical reagent grade.
Methods
Recovery of AP
The solubility and uniformity of AP in the oil samples were determined according to AOCS (1998). The recovered concentration percentage of AP in CO and OO were 97 and 99 %, respectively. The %recovered was calculated by dividing the recovered concentration of AP to the amount of added AP.
Chemical interesterification
Interesterification reactions were carried out in a 1-liter suction flask using a hot plate stirrer. CO and OO samples (500 g) with, and without 400 ppm AP were separately dried by heating under vacuum for 20 min at 90 °C to remove traces of moisture from the oil. NaOCH3 (0.5 %) was added as a catalyst, and the mixture was stirred at 80–90 °C for 5–8 min, upon which the color of the mixture became brownish because of the formation of active catalyst and the triacylglycerols. Initially and after 10, 20 and 30 min CI, oil samples were taken for fatty acid composition, tocopherol, PV and MAD analysis. After a 30-min reaction, the catalyst was inactivated by adding citric acid (0.4 % of total sample volume) and stirring for 15 min at 80 °C under vacuum. The reaction mixture was washed three times with warm water (50 °C, 250 mL) to remove the citric acid and sodium methoxide. A filter aid (2 %) (celite analytical filter aid 300 G, Fisher Scientific Co., Pittsburgh, PA) was added and mixed well, and the mixture was filtered through a Buchner filter. The filtrate was dried under vacuum in a rotary evaporator. Residual water was removed with excess anhydrous sodium sulfate followed by filtration through a Whatman no. 2 filter paper (Whatman International Ltd., Maidstone, UK) (Zeitoun et al. 1993; Rousseau and Marangoni 1999). The interesterified oils were kept at −18 °C until required.
Oxidation conditions
Fifteen grams of non-interesterified and interesterified CO and OO, with or without AP were separately transferred into 30 mL serum bottles. The bottles were sealed, air tight with a teflon-coated rubber septum and aluminum caps (Supleco Inc., Bellefonte, PA). Samples were stored in thermostated oven (EN 400 Y, Nuve, Istanbul, Turkey) in the dark at 60 °C for 28 days. Separate containers were used for the fatty acid composition, tocopherol, PV and MAD analysis, initially and after 7, 14, 21 and 28 days of storage (Basturk et al. 2007). Samples were kept at −18 °C until required. The experiment was repeated twice and the analysis was duplicated.
Fatty acid composition
For the preparation of fatty acid methyl esters (FAMEs), 0.4 g sample was dissolved in 4 mL of isooctane and methylated in 0.2 mL 2-M methanolic KOH. Analysis of FAME was performed on an Agilent 6890 series gas-chromatography (Agilent Technologies, Palo Alto, CA) equipped with flame ionization detector and a 60 m capillary column (ID = 0.25 mm) coated with 0.25 μm of 50 %-cyanopropyl-methylpolysiloxane (J&W Scientific, Folsom, CA, USA). Helium was used as a carrier gas at a flow rate of 1.5 mL/min and a split ratio of 1:10. Injector temperature was 250 °C, detector temperature was 260 °C and the oven temperature was programmed at 120 °C for a hold of 5 min and increased to 240 °C at a rate of 15 °C/min and hold at the final temperature for 20 min. Samples were injected into the column inlet using an Agilent 7683 B series automatic injector. FAMEs were identified by comparison of their retention time and equivalent chain length with respect to standard FAMEs (47885-U, Supelco). FAMEs from samples were quantified according to their percentage area (AOAC 1990).
Tocopherols
Tocols were extracted according to the method described by Surai et al. (1996). In brief, 0.5 g of sample was saponified with ethanolic KOH in the presence of pyrogallol and the tocols were extracted from the mixture with hexane (5 mL). The extraction was repeated twice more with 5 mL hexane. Hexane extracts were combined, evaporated and re-dissolved in a mixture of methanol/dichloromethane (1:1, v/v). The extract was dried under nitrogen and redissolved in methanol. Normal phase was used to analyze tocopherols using a ThermoFinnigan HPLC (ThermoFinnigan, San Jose, CA). The chromatographic separation was achieved with a Phenomatographic Luna silica gel column (4.6 mm i.d. × 250 mm, 5 μm particle size, Phenomenex, Torrance, CA). Chromatography was performed using a mobile phase of n-hexane/ethyl acetate/acetic acid (97.3:1.8:0.9 v/v/v) at a flow rate of 1.6 mL/min. Fluorescence detection utilised excitation and emission wavelengths of 295 and 330 nm, respectively (Panfili et al. 2003). Calibration was performed using standard solutions of tocopherols and tocotrienols.
Oxidative stability measurements
Oxidative stability of oil samples was evaluated by measurement of PVs (AOCS 1994) and MAD contents (Özkanlı and Kaya 2007).
Statistical analysis
Data from two replications were analyzed by one-way analysis of variance using SPSS for Windows program. If a significant was detected, the Duncan’s multiple range test (Duncan 1955) was employed to determine differences between treatments. Significance level was established at p < 0.05.
