Abstract
Fagopyrum tataricum is used for the treatment of type 2 diabetes mellitus in Taiwan. The aim of this study was to evaluate the inhibitory effects of 75 % ethanol extract of buckwheat (EEB) and rutin on carbohydrate-metabolized enzymes, including α-amylase and α-glucosidase, which are related to hyperglycemia. The rutin dosage (40 μg/mL) was equivalent to that of EEB (200 μg/mL). In addition, the antioxidant and antiglycation activities of EEB and rutin were investigated. Results showed that both EEB and rutin exerted free radical (DPPH and ABTS) scavenging activity. They also attenuated protein glycation to lower the generation of advanced glycation end-products (AGEs) through the suppression of fructosamine and α-dicarbonyl compounds. Moreover, EEB and rutin also inhibited α-amylase and α-glucosidase activity. Taken together, these findings suggest that EEB and rutin may reduce oxidative stress, AGEs formation, and carbohydrate-metabolized enzymes hence EEB may use as protection agent in diabetic patients.
Keywords: Fagopyrum tataricum (buckwheat), Rutin, α-Amylase, α-Glucosidase, Advanced glycation endproducts (AGEs)
Introduction
Glycation is a nonenzymatic reaction that occurs mainly between amino and carbonyl groups of proteins and reducing sugars, respectively. Advanced glycation end-products (AGEs) are defined as the products of the irreversible formation of a series of complex end-products resulting from glycation of proteins. AGEs are regarded as the outcome of post-translational modifications of proteins (Brownlee et al. 1988). In general, these nonenzymatic glycation reactions proceed relatively slowly, taking a few hours to a few days, which called Amadori products. The Amadori products further interact with the ε-amino group of lysine residues of proteins to form irreversible products. At the same time, amines and α-dicarbonyl compounds are formed by rearrangement and dehydration of sugars to form fluorescent pentosidine of AGEs (Wu et al. 2011a).
Flavonoids consist of 3 benzene rings with 1 or more hydroxyl groups. The antiglycation effects of naturally occurring flavonoids are attributed in part to their antioxidant properties. The inhibitory effects of the flavonoids on AGEs formation approximately follow the order flavone > flavonols > flavanols > flavanone with a few exceptions (Wu and Yen 2005). Considerable effort has been made to identify clinically useful inhibitors of protein AGEs to delay or prevent glycation to alleviate insulin resistance (Dávalos et al. 2009). These include silymarin (Wu et al. 2011b), alagebrium (Dhar et al. 2010), quercetin (Wu and Yen 2005), and rutin (Pashikanti et al. 2010).
Hydrolysis of dietary carbohydrates is the major source of glucose in the blood. This hydrolysis is carried out by a group of hydrolytic enzymes, namely α-amylase and α-glucosidase. Inhibition of these enzymes is increasingly believed to be the best strategy for management of type 2 diabetes, it is achieved through retarding the digestion of dietary carbohydrates and thus reducing the increase in postprandial blood glucose level (Krentz and Bailey 2005; Cheng and Fantus 2005). Plants and microorganisms are rich sources of α-glucosidase inhibitors (Lo and Wasser 2011; Takahashi and Miyazawa 2012). Hence, the search for novel anti-diabetic drug with lesser undesirable effects from natural resources remains an active subject of research.
Fagopyrum tataricum (buckwheat) is an herbaceous plant that belongs to the Polygonaceae family. It has now been introduced in many countries, because the seeds of this herb are a healthy and nutritionally important food item. Rutin has been found to be the major ingredient of buckwheat (Shen et al. 2012). Tartary buckwheat (F. tataricum) contains more rutin and quercetin than common buckwheat (F. esculentum) dose; rutin is known to have antioxidative activity (Liu et al. 2008). Recently, we have reported the anti-diabetic activity of 75 % ethanol extract from buckwheat (EEB) in high-fructose diet-induced C57BL/6 mice (Lee et al. 2012). Albeit EEB has been found to improve diabetes but suppressions of protein glycation and carbohydrate absorption are unclear; therefore, we investigated the antioxidant, anti-glycation, inhibitions of amylase and glucosidase by EEB in this study.
Materials and methods
Chemicals
Potassium diphosphate and dipotassium hydrogen phosphate were purchased from Hayashi Pure Chemical Industries Ltd. (Osaka, Japan). Quercetin, 2,2′-azino-bis (3-ethylbenzthiasoline-6-sulphonic acid) (ABTS+), rutin, fetal serum albumin (BSA), fructose, 3,5-dinitrosalicylic acid (DNS), 1,1-diphenyl-2-picryl hydrazyl (DPPH), p-nitro-blue tetrazolium chloride (NBT), aminoguanidine, butylated hydroxyl anisole (BHA), and Folin–Ciocalteu’s reagent, p-nitrophenyl-α-D-glucopyranoside (PNPG) were purchased from Sigma Corporation (St. Louis, MO, USA). Potassium dihydrogen phosphate (KH2PO4) and dipotassium hydrogen phosphate (K2HPO4) were purchased from Merck Corporation (Darmstadt, Germany).
Sample preparation
The procedure reported by Lee et al. (2012). The seeds of F. tataricum (buckwheat) were provided by Taiwan Golden Buckwheat Limited company and then were freeze-dried and ground. Approximately 1 kg of the buckwheat powder was extracted by 10 L of 75 % ethanol for 2 days, then centrifuged and filitered. After extraction, the ethanol extract were vacuum-concentrated and freeze-dried. The extract powder was stored at −20 °C until used. The 75 % ethanol extract of buckwheat was shown as EEB in this study.
Total flavonoid content
The total flavonoid content was determined using the method described by Abu Bakar et al. (2009). Briefly, 0.5 mL of the extract was mixed with 2.25 mL of distilled water in a test tube followed by addition of 0.15 mL of 5 % NaNO2 solution. After 6 min, 0.3 mL of a 10 % AlCl3. 6H2O solution was added and allowed to stand for another 5 min before 1.0 mL of 1 M NaOH was added. The absorbance of the reaction mixture at 510 nm was determined.
High performance liquid chromatography (HPLC) assay
HPLC was performed with a Hitachi liquid chromatograph (Hitachi, Ltd., Tokyo, Japan) consisting of a model L-6200 pump and a model L-4200 UV–vis detector set at 320 nm. The analyses were carried out on a LiChrospher RP-18 column (250 mm · 4.6 mm i.d., 5 μm, E. Merck Co., Darmstadt, Germany). Extract were filtered through a 0.45 μm filter before use. The mobile phase A was 2 % acetic acid, and the mobile phase B was 0.5 % acetic acid/water (1 : 1; v/v). Rutin, quercetin, and quercetin-3-glucoside were determined by ultraviolet detector (Hitachi L-7455 diode array detector). Rutin, quercetin, and quercetin-3-glucoside were identified by comparison of their retention time (Rt) values and UV spectra