Abstract
Strong antioxidant activity, antimicrobial, antiviral, antitumor, and some antiallergenic potential actions have been reported in peppermint. For daily acceptance of the benefits of this plant, UF-Feta cheese, enriched by whole peppermint extract, was prepared with different levels of Peppermint Extract (PE) (220–660 μg/g cheese), starter (1.3–2.7 g/100 Kg retentate), rennet (1.3–2.5 g/100 Kg retentate), and Ripening Time (RT) (10–50 days). Simultaneous effects of the considered variables were also investigated on Water-soluble Phenolic Content (WSPC), Antioxidant Activity (AOA), and Sensory Score (SS) by means of Response surface methodology. The results showed that although rennet concentration had a little positive effect on WSPC, its effect on AOA was significantly negative. It was determined that PE had a crucial role in acceptance of cheese samples and showed a negative effect on SS. More maturation of produced cheese samples effectively increased AOA. It was found that for producing cheese with maximum SS and AOA, the optimum values of variables should be applied as follows: PE, 227 μg/g cheese; starter, 2.7 g/100 Kg retentate; rennet, 1.3 g/100 Kg retentate; RT, 41.7 days.
Keywords: Antioxidant activity, UF-Feta cheese, Peppermint extract, Response surface methodology
Introduction
Nowadays, nutraceuticals production should be highly regarded by food industries since they play a significant role in modifying and maintaining physiological function that keep the body healthy (Das et al. 2012). One of the primary ingredients in nutraceuticals production is herbal extracts. Some of these extracts have antioxidant properties. Peppermint is a plant with strong antioxidant activity (Sroka et al. 2005; Fadavi and Beglaryan 2010) as well as antimicrobial, antiviral, antitumor, and some antiallergenic potential. Animal model studies have demonstrated several positive effects such as relaxation on gastrointestinal (GI) tissue, analgesic and anesthetic reactions in the central and peripheral nervous system, immunomodulating and chemopreventive functions (McKay and Blumberg 2006). To accept these benefits, the application of peppermint extract as an additive in the most consuming processed food has been proposed. People in Iran have been traditionally consuming cheese with vegetables specially peppermint. However, changes in lifestyle and lack of time to sufficiently wash and prepare vegetables, due to the increasing urbanization have led to a decrease in their consumption. According to the statistics, Feta cheese produced by ultrafiltration (UF) is a favourite cheese among Iranians and this considerable demand has led to an increase in its production from 50,000 tons in 2001 to 400,000 tons in 2008 (Dairy-sector, Iran 2011). In this research, the Response Surface Methodology (RSM) was used to analyze, model, and optimize the different parameters on water-soluble phenolic content, antioxidant activity and sensory evaluation in UF-Feta cheese production enriched with whole peppermint extract. The aim of this study was to investigate the effects of three different concentrations of peppermint extract, rennet and starter, and duration of ripening time on antioxidant activity of UF-Feta cheese and to predict suitable levels of applied factors in which maximum antioxidant activity and sensory score would be obtained.
Materials and methods
Chemicals
Folin–Ciocalteu’s phenol reagent (Merck), Gallic acid (Fluka, 98 %), Anhydrous Sodium Carbonate (Acros Organics, 98 %). 1, 1-diphenyl-2-picrylhydrazyl (Sigma Chemical Co.).
Lactococcus lactis subsp. Cremoris; L. lactis subsp. lactis (DM-230); Thermophilic Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus (Y-502) obtained from Danisco, France (Choozit RA 26 LYO 125 DCU) were used as starter. Rennet [Fromase- 2200 TL granulate; P2200 International Milk Coagulating Unit (IMCU) g −1] as microbial coagulant from Rhizomucor miehei, was obtained from DSM Food Specialities (Seclin, France). Raw cow’s milk, equipment, and filtration moduli were provided by Nasre-Novin Golestan dairy plant (Golestan, Iran).
Preparation of peppermint extract
Fresh leaves of peppermint without any spots or spoilage were harvested from farms located in the suburb of Gorgan, Iran (longitude: 54°, 29.0˝E, latitude: 36°, 50.0˝N) with an altitude between of 50–150 M above the sea level. After washing, they were homogenized into small pieces by using a crasher (Moulinex, type 320, Spain) and ground to a paste. The whole extract was obtained from the paste using a juicer (Myson, model MJE 900, Korea). The extract was filtered through Whatman No. 2 filter paper, pasteurized at 70 °C for 10 min, and then frozen at −20 °C for further use.