Results and discussion
Fatty acid composition
In this study the fatty acids of oil samples were categorized as saturated (SFA), unsaturated (UFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids. The UFA/SFA ratio was used to evaluate the effect of 400 ppm AP addition on fatty acids of samples during 30 min CI, and under accelerated oxidation condition (28 days storage at 60 °C). UFA/SFA includes the percentage of all the fatty acids, therefore it represents the fatty acid profile of oil sample as a single data, consequently easy to interprete. The differences between UFA/SFA ratios of non-interesterified and interesterified CO and OO, with or without AP during 30 min CI were not significant (p > 0.05) (data not shown). CI does not affect the degree of unsaturation and does not cause any isomerization in oils and fats (Basturk et al. 2007). The initial UFA/SFA ratios of CO and OO ranged between 2.66–2.75 and 5.47–5.67, respectively (Tables 1 and 2). PUFAs (C18:2) were predominant fatty acids in CO, and OO was rich in MUFA (C18:1). The UFA/SFA ratios of all the treatments showed significant (p < 0.05) reduction during storage at 60 °C. This is mainly due to decrease in PUFA contents of oil samples. Non-interesterified and interesterified samples with AP had higher UFA/SFA than their counterparts without AP. AP seemed to protect PUFAs against oxidation.
Table 1.
Fatty acid composition of cottonseed oil samples stored at 60 °C for 28 days
| Days | Fatty acids methyl esters ( %) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cottonseed oil | Cottonseed oil + Ascorbyl palmitat | INT-Cottonseed oil | INT-Cottonseed oil + Ascorbyl palmitat | |||||||||||||
| SFA | MUFA | PUFA | UFA/SFA | SFA | MUFA | PUFA | UFA/SFA | SFA | MUFA | PUFA | UFA/SFA | SFA | MUFA | PUFA | UFA/SFA | |
| 0 | 27.3 | 17.3 | 55.4 | 2.66aA | 27.0 | 16.8 | 55.6 | 2.68aA | 27.1 | 17.1 | 55.8 | 2.69aA | 26.6 | 17.3 | 55.8 | 2.75aA |
| 7 | 27.2 | 17.0 | 55.6 | 2.67aB | 27.2 | 17.2 | 54.9 | 2.65aB | 27.2 | 17.4 | 55.2 | 2.67aB | 26.5 | 17.8 | 55.4 | 2.76aA |
| 14 | 27.8 | 17.4 | 54.8 | 2.60abB | 27.3 | 16.9 | 55.7 | 2.66aAB | 27.5 | 17.2 | 55.1 | 2.63bB | 26.8 | 17.6 | 55.6 | 2.73abA |
| 21 | 28.5 | 17.8 | 53.6 | 2.50cC | 27.5 | 17.6 | 54.8 | 2.63abB | 27.7 | 17.4 | 54.9 | 2.61bB | 26.8 | 18.0 | 55.2 | 2.73abA |
| 28 | 28.2 | 17.6 | 53.9 | 2.53bcC | 27.9 | 17.5 | 54.6 | 2.58bBC | 27.6 | 17.6 | 54.8 | 2.62bB | 27.1 | 17.9 | 55.0 | 2.69bA |
a dDifferent superscript letters in the same column indicate significant difference between values (UFA/SFA) at p < 0.05 level
A CDifferent superscript letters in the same raw indicate significant difference between values (UFA/SFA) at p < 0.05 level
INT Interesterified, UFA/SFA Unsaturated fatty acids/Saturated fatty acids, MUFA Monounsaturated fatty acids, PUFA Polyunsaturated fatty acids
Table 2.
Fatty acid omposition of olive oil samples stored at 60 °C for 28 days
| Days | Fatty acids methyl esters ( %) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olive oil | Olive oil + Ascorbyl palmitat | INT-Olive oil | INT-Olive oil + Ascorbyl palmitat | |||||||||||||
| SFA | MUFA | PUFA | UFA/SFA | SFA | MUFA | PUFA | UFA/SFA | SFA | MUFA | PUFA | UFA/SFA | SFA | MUFA | PUFA | UFA/SFA | |
| 0 | 15.4 | 73.8 | 10.4 | 5.47aB | 15.1 | 73.5 | 10.9 | 5.59aAB | 15.0 | 74.2 | 10.8 | 5.67aA | 15.0 | 73.7 | 11.2 | 5.66aA |
| 7 | 15.6 | 74.2 | 10.2 | 5.41bC | 15.4 | 74.1 | 10.4 | 5.49abB | 15.0 | 74.2 | 10.8 | 5.67aA | 15.0 | 73.8 | 11.2 | 5.65aA |
| 14 | 15.8 | 73.5 | 10.3 | 5.30cC | 15.4 | 73.9 | 10.7 | 5.49abB | 15.5 | 74.5 | 10.0 | 5.45bB | 15.0 | 74.0 | 10.9 | 5.66aA |
| 21 | 16.0 | 74.2 | 9.8 | 5.25cC | 15.6 | 74.6 | 9.60 | 5.40bcB | 15.6 | 74.2 | 10.2 | 5.41bB | 15.2 | 73.8 | 11.0 | 5.58bA |
| 28 | 16.2 | 73.4 | 9.9 | 5.14dB | 15.8 | 75.0 | 9.10 | 5.32cA | 15.7 | 74.2 | 10.1 | 5.37bA | 15.7 | 73.6 | 10.7 | 5.37cA |
a dDifferent superscript letters in the same column indicate significant difference between values (UFA/SFA) at p < 0.05 level
A CDifferent superscript letters in the same raw indicate significant difference between values (UFA/SFA) at p < 0.05 level
INT Interesterified, UFA/SFA Unsaturated fatty acids/Saturated fatty acids, MUFA Monounsaturated fatty acids, PUFA Polyunsaturated fatty acids