Production of UF-Feta cheese
According to the method used in Iranian UF-Feta cheese making plants, cheese samples were prepared at Nasre-Novin Golestan dairy plant (Gorgan, Iran). Cow’s milk, after passing the stages of filtration, cooling, pasteurization (72 °C, 15 s), ultrafiltration (with the concentration factor of 4.5 kg of milk to 1.0 kg of retentate), homogenization (50–70 bar), and repasteurization (85 °C, 60 s), entered into the starter tank, where, by adding the starter, the pH of milk reached the 6.2 level. Then, in the filler, the prepared peppermint extract and rennet (≥2,200 international milk-clotting units/g) were added to each cheese container. After this, they were sent to the coagulation tunnel, where containers were set at 37 °C for 30 min, and allowed the retentate to be converted to a pre-cheese mixture. In the sealing machine, the parchment paper on the top of precheese was coated by 8 g of salt. The aluminum foil was then placed on the cheese container with hot plate of sealing machine on it. The samples were sent to the preripening period (37 °C), where cheese pH decreased to 4.80. After that, cheese samples were transferred to a cold room (9 ± 1 °C) to be cooled and kept for some specified period of ripening. Experimental cheese samples were made at three different levels of peppermint extract (220, 440, and 660 μg/g cheese), starter (1.3, 2.0, and 2.7 g/100 Kg retentate), rennet (1.3, 1.9, and 2.5 g/100 Kg retentate), ripening time (10, 30, and 50 days) in three replicates.
Sample preparation
Twenty grams of each cheese sample was homogenized in a Stomacher (Model 400 circulator, Seward, England) with 20 ml of water for 2 min. The homogenized samples were centrifuged using a refrigerated centrifuge, (Model; Sigma 3K30, Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany) two times at 10,000 g. at 4 °C for 10 min, and the supernatant was collected and kept at 4 °C. All experiments were performed within 4 days of sample extraction (Apostolidis et al. 2007).
Determination of water-soluble phenolic content
The water-soluble phenolic content was determined by an assay modified by Shetty et al. (1995). Homogenized water extract (1 ml) was transferred into a test tube and mixed with 1 ml of 95 % ethanol and 5 ml of distilled water. To each sample, 0.5 ml of 50 % (V/V) Folin- Ciocalteu’s reagent was added and mixed. After 5 min, 1 ml of 5 % Na2CO3 was added to the reaction mixture and allowed to stand for 60 min. The absorbance was read at 725 nm in a spectrophotometer (Jenway, Model 6305, UV/Vis., England). The absorbance values were converted to water-soluble phenolics and were expressed in mg gallic acid equivalents per gram of dry matter of sample. Standard curves were established using various concentrations of gallic acid in water.
Determination of Antioxidant activity (AOA) by DPPH radical scavenging assay
The capacity to scavenge the 2,2-diphenyl-1- picrylhydrazyl (DPPH) free radical was monitored according to the method reported by Apostolidis et al. (2007). To 3 ml of 60 μM DPPH in ethanol, 250 μl of each homogenized water extract was added, the decrease in absorbance was monitored at 517 nm in a spectrophotometer (Jenway, Model 6305, UV/Vis., England) until a constant reading was obtained. DPPH scavenging effect was calculated as percentage of DPPH discoloration using the equation: %scavenging effect = [(ADPPH − AS)/ADPPH] × 100, where AS is the absorbance of the solution when the sample extract has been added at a particular level and ADPPH is the absorbance of the DPPH solution.
Sensory evaluation
A tool applied in this research was the 7-point hedonic scale, which is a common tool to quantify consumer acceptance. It is a kind of rating scale that has been used in sensory evaluation in the food industry to determine the acceptance of a food and to provide a bench mark for comparison. Its use has been validated in the scientific literature (Stone and Sidel 1993). The test, used in this study, includes the rating for the overall appearance, smoothness of color (whiteness), odor/aroma, taste, overall texture/mouth feel and overall liking with a 7-point hedonic scale (Very bad (1), Bad (2), Little bad (3), Not bad not good (4), Little good (5), Good (6), Very good (7)). Untrained consumers (n = 60) were randomly recruited from Islamic azad University, Azadshahr Branch. Requirements for the panelists were: (1) to be at least 18 years old, (2) not having allergic reactions to cheese and peppermint and (3) positive attitude with disposition of time to complete a questionnaire. The samples were served according to the ripening time and in each test, 3 samples were examined in different times. The consumers were also informed on the procedure of testing. In this study, sensory score refers to the score mean of each sample in overall liking and 60 testers were employed in this assay.