Tocopherols
α- and γ-Tocopherols were the tocols found in CO and OO. The α- and γ-tocopherol contents of non-interesterified CO and OO were 159.47, 73.23 ppm, and 35.6, 2.33 ppm, respectively (Table 3). The tocopherol content of CO significantly decreased (p < 0.05) during CI. CO samples with 400 ppm AP contained higher α- and γ-tocopherols than their counterparts without AP. After 30 min CI, α- and γ-tocopherol losses of CO samples were about 13 % and 40 %, respectively. The higher loss in γ-tocopherol is probably due to its higher sensivity to oxidation than α-tocopherol. Simmone and Eitenmiller (1998) reported that the relative stability of the α-forms of tocols were highest under frying conditions and their antioxidative potential was the weakest. The losses in α- and γ-tocopherols of OO during CI were not significant (p > 0.05). OO samples with AP showed slightly higher tocopherol contents than their counterparts without AP. Basturk et al. (2007) reported α-tocopherol (61.32 mg/100 g oil) and β-tocopherol (19.87 mg/100 g oil) as tocols found in CO with 23.71 % and 18.67 % reduction for them after 30 min CI, respectively. Tocopherols in oils are found to react with carboxylic acids present in the medium, thus leading to the formation of tocopheryl esters that are not analyzed as free tocopherols and do not render any stability to the resultant modified oils as they lack any free hydroxyl groups on the phenolic ring of the molecule (Hamam and Shahidi 2006). The α- and γ- tocopherol contents of cottonseed and olive oils reported by Sheppard and Pennington (1993) were 389, 387 mg/kg, and 119, 7.0 mg/kg, respectively. The differences in the antioxidant contents of vegetable oils are due to the differences in species of oilseeds, extraction methods (Lee et al. 2007) and analytical techniques (Speak et al. 1999). Tocopherol content of most of the samples significantly (p < 0.05) decreased during 28 days storage at 60 °C (Tables 4 and 5). In spite of their lower initial tocopherol contents due to losses during CI, interesterified oils showed higher tocopherol contents than their non-interesterified counterparts throughout the storage period. The lower tocopherol losses in interesterified oils may be due to the removal of some compounds such as FFA, PV and MAD during CI, which could have prooxidant effects. The partial vacuum and bleaching which was applied during interesterification reduced the primary and secondary oxidation products of resultant modified oils (Basturk et al. 2007). Lower prooxidant content probably led to lower tocopherol losses. Samples with AP contained higher tocopherol contents than their counterparts without AP in most of the CO and OO groups. In the absence of AP the α-tocopherol content of OO fell below 1 ppm (97.57 % loss). However, in non-interesterified and interesterified OO treatments with AP, α-tocopherol losses were 63.72 and 29.69 %, respectively. AP is considered to have protective effect on α-tocopherol of oil samples. The protective effect of AP on α-tocopherol in heated (95 °C) sunflower oil (Beddows et al. 2001), and its strong synergistic effects for binary mixtures with α-tocopherol in the oxidation of butter oil triacylglycerols (Karabulut 2010) have been previously reported.
Table 3.
Tocopherol contents of cottonseed and olive oil samples duirng chemical interesterification (mg/kg oil)
| Days | Cottonseed oil | Cottonseed oil + Ascorbyl palmitat | Olive oil | Olive oil + Ascorbyl palmitat | ||||
|---|---|---|---|---|---|---|---|---|
| α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | |
| 0 | 159.47aA | 73.23aA | 161.62aA | 74.58aA | 35.60aA | 2.33aA | 35.78aA | 2.47aA |
| 10. | 153.61aA | 42.85bA | 155.21abA | 45.06bA | 31.20aA | 2.05aA | 33.44aA | 2.35aA |
| 20. | 143.57bA | 42.84bB | 148.53bcA | 46.96bA | 31.88aA | 1.93aA | 35.23aA | 2.38aA |
| 30. | 139.66bA | 41.76bB | 140.20cA | 44.80bA | 30.43aA | 1.97aA | 34.65aA | 2.45aA |
a dDifferent superscript letters in the same column indicate significant difference between values at p < 0.05 level
A DDifferent superscript letters in the same raw for each oil indicate significant difference between values at p < 0.05 level for the same compound
INT Interesterified
Table 4.
Tocopherol contents of cottonseed oil samples stored at 60 °C for 28 days (mg/kg oil)
| Days | Cottonseed oil | Cottonseed oil + Ascorbyl palmitat | INT-Cottonseed oil | INT-Cottonseed oil + Ascorbyl palmitat | ||||
|---|---|---|---|---|---|---|---|---|
| α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | |
| 0. | 159.47aA | 73.22aA | 161.62aA | 74.58aA | 139.66aB | 41.76aB | 140.2aB | 44.80aB |
| 7. | 111.25bAB | 31.74bcB | 124.30bA | 43.60bA | 108.64bB | 41.15aAB | 114.17bAB | 44.30aA |
| 14. | 93.85cB | 33.20bA | 106.91cA | 39.65bA | 102.25bAB | 38.48aA | 107.66bA | 41.17aA |
| 21. | 65.82dB | 28.91bcB | 103.5cA | 39.89bA | 102.97bA | 38.82aA | 112.71bA | 43.51aA |
| 28. | 63.80dB | 23.17cC | 99.58cA | 34.00bB | 70.45cB | 37.32aA | 91.64cA | 42.08aA |
a dDifferent superscript letters in the same column indicate significant difference between values at p < 0.05 level
A DDifferent superscript letters in the same raw indicate significant difference between values at p < 0.05 level for the same compound
INT Interesterified
Table 5.
Tocopherol contents of olive oil samples stored at 60 °C for 28 days (mg/kg oil)
| Days | Olive oil | Olive oil + Ascorbyl palmitat | INT-Olive oil | INT-Olive oil + Ascorbyl palmitat | ||||
|---|---|---|---|---|---|---|---|---|
| α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | α- tocopherol | γ- tocopherol | |
| 0. | 35.60aA | 2.33aA | 35.78aA | 2.47aA | 30.43aA | 1.97aA | 34.65aA | 2.45aA |
| 7. | 21.31bB | 1.32bA | 35.95aA | 1.91abA | 22.68bB | 1.20bA | 30.52abAB | 1.66bA |
| 14. | 17.19bB | 0.94bcA | 20.20bAB | 1.40bcA | 21.96bAB | 1.07bcA | 28.62bcA | 1.20bA |
| 21. | 10.63bcC | 0.82bcA | 20.13bB | 1.15cA | 21.89bAB | 0.85bcA | 26.93bcA | 0.94bA |
| 28. | 0.865cC | 0.65cA | 12.98bB | 0.83cA | 22.10bA | 0.68cA | 24.36cA | 0.89bA |
a dDifferent superscript letters in the same column indicate significant difference between values at p < 0.05 level
A DDifferent superscript letters in the same raw indicate significant difference between values at p < 0.05 level for the same compound
INT Interesterified
Peroxide value (PV)
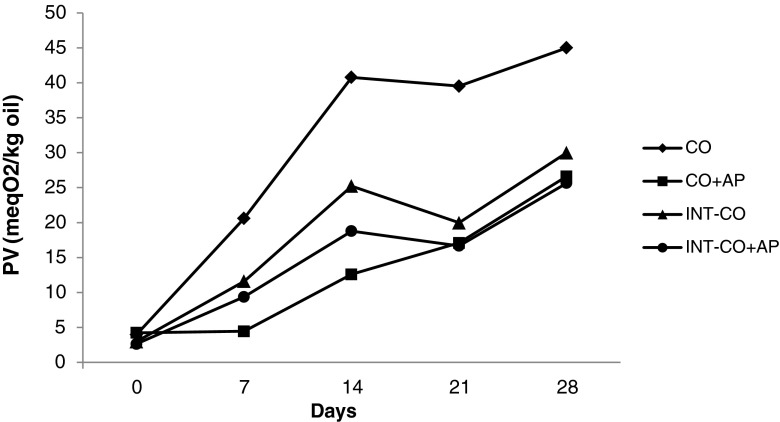
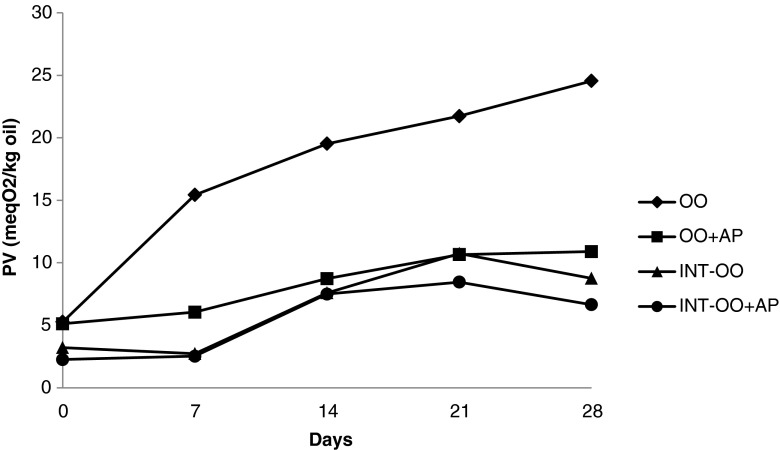
Hydroperoxides were measured to determine the initial rate of oxidation because they are generally accepted as the first products formed by oxidation (Rossell 1986). However, the hydroperoxide generation and degradation rates were different in each sample; there were some general trends. The final PVs of all the samples were less than their initial values after 30 min CI (Table 6). CI was carried out under vacuum at moderately high temperature (90 °C); this could remove peroxide and peroxide decomposition products. This application can be considered as a mild deodorization process (Basturk et al. 2007). Johnson (2002) noted that freshly deodorized oil should have a PV of zero. The PV of samples significantly increased (p < 0.05) up to 20 min, followed by a reduction at 30 min of CI. Samples with added AP showed lower PV than their counterparts without AP. The PV of all the treatments significantly (p < 0.05) increased during storage at 60 °C (Figs. 1 and 2). Samples with added AP had lower PV than their counterparts without AP. Non-interesterified CO and OO showed significantly higher (p < 0.05) PV than those of interesterified groups at all the sampling intervals. Inspite of its lower PUFA content OO showed higher initial PV than CO, because OO did not go through refining process. List et al. (1993) indicated that 67 % of peroxides in soybean oil were removed during the oil refining such as bleaching. CO showed higher PV formation than OO throughout the storage period due to its higher PUFA content. According to Lee et al. (2007) OO was more stable in spite of its higher initial amount of free fatty acids and peroxides than sunflower and soybean oils. The initial and final PV for non-interesterified and interesterified CO kept at 60 °C for 21 days reported by Basturk et al. (2007) were 6.0, 109.4, and 5.0, 43.1 meq O2/kg oil, respectively.