Experimental design and statistical analysis
Response Surface Methodology (RSM), an empirical modeling technique used to estimate the relationship between a set of controllable experimental factors and observed results (Li et al. 2002; Lee et al. 2003), is currently one of the most popular optimization techniques in the field of food science because of its comprehensive theory, reasonably high efficiency and simplicity (Arteaga et al. 1994). The most common experimental design used in RSM is the Central Composite Design (CCD) which has equal predictability in all directions from the center (Liu and Tzeng 1998; Reddy et al. 2000). In addition, CCDs are optimized designs for fitting quadratic models. The number of experimental points in the CCD is sufficient to test statistical validity of the fitted model and lack-of-fit of the model (Arteaga et al. 1994). The central point in CCD is replicated several times to estimate the error due to experimental or random variability.
The statistical analysis of the data was performed using the Design-Expert software (Version 7.0.0, Stat-ease Inc., Minneapolis, MN, USA, 2005). A 3-level-4-factor experimental design with 5 replicates at the center point was used (Box and Behnken 1960).
Four different parameters of peppermint extract, starter, rennet, and ripening time were chosen as main variables and designated as X1, X2, X3, and X4, respectively. The low, middle, and high levels of each variable were designated as -1, 0, and +1, respectively (Table 1). The variables were coded according to the Eq. (1):
| 1 |
Table 1.
Code and levels of variables chosen for the trails
| Factors | Symbols | Levelsa | |||
|---|---|---|---|---|---|
| Coded | Uncoded | −1 | 0 | +1 | |
| Peppermint extract (μg/g cheese) | x 1 | X1 | 220 | 440 | 660 |
| Starter (g/100 Kg retentate) | x 2 | X2 | 1.30 | 2.00 | 2.70 |
| Rennet (g/100 Kg retentate) | x 3 | X3 | 1.30 | 1.90 | 2.50 |
| Ripening time (day) | x 4 | X4 | 10 | 30 | 50 |
a x 1 = (X 1 -440)/220; x 2 = (X 2 -2.00)/0.70; x 3 = (X 3 -1.90)/0.60; x 4 = (X 4 -30)/20
Where xi = (dimensionless) coded value of the variable Xi, and X0 = the value of Xi at the centre point and ΔX = the step change. Table 2 shows the actual design of the experiments. The behavior of the system was explained by the following second degree polynomial equation:
| 2 |
Table 2.
Box–Behnken design arrangement of process variables and actual values and predicted values of responses
| Standard order | Variables | WSPCa (μgb Gallic acid/g dry matter) | Antioxidant activity (%) | Sensory score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x 1 | x 2 | x 3 | x 4 | Actual value | Predicted value | Actual value | Predicted value | Actual value | Predicted value | |
| 1 | 0 | −1 | −1 | 0 | 21.33 | 22.71 | 48.11 | 46.64 | 4.04 | 4.49 |
| 2 | 0 | −1 | +1 | 0 | 27.01 | 26.10 | 41.89 | 38.06 | 4.41 | 3.98 |
| 3 | 0 | +1 | −1 | 0 | 23.50 | 24.89 | 42.70 | 48.37 | 4.26 | 4.68 |
| 4 | 0 | +1 | +1 | 0 | 25.21 | 24.32 | 20.00 | 23.31 | 4.87 | 4.41 |
| 5 | −1 | 0 | 0 | 10 | 21.69 | 21.97 | 20.27 | 21.17 | 4.70 | 4.83 |
| 6 | +1 | 0 | 0 | 10 | 23.23 | 23.18 | 24.32 | 23.56 | 4.27 | 4.04 |
| 7 | −1 | 0 | 0 | +1 | 23.41 | 23.94 | 28.69 | 31.29 | 4.60 | 4.82 |
| 8 | +1 | 0 | 0 | +1 | 20.16 | 20.37 | 31.90 | 32.85 | 4.25 | 4.11 |
| 9 | 0 | 0 | −1 | 10 | 24.40 | 23.51 | 55.51 | 47.41 | 5.10 | 4.64 |
| 10 | 0 | 0 | +1 | 10 | 23.05 | 23.75 | 20.27 | 30.39 | 3.60 | 4.05 |
| 11 | 0 | 0 | −1 | +1 | 22.68 | 21.91 | 63.81 | 56.92 | 5.05 | 4.48 |
| 12 | 0 | 0 | +1 | +1 | 23.68 | 24.50 | 28.95 | 40.29 | 3.95 | 4.29 |
| 13 | −1 | −1 | 0 | 0 | 22.65 | 23.08 | 20.27 | 24.15 | 4.60 | 4.31 |
| 14 | −1 | +1 | 0 | 0 | 25.12 | 25.01 | 19.46 | 19.00 | 5.34 | 5.39 |
| 15 | +1 | −1 | 0 | 0 | 23.59 | 23.63 | 23.78 | 27.48 | 4.50 | 4.33 |
| 16 | +1 | +1 | 0 | 0 | 22.59 | 22.10 | 20.27 | 19.62 | 3.71 | 3.88 |
| 17 | −1 | 0 | −1 | 0 | 22.50 | 21.60 | 20.27 | 24.75 | 5.66 | 5.62 |