Table 6.
The peroxide values of cottonseed and olive oil samples during interesterification (meq O2/kg oil)
| Time (min) | Oils | |||
|---|---|---|---|---|
| Cottonseed | Cottonseed + AP | Olive | Olive + AP | |
| 0 | 3.98bA | 4.23bcA | 5.27bA | 5.12aA |
| 10 | 8.42abA | 7.56abB | 5.68abA | 5.42aA |
| 20 | 12.1aA | 9.66aB | 6.98aA | 5.62aA |
| 30 | 2.98bA | 2.65cA | 3.22cA | 2.26bA |
a bDifferent superscript letters in the same column indicate significant difference between values at p < 0.05 level
A BDifferent superscript letters in the same raw for each oil indicate significant difference between values at p < 0.05 level for the same oil
AP Ascorbyl palmitate, INT Interesterified
Fig. 1.
Peroxide values (PVs) of cottonseed oil samples during storage at 60 °C (CO cottonseed oil; AP Ascorbyl palmitate; INT Interesterified)
Fig. 2.
Peroxide values (PVs) of olive oil samples during storage at 60 °C (OO Olive oil; AP Ascorbyl palmitate; INT Interesterified)
Malonaldehyde (MAD)
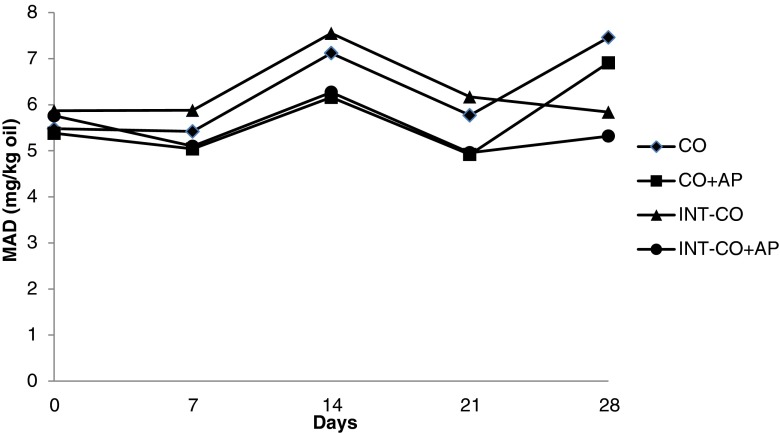
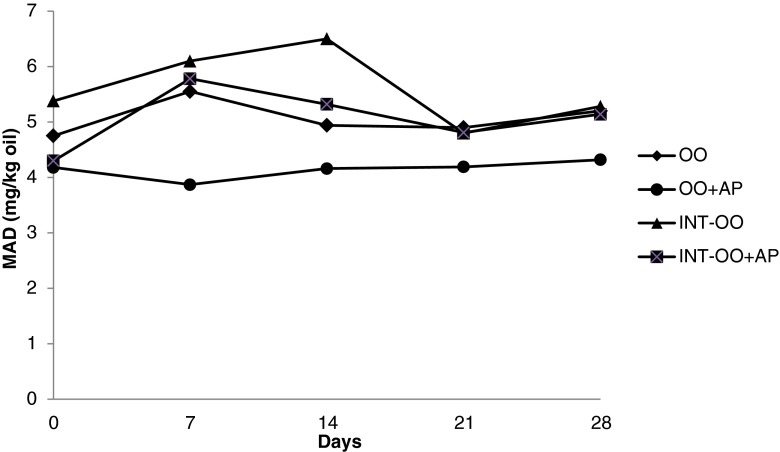
The changes in MAD contents of CO and OO during 30 min CI are given in Table 7. MAD formation during CI showed similar trend as PV. An increase at the early stage of oxidation, followed by a reduction at the later stage. This reduction may be due to the partial removal of MAD during CI, which was performed under low pressure and at moderately high temperature (90 °C). CO and OO samples with AP had lower MAD contents than their counterparts without AP, with no significant change during CI (p > 0.05). Basturk et al. (2007) reported reduction in AVs of CO, palm oil and soybean oil after 30 min CI. Under accelerated oxidation the MAD contents of samples did not show a regular increase, as was already observed in PV. The MAD levels of all the treatments varied irregularly in a narrow range during storage at 60 °C (Figs. 3 and 4). Oxidation is a mixed reaction that involves series and parallel reactions. The cumulative effect of various internal and external parameters in the oil systems makes oxidation a highly complex process (Adhvaryu et al. 2000). In some oils the generation of secondary oxidation products begins almost simultaneously with the generation of hydroperoxides, and in others, the degradation of hydroperoxides begins when the concentration of these compounds is appreciable. However, a high rate of hydroperoxides does not always involve a high rate of generation of secondary oxidation products (Guillen and Cabo 2002). During storage at 60 °C interesterified samples showed significantly (p < 0.05) lower MAD contents than their non-interesterified counterparts. This could be due to the lower intial PV of interesterified oils. Hydroperoxides which are transitory intermediates in oxidized oils can break down to give two free radicals (ROº and OHº) or two free radicals (ROOº, ROº) and water. This branching steps lead to proliferation of free radicals which may participate in the propagation step (Hamilton et al. 1997). Therefore, the lower initial hydroperoxide concentration could directly affect the reaction rate of secondary oxidation products. Jacobsen et al. (2003) reported that the higher initial levels of lipid hydroperoxides and secondary volatile oxidation compounds may reduce the oxidative stability of mayonnaise. Samples with AP had lower MAD contents than those without AP. This could be due to their higher tocopherol contents.