| 18 | −1 | 0 | +1 | 0 | 26.47 | 26.25 | 18.92 | 7.53 | 5.59 | 5.52 |
| 19 | +1 | 0 | −1 | 0 | 23.86 | 23.66 | 20.00 | 26.32 | 4.95 | 5.16 |
| 20 | +1 | 0 | +1 | 0 | 21.33 | 21.83 | 19.46 | 9.91 | 4.32 | 4.49 |
| 21 | 0 | −1 | 0 | 10 | 22.86 | 22.59 | 46.76 | 47.08 | 3.26 | 3.47 |
| 22 | 0 | +1 | 0 | 10 | 24.67 | 24.90 | 44.05 | 41.58 | 3.87 | 3.77 |
| 23 | 0 | −1 | 0 | +1 | 24.94 | 24.28 | 60.39 | 57.79 | 3.27 | 3.50 |
| 24 | 0 | +1 | 0 | +1 | 22.50 | 22.37 | 55.68 | 50.29 | 3.90 | 3.82 |
| 25 | 0 | 0 | 0 | 0 | 22.16 | 23.95 | 24.05 | 19.34 | 5.40 | 5.15 |
| 26 | 0 | 0 | 0 | 0 | 24.94 | 23.95 | 15.14 | 19.34 | 5.10 | 5.15 |
| 27 | 0 | 0 | 0 | 0 | 24.49 | 23.95 | 15.35 | 19.34 | 5.00 | 5.15 |
| 28 | 0 | 0 | 0 | 0 | 22.95 | 23.95 | 24.05 | 19.34 | 5.50 | 5.15 |
| 29 | 0 | 0 | 0 | 0 | 25.21 | 23.95 | 18.11 | 19.34 | 4.75 | 5.15 |
Variables: x 1, peppermint extract (μg/g cheese); x 2, starter (g/100 Kg retentate); x 3, rennet (g/100 Kg retentate); x 4, Ripening time (day)
a WSPC Water-soluble phenolic content
b μg; 10−6 gram
Where Y is response; β0, βi, βii and βij are constant coefficients and xi the coded independent variables.
By the above mentioned software, regression analyses of the data and the coefficients of the regression equation were estimated. The fit of the regression model attained was checked by the adjusted coefficient of determination (R2Adj). The two-dimensional graphical representation of the system behavior, called the response surface, was used to describe the individual and cumulative effects of the variables as well as the mutual interactions between the variables on the dependant variable. Using this software, statistically significant and suitable polynomial response surface models for antioxidant activity, water-soluble phenolic content, and sensory evaluation response variables were detected. The results in AOA and WSPC were means of three replicates.
Results and discussion
Predictive models of regression
The Water-soluble phenolic content (WSPC), Antioxidant activity (AOA) and Sensory Score (SS) response values, obtained under the different experimental conditions, are summarized in Table 2. The application of RSM offers, on the basis of parameter estimate, an empirical relationship between the response variable and the studied test variables. By applying multiple regression analysis on the experimental data, the response variable of WSPC, AOA, SS, and their test variables are related by the following second-order polynomial equation:
| 3 |
| 4 |
| 5 |
Some parts of analysis of variance (ANOVA) and test of significance for regression coefficients for the selected quadratic predictive model is shown in Table 3. Statistical testing of models was done in the form of analysis of variance (ANOVA) which is required to test the significance and adequacy of the models. The ANOVA of the regression model demonstrates that the models are significant, as it is evident from the probability values which are shown in Table 2. These models also showed statistically insignificant lack of fit and were found to be adequate for prediction within the range of variables employed. The coefficient values of Eq. (2) for each response were calculated and tested for their significance using Design Expert; and are listed in Table 3. The correlation measure for testing the goodness of fit of the regression equation is the adjusted determination coefficient (R2Adj). The value of R2Adj for Eqs. of 3, 4 and 5 indicates a moderate degree of correlation between the observed and predicted values. Adequate Precision is a signal to noise ratio and compares with the range of the predicted values at the design points to the average prediction error as seen from Table 3. For all the models, this ratio is greater than 4, which indicates adequate model discrimination. These models can be used to navigate the design space.
Table 3.