Table 7.
The malonaldehyde contents of cottonseed and olive oil samples during interesterification (mg/kg oil)
| Time (min) | Oils | |||
|---|---|---|---|---|
| Cottonseed | Cottonseed + AP | Olive | Olive + AP | |
| 0 | 5.48bA | 5.38aA | 4.74bA | 4.18aA |
| 10 | 7.66abA | 5.34aA | 5.57aA | 4.49aA |
| 20 | 8.27aA | 6.29aA | 5.51abA | 5.03aA |
| 30 | 5.87abA | 5.76aA | 5.38abA | 4.30aB |
a bDifferent superscript letters in the same column indicate significant difference between values at p < 0.05 level
A BDifferent superscript letters in the same raw for each oil indicate significant difference between values at p < 0.05 level for the same oil
AP Ascorbyl palmitate, INT Interesterified
Fig. 3.
Malonaldehyde (MAD) contents of cottonseed oil samples during storage at 60 °C (CO cottonseed oil; AP Ascorbyl palmitate; INT Interesterified)
Fig. 4.
Malonaldehyde (MAD) contents of olive oil samples during storage at 60 °C (OO olive oil; AP Ascorbyl palmitate; INT Interesterified)
Conclusion
Chemical interesterification reduced the tocopherol contents of resultant modified oils. At the presence of AP, oil samples showed lower tocopherol losses during chemical interesterification and storage at 60 °C than their counterparts without AP. AP increased the oxidative stability of oils during chemical interesterification and storage under accelerated oxidation by reducing the tocopherol losses, and decreasing the PV and MAD formation.
Acknowledgments
The authors wish to thank The Science and Technological Research Council of Turkey (Project No: TOVAG 106O823) and the Yüzüncü Yıl University Research Fund (Project No: 2008-ZF-B118) for the financial supports.
References
- Adhikari S, Adhikari J. Detection of interesterified fats in hydrogenated fats. J Am Oil Chem Soc. 1992;69(10):1051–1053. doi: 10.1007/BF02541079. [DOI] [Google Scholar]
- Adhvaryu A, Erhan SZ, Liu ZS, Perez JM. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta. 2000;364(1–2):87–97. doi: 10.1016/S0040-6031(00)00626-2. [DOI] [Google Scholar]
- Alpaslan M, Karaali A. The interesterification-induced changes in olive and palm oil blends. Food Chem. 1998;61(3):301–305. doi: 10.1016/S0308-8146(97)00081-2. [DOI] [Google Scholar]
- Anonymous (2003) Edible Cottonseed Oil Standard. Turkish Standard Institute, TS 887, Ankara
- Anonymous (2011) Edible Olive Oil Standard. Turkish Standard Institute, TS 341, Ankara
- AOAC . Official methods of analysis. Washington, DC: Association of Official Analytical Chemists; 1990. [Google Scholar]
- AOCS . The official methods and recommended practices of the American Oil Chemists’ Society. 4. Champaign: AOCS Press; 1994. [Google Scholar]
- AOCS . The official methods and recommended practices of the American Oil Chemists’ Society. 5. Champaign: AOCS Press; 1998. [Google Scholar]
- Bartee SD, Kim HJ, Min DB. Effects of antioxidants on the oxidative stability of oils containing arachidonic, docosapentaenoic and docosahexaenoic acids. J Amer Oil Chem Soc. 2007;84:363–368. doi: 10.1007/s11746-007-1046-4. [DOI] [Google Scholar]
- Baştürk A (2011) The effects of heavy metal ions, ascorbyl palmitate, temperature and time on oxidative stability of vegetable oils. PhD Dissertation, Yüzüncü Yıl University, Institute of Applied Sciences, Van, Turkey
- Basturk A, Javidipour I, Boyacı IH. Oxidative stability of natural and chemically interesterified cottonseed, palm and soybean oils. J Food Lipids. 2007;14(2):170–188. doi: 10.1111/j.1745-4522.2007.00078.x. [DOI] [Google Scholar]
- Beddows CG, Jagait C, Kelly MJ. Effect of ascorbyl palmitate on the preservation of α-tocopherol in sunflower oil, alone and with herbs and spices. Food Chem. 2001;73:255–261. doi: 10.1016/S0308-8146(00)00295-8. [DOI] [Google Scholar]
- Coppen P. The use of antioxidants. In: Allen JC, Hamilton RJ, editors. Rancidity in foods. 3. London: Blackie Academic Press; 1994. pp. 84–103. [Google Scholar]
- Dinç S, Javidipour I, Özbas OO, Tekin A. Utilization of zero-trans non-interesterified and interesterified shortenings in cookie production. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan IS, Javidipour I, Akan T. Effects of interesterified palm and cottonseed oil blends on cake quality. Int J Food Sci Technol. 2007;42:157–164. doi: 10.1111/j.1365-2621.2006.01178.x. [DOI] [Google Scholar]