Analysis of variance (ANOVA) and test of significance for regression coefficients
| Source | Water-soluble Phenolic Content (μg Gallic acid/g dry matter) | Antioxidant activity | Sensory score | |||
|---|---|---|---|---|---|---|
| p-value (Prob > F) | Coefficient estimate | p-value (Prob > F) | Coefficient estimate | p-value (Prob > F) | Coefficient estimate | |
| Model | 0.0299* | – | 0.0007*** | – | 0.0060** | – |
| Intercept | – | 23.9495 | – | 19.3405 | – | 5.15 |
| x 1 | 0.0898 | −0.5900 | 0.6661 | 0.9888 | 0.0081** | −0.3741 |
| x 2 | 0.7606 | 0.1006 | 0.1691 | −3.2522 | 0.2198 | 0.1558 |
| x 3 | 0.0469* | 0.7057 | 0.0022** | −8.4088 | 0.1334 | −0.1933 |
| x 4 | 0.5267 | −0.2102 | 0.0483* | 4.8526 | 0.8820 | 0.0183 |
| x 1 x 2 | 0.1453 | −0.8648 | 0.8644 | −0.6756 | 0.0902 | −0.3825 |
| x 1 x 3 | 0.0118* | −1.6216 | 0.9591 | 0.2027 | 0.5161 | −0.14 |
| x 1 x 4 | 0.0515 | −1.1936 | 0.9578 | −0.2092 | 0.9255 | 0.02 |
| x 2 x 3 | 0.0990 | −0.9909 | 0.3067 | −4.1216 | 0.7794 | 0.06 |
| x 2 x 4 | 0.0800 | −1.0585 | 0.8994 | −0.4999 | 0.9814 | 0.005 |
| x 3 x 4 | 0.3141 | 0.5855 | 0.9803 | 0.0976 | 0.6415 | 0.1 |
| x 21 | 0.0790 | −0.8343 | 0.0083** | −9.3742 | 0.6923 | 0.0666 |
| x 22 | 0.4571 | 0.3367 | 0.0010*** | 12.5945 | 0.0005*** | −0.7408 |
| x 23 | 0.6292 | 0.2174 | 0.0342* | 7.1607 | 0.9072 | −0.0195 |
| x 24 | 0.1102 | −0.7510 | 0.0001*** | 17.2499 | 0.0004*** | −0.7670 |
| Lack of Fit | 0.7653 | – | 0.1029 | – | 0.2226 | – |
| R-Squared | – | 0.74 | – | 0.86 | – | 0.81 |
| Adj R-Squared | – | 0.48 | – | 0.73 | – | 0.61 |
| Adeq Precision | – | 7.29 | – | 8.99 | – | 7.12 |
Variables: x 1, peppermint extract (μg/g cheese); x 2, starter ( g/100 Kg retentate ); x 3, rennet ( g/100 Kg retentate ); x 4, Ripening time (day)
*** P ≤ 0.001, ** P ≤ 0.01, * P ≤ 0.05
Water-soluble phenolic content (WSPC)
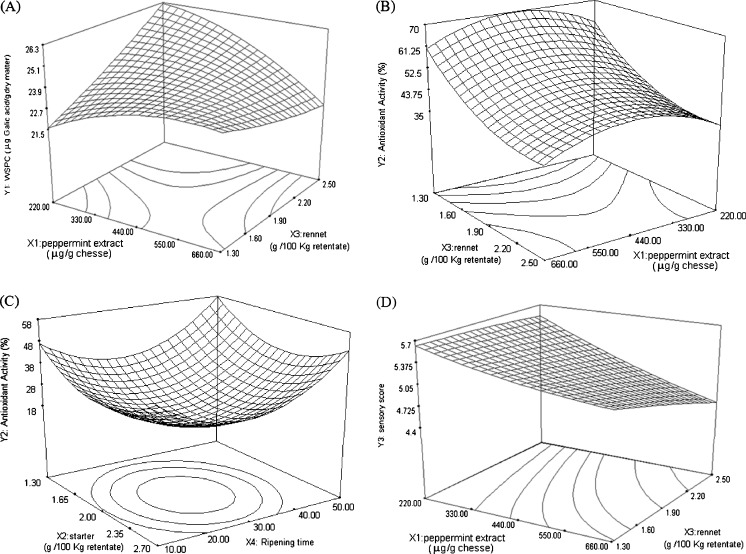
The data in WSPC are listed in Table 2, which is in the range of 20 to 27 μg/dry matter of cheese. As shown in Fig. 1a maximum value of WSPC was achieved at the lowest level of PE and the highest level of rennet (at the middle and highest levels of other variables), but minimum WSPC could be attained at the highest level of all variables. It was found that linear term of rennet had a positive effect on WSPC (p < 0.05, Table 3). As shown in Fig. 1a, with the increase in rennet level, WSPC increased. Rennet producing medium chain peptides (Fox 2004) may act as phenolic compound and cause this increase in WSPC. The interaction of rennet with peppermint showed a negative effect (p < 0.05) on WSPC (Table 3). As seen from Fig. 1a, at low and medium levels of PE, increase in rennet level led to increase in WSPC (at the middle level of starter and ripening time), but by increasing PE level, (the procedure became reverse) during rising of rennet value, WSPC decreased. These changes were to some extent unusual, since peppermint has a good source of phenolic content (Sroka et al. 2005; McKay and Blumberg 2006; Fadavi and Beglaryan 2010). This paradox may be due to the absorption of phenolic compounds of PE by some peptides (produced by rennet and starter during ripening) which not only neutralizes the phenolic compounds of PE, but also deactivates the phenolic compounds presented in cheese. The retention of phenolic compounds in cheese is related to the interactions between phenolic compounds and proteins, which can be induced by hydrogen bonding, hydrophobic, ionic, and covalent interactions (Hagerman 1992). Besides, these interactions can be affected by several factors such as; pH, temperature, phenolic structure, molecular weight, and amino acids compositions in that medium (Bartolome et al. 2000).