- Duncan DB. Multiple range and multiple F-test. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Gavriilidou V, Boskou D. Effect of chemical interesterification on the autoxidative stability of olive oil-tristearin blends. In: Charalambous G, editor. Food flavours, ıngredients and composition. Amsterdam: Elsevier Science Publishers; 1993. pp. 313–314. [Google Scholar]
- Gordon MH, Kourimska L. Effect of antioxidants on losses of tocopherols during deep-fat frying. Food Chem. 1995;52:175–177. doi: 10.1016/0308-8146(94)P4200-Y. [DOI] [Google Scholar]
- Guillen MD, Cabo N. Fourier transform infrared spectra data versus peroxide and anisidine values to determine stability of edible oils. Food Chem. 2002;77:503–510. doi: 10.1016/S0308-8146(01)00371-5. [DOI] [Google Scholar]
- Hamam F, Shahidi F. Acidolysis reactions lead to esterification of endogenous tocopherols and compromised oxidative stability of modified oils. J Agri Food Chem. 2006;54(19):7319–7323. doi: 10.1021/jf061730e. [DOI] [PubMed] [Google Scholar]
- Hamilton RJ, Kalu C, Prisk E, Padley FB, Pierce H. Chemistry of free radicals in lipids. Food Chem. 1997;60:193–199. doi: 10.1016/S0308-8146(96)00351-2. [DOI] [Google Scholar]
- Jacobsen C, Xu X, Nielsen NS, Timm-Heinrich M. Oxidative stability of mayonnaise containing structured lipids produced from sunflower oil and caprylic acid. Eur J Lipid Sci. 2003;105:449–458. doi: 10.1002/ejlt.200300796. [DOI] [Google Scholar]
- Javidipour I, Tunçtürk Y. Effects of using interesterified and non-interesterified corn and palm oil blends on quality and fatty acid composition of Turkish White cheese. Int J Food Sci Technol. 2007;42:1465–1474. doi: 10.1111/j.1365-2621.2006.01366.x. [DOI] [Google Scholar]
- Javidipour I, Vural H. Effects of incorporation of interesterified plant oils on quality and fatty acid composition of Turkish-type salami. Nahrung/Food. 2002;46(6):404–407. doi: 10.1002/1521-3803(20021101)46:6<404::AID-FOOD404>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Javidipour I, Vural H, Ozbas OO, Tekin A. Effects of interesterified vegetable oils and sugar beet fiber on the quality of Turkish-type salami. Int J Food Sci Technol. 2005;40:177–185. doi: 10.1111/j.1365-2621.2004.00928.x. [DOI] [Google Scholar]
- Johnson LA. Recovery, refining, converting, and stabilizing edible fats and oils. In: Akoh CC, Min DB, editors. Food lipids-chemistry, nutrition, and biotechnology. 2. New York: Marcel Dekker, Inc; 2002. pp. 223–273. [Google Scholar]
- Karabulut I. Effects of α-tocopherol, β-carotene and ascorbyl palmitate on oxidative stability of butter oil triacylglycerols. Food Chem. 2010;123:622–627. doi: 10.1016/j.foodchem.2010.04.080. [DOI] [Google Scholar]
- Kimoto H, Endo Y, Fujımoto K. Influence of interesterification on the oxidative stability of marine oil triacylglycerols. J Am Oil Chem Soc. 1994;71(5):469–473. doi: 10.1007/BF02540655. [DOI] [Google Scholar]
- Lau FY, Hammond EG, Ross PF. Effect of randomization on the oxidation of corn oil. J Am Oil Chem Soc. 1982;59(10):407–411. doi: 10.1007/BF02634423. [DOI] [Google Scholar]
- Lee KH, Jung MY, Kim SY. Quenching mechanisms and kinetics of ascorbyl palmitate for the reduction of the photo-sensitized oxidation of oils. J Am Oil Chem Soc. 1997;74(9):1053–1057. doi: 10.1007/s11746-997-0024-1. [DOI] [Google Scholar]
- Lee J, Lee Y, Choe E. Temperature dependence of the autoxidation and antioxidants of soybean, sunflower, and olive oil. Eur Food Res Technol. 2007;226:239–246. doi: 10.1007/s00217-006-0532-5. [DOI] [Google Scholar]
- List GR, King JW, Johnson JH, Warner K, Mounts TL. Supercritical CO2 degumming and physical refining of soybean oil. J Am Oil Chem Soc. 1993;70(5):473–476. doi: 10.1007/BF02542578. [DOI] [Google Scholar]
- Norizzah AR, Chong CI, Cheow CS, Zaliha O. Effects of chemical interesterification on physicochemical properties of palm stearin and palm kernel olein blends. Food Chem. 2004;86(2):229–235. doi: 10.1016/j.foodchem.2003.09.030. [DOI] [Google Scholar]