Fig. 1.
Surface response plot of a interactions of peppermint extract and rennet on WSPC (water-soluble phenolic content), b PE (peppermint extract) × rennet and c Starter × ripening time on AOA (antioxidant activity) and d PE (peppermint extract) × Rennet on sensory score in UF-Feta cheese
Starter had no effect on WSPC, but its coefficient was weakly positive. As shown in Table 3, starter interaction with rennet and ripening time had minor negative effects (p < 0.1) on WSPC. The increase in the starter level caused rising in WSPC (at the middle levels of other variables), but with an increase in ripening time, at high levels of ripening time, the positive effect of starter on WSPC decreased and even reversed.
Antioxidant activity (AOA)
The AOA of produced cheese samples, according to the actual values, ranged from 15.14 % to 63.81 % (Table 2). Although peppermint has a rich source of antioxidant compounds (Sroka, et al. 2005; Fadavi and Beglaryan 2010) among the variables, linear term of PE (with positive coefficient) showed an insignificant effect on AOA, and even its quadratic term had a negative effect (p < 0.01) on AOA (Table 3). This might be the result of interactions occurred between phenolic molecules of PE and proteins (Hagerman 1992), especially whey proteins containing active group (-SH). These reactions led to reducing the influence of antioxidant compound of PE. Several enzymes like tyrosinase may convert polyphenols especially luteolin, the important flavones having AOA in PE (Sroka et al. 2005), to highly active quinines (Shahidi and Naczk 2006). These enzymes can be produced by starters. Quinones can react with amino and sulfhydryl groups of proteins and enzymes as well as with anthocyanins. These secondary reactions may bring about changes in physical, chemical, and nutritional characteristics of food proteins and may also affect sensory properties of food products (Mayer and Harel 1979). Figure 1b shows that the most AOA could be achieved at the middle level of PE.
Starter (with negative coefficient) had no effect on the AOA in linear form, but its quadratic form had a positive effect (p < 0.001) on AOA (Table 3). Linear term of rennet had a negative effect (p < 0.01) but in quadratic form had positive effect (p < 0.01) on AOA (Table 3). Figure 1b clearly shows that with an increase in rennet values, AOA decreases (at the different levels of other variables). This reduction may be due to the interaction of produced intermediate size peptides of cheese with effective polyphenols of PE, which decreases the AOA. This is because in most cheese varieties, rennet produces large (water-insoluble) and intermediate-sized (water-soluble) peptides which are subsequently hydrolyzed to small size by the coagulant and enzymes from the starter (Fox 2004).
The ripening time, is the only variable which had a positive effect on AOA both in linear (p < 0.05) and quadratic forms (p < 0.0001). The high AOA values (more than 55 %) were achieved at the highest level of ripening time (at the middle levels of PE and rennet), which is evident in Fig. 1c. It seems that activation of sulphur-groups during heating process and production of amino acids containing SH-groups during ripening is the main reason for AOA increase. These processes gradually decrease the redox potential which prevents oxidation in cheese (Thomas et al. 1975). Sulphur-groups in proteins, activated in heating process of 75–80 °C in a few minutes, can absorb oxygen, oxygen radicals such as hydrogen peroxide and other peroxides components which result in the production of H2S (Patrick and Swaisgood 1976). Most proteins containing sulphur-groups, derived from β-lacto-globulins and fat-globular membrane proteins, exist in whey (Badings and Vanderpol 1973). Because the total whey proteins left in UF cheese and milk is heated before ultrafiltration (85 °C/1 min), these reactions occur more severely than in other cheeses.
Comparing WSPC and AOA of cheese extracts shows no correlation (data are not shown). This is similar to previous findings reported by Apostolidis et al. (2007) who investigated the amount of AOA and WSPC in 3 different samples of cheese enriched by herbal and fruit extracts.
Sensory evaluation
The obtained data in sensory evaluations (collected from 60 panelists) were means of overall liking score of the produced cheese samples (Table 2). The range of actual values in this test was between 3.26 and 5.66. PE plays a key role in Sensory Score (SS), because it showed a significant effect (P < 0.01) on SS with a negative coefficient (Table 3). It is evident from Fig. 1d that increasing PE level decreases SS, which indicates that more PE produces more unpleasant taste. This is because of the contribution of phenolic compounds in the aroma and taste of numerous foods (Crouzet et al. 1997; Teranishi et al. 1989). The interaction of PE with starter had a minor negative effect on SS (Table 3).
Although the linear term of starter had no effect on SS, its quadratic term had a negative effect (p < 0.001) on SS (Table 3). Rennet had no effect on SS (Table 3) but decreasing rennet level (at the middle levels of other variable), can increase SS (Fig. 1d). Ripening time acted like starter and its effect was not significant on SS, but its quadratic term had a highly negative effect (p < 0.001) on SS (Table 3). From the lowest level to the medium level of ripening time, SS increased (regardless of other variable levels), but values more than the medium level decreased SS (data are not shown). According to the proposed SS model, the utmost sensory score (5.7) would be achieved when the PE and rennet is at the lowest level and starter and ripening time are at the middle levels of ripening (data are not shown).
Optimization
Optimum conditions to produce UF-Feta cheese, enriched with peppermint extract, were determined to obtain the criteria; maximum sensory score (with importance grade of 4) and maximum antioxidant activity (with Importance grade of 3). Second order polynomial models, obtained in this study, were utilized for each response in order to determine the optimum value of each parameter (investigated in this study) for the cheese production. In this study, the optimization was applied for selected ranges of peppermint extract (220–660 μg/g cheese), starter (1.3–2.7 g/100 Kg retentate), rennet (1.3–2.5 g/100 Kg retentate), and ripening time (10–50 days). Using the desirability function method, five solutions were obtained for the optimum covering criteria with desirability value of 0.71 (data are not shown). The best solution was found as follows: 227 μg/g cheese for peppermint extract, 2.7 g/100 Kg retentate for starter, 1.3 g/100 Kg retentate for rennet, and 41.7 days for ripening time. In these circumstances, the solution which had maximum sensory score of 5.02 and maximum antioxidant activity of 48 % had the highest acceptability level and the highest nutritional value for the consumers, which were the main goals of this research.
Conclusion
In this study, the main objective was to produce a UF-Feta cheese containing peppermint extract, with high antioxidant property and acceptability by the consumers. Important factors in UF-Feta cheese production such as starter, rennet, ripening time, and peppermint extract, as a new additive, were optimized. RSM and the conventional graphic and desirability functions methods have been effective in determining the optimum zone within the experimental region. Optimal conditions to achieve the criteria for producing UF-Feta cheese, enriched with peppermint extract, were determined. The best solution was predicted to be 227 μg/g cheese for peppermint extract, 2.7 g/100 Kg retentate for starter, 1.3 g/100 Kg retentate for rennet, and 41.7 days for ripening time. In these circumstances, the solution had maximum sensory score of 5.02, maximum antioxidant activity of 48 %, and desirability value of 0.71.
Acknowledgments
The authors are grateful to Nasr-e-Novin Golestan dairy plant (Golestan, Iran) for providing raw materials, production process equipments, and laboratory space. They are also thankful to Islamic Azad University, Azadshahr Branch, Iran for laboratorial support. This study is part of a PhD thesis, entitled Optimization of Cheese Production, Enriched by Herbal Antioxidant Extract, which was approved by scientific committee of Armenian State Agrarian University as Scientific Research Project (No.331/ts-4 dated 2009/2/30).