- Özkanlı O, Kaya A. Storage stability of butter oils produced from sheep’s non-pasteurized and pasteurized milk. Food Chem. 2007;100:1026–1031. doi: 10.1016/j.foodchem.2005.10.052. [DOI] [Google Scholar]
- Öztürk S, Özboy-Özbas Ö, Javidipour I, Köksel H. Utilization of sugarbeet fiber and zero-trans interesterified and non-interesterified shortenings in cookie production. Zuckerindustrie. 2008;133(11):704–709. [Google Scholar]
- Ozturk S, Ozbas OO, Javidipour I, Koksel H. Effects of zero-trans interesterified and non-interesterified shortenings and brewer’s spent grain on cookie quality. J Food Lipids. 2009;16(3):297–313. doi: 10.1111/j.1745-4522.2009.01148.x. [DOI] [Google Scholar]
- Panfili G, Fratianni A, Irano M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J Agri Food Chem. 2003;51(14):3940–3944. doi: 10.1021/jf030009v. [DOI] [PubMed] [Google Scholar]
- Park DK, Terao J, Matsushita S. Influence of interesterification on the antioxidative stability of vegetable oils. Agri Biol Chem. 1983;47(1):121–123. doi: 10.1271/bbb1961.47.121. [DOI] [Google Scholar]
- Perricone N, Nagy K, Horvath F, Dajko G, Uray I, Zs-Nagy I. The hydroxyl free radical reactions of ascorbyl palmitate as measured in various in vitro models. Biochem Biophys Res Commun. 1999;262:661–665. doi: 10.1006/bbrc.1999.1277. [DOI] [PubMed] [Google Scholar]
- Rossell JB. Classical analysis of oils and fats. In: Hamilton RJ, Rossell JB, editors. Analysis of oils and fats. New York: Elsevier Applied Science Publishers; 1986. pp. 1–90. [Google Scholar]
- Rousseau D, Marangoni AG. The effects of interesterification on physical and sensory attributes of butterfat and butterfat-canola oil spreads. Food Res Int. 1999;31(5):381–388. doi: 10.1016/S0963-9969(98)00100-8. [DOI] [Google Scholar]
- Sheppard AJ, Pennington JAT. Analyses and distribution of vitamin E in vegetable oils and foods. In: Packer L, Fuchs J, editors. Vitamin E in health and disease. New York: Marcel Dekker Inc; 1993. pp. 9–31. [Google Scholar]
- Simmone AH, Eitenmiller RR. Retention of vitamin E and added retinyl palmitate in selected vegetable oils during deep-fat frying and in fried breaded products. J Agri Food Chem. 1998;46(12):5273–5277. doi: 10.1021/jf9802528. [DOI] [Google Scholar]
- Speak BK, Surai PF, Noble RC, Beer JV, Wood NAR. Differences in egg lipid and antioxidant composition between wild and captive pheasants and geese. Comp Biochem Physiol Part B. 1999;124(1):101–107. doi: 10.1016/S0305-0491(99)00108-X. [DOI] [Google Scholar]
- Surai PF, Noble RC, Speake BK. Tissue-specific differences in antioxidant distribution and susceptibility to lipid peroxidation during development of the chick embryo. Biochimica Et Biophysica Acta. 1996;1304(1):1–10. doi: 10.1016/S0005-2760(96)00099-9. [DOI] [PubMed] [Google Scholar]
- Tautorus CL, Mccurdy AR. Effect of randomization on oxidative stability of vegetable oils at two different temperatures. J Am Oil Chem Soc. 1990;67(8):525–530. doi: 10.1007/BF02540760. [DOI] [Google Scholar]
- Vural H, Javidipour I. Replacement of beef fat in frankfurters by interesterified palm, cottonseed and olive oils. Eur Food Res Technol. 2002;214:465–468. doi: 10.1007/s00217-002-0502-5. [DOI] [Google Scholar]
- Vural H, Javidipour I, Ozbas OO. Effects of interesterified vegetable oils and sugarbeet fiber on the quality of frankfurters. Meat Sci. 2004;67:65–72. doi: 10.1016/j.meatsci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Wada S, Koizumi C. Influence of the position of unsaturated fatty acid esterified glycerol on the oxidation rate of triglyceride. J Am Oil Chem Soc. 1983;60(6):1105–1109. doi: 10.1007/BF02671335. [DOI] [Google Scholar]
- Zalewski S, Gaddis AM. Effect of transesterification of lard on stability, antioxidant-synergist efficiency, and rancidity development. J Am Oil Chem Soc. 1967;44(10):576–580. doi: 10.1007/BF02901253. [DOI] [Google Scholar]
- Zeitoun MAM, Neff WE, List GR, Mounts TL. Physical properties of interesterified fat blends. J Am Oil Chem Soc. 1993;70(5):467–471. doi: 10.1007/BF02542577. [DOI] [Google Scholar]