References
- Apostolidis E, Kwon Y-I, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Arteaga G-E, Li-Chan E, Vazquez M-C, Nakai S. Systematic experimental designs for product formula optimization. Trends Food Sci Technol. 1994;5:243–254. doi: 10.1016/0924-2244(94)90017-5. [DOI] [Google Scholar]
- Badings HT, Vanderpol JJ. Effects of cooling milk on the heat-release of hydrogen sulfide from the fat-globule membrane. Neth Milk Dairy J. 1973;27:45–53. [Google Scholar]
- Bartolome B, Estrella I, Hernandez M-T. Interaction of low molecular weight phenolics with proteins (BSA) J Food Sci. 2000;65:617–621. doi: 10.1111/j.1365-2621.2000.tb16060.x. [DOI] [Google Scholar]
- Box G-E, Behnken DW. Some new three level designs for the study of quantitative variables. Technometrics. 1960;7:455–475. doi: 10.1080/00401706.1960.10489912. [DOI] [Google Scholar]
- Crouzet J, Sakho M, Chassagne D. Fruit aroma precursors with special reference to phenolics, in phytochemistry of fruit and vegetables. In: Tomas-Barberan FA, Robins RJ, editors. Proceedings of the phytochemical society of Europe. Oxford: Clarendon; 1997. pp. 109–124. [Google Scholar]
- Dairy-sector . Dairy production statistics in milk industry. Tehran: Ministry of Jahad-e –Keshavarzi; 2011. [Google Scholar]
- Das L, Bhaumik E, Raychauduri U, Chakraborty R. Role of nutraceuticals in human health. J Food Sci Technol. 2012;49(2):173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadavi A, Beglaryan R. Investigation of antioxidant activity and total phenolic content of extracs from various plant leaves. Bull State Agrar Univ Armenia. 2010;3(31):153–157. [Google Scholar]
- Fox P-F. Cheese: chemistry, physics, and microbiology. 3. London: Elsevier Academic; 2004. pp. 19–45. [Google Scholar]
- Hagerman AE. Tannin-protein interactions. In: Ho CT, Lee CY, Huang MT, editors. Phenolic compounds in food and their effects on health I. Washington D.C: Analysis, Occurrence, and Chemistry, American Chemical Society; 1992. pp. 236–248. [Google Scholar]
- Lee H, Song M, Hwang S. Optimizing bioconversion of deproteinated cheese whey to mycelia of Ganoderma lucidum. Process Biochem. 2003;38:1685–1693. doi: 10.1016/S0032-9592(02)00259-5. [DOI] [Google Scholar]
- Li C, Bai J, Cai Z, Ouwang F. Optimization of a cultural medium for bacteriocin production by Lactococcus lactis using response surface methodology. J Biotechnol. 2002;93:27–34. doi: 10.1016/S0168-1656(01)00377-7. [DOI] [PubMed] [Google Scholar]
- Liu BL, Tzeng Y-M. Optimization of growth medium for the production of spores from Bacillus thuringiensis using response surface methodology. Bioprocess Eng. 1998;18:413–418. [Google Scholar]
- Mayer A-M, Harel E. Review: polyphenol oxidase in plants. Phytochemistry. 1979;18:193–225. doi: 10.1016/0031-9422(79)80057-6. [DOI] [Google Scholar]
- McKay L-D, Blumberg J-B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- Patrick PS, Swaisgood HE. Sulfhydryl and disulfide groups in skim milk as affected by direct UHT-heating and subsequent storage. J Dairy Sci. 1976;59:594–600. doi: 10.3168/jds.S0022-0302(76)84246-4. [DOI] [Google Scholar]
- Reddy PRM, Mrudula S, Ramesh B, Reddy G, Seenayya G. Production of thermostable pullulanase by Clostridium thermosulfurogenes SV2 in solid-state fermentation: optimization of enzyme leaching conditions using response surface methodology. Bioprocess Eng. 2000;23:107–112. doi: 10.1007/PL00009116. [DOI] [Google Scholar]
- Shahidi F, Naczk M. Phenolics in food and nutraceuticals. Boca Raton: CRC Press LLC; 2006. pp. 439–478. [Google Scholar]
- Shetty K, Curtis O-F, Levin RE, Witkwosky R, Ang W. Prevention of vitrification associated with in vitro shoot culture of oregano (Origanum vulgare) by Pseudomonas spp. J Plant Physiol. 1995;147:447–451. doi: 10.1016/S0176-1617(11)82181-4. [DOI] [Google Scholar]
- Sroka Z, Fecka I, Cisowski W. Antiradical and anti-H2O2 properties of polyphenolic compounds from an aqueous peppermint extract. Z Naturforsch [C] 2005;60(11–12):826–832. doi: 10.1515/znc-2005-11-1203. [DOI] [PubMed] [Google Scholar]
- Stone H, Sidel JL. Sensory evaluation practices. 2. San Diego: Academic; 1993. [Google Scholar]
- Teranishi R, Buttery RG, Shahidi F. Flavor chemistry: trends and developments. Washington, D.C: American Chemical Society; 1989. [Google Scholar]
- Thomas EL, Burton H, Ford J-E, Perkin A-G. The effect of oxygen content on flavor and chemical changes during aseptic storage of whole milk after UHT-processing. J Dairy Res. 1975;42:285–295. doi: 10.1017/S0022029900015326. [DOI] [Google Scholar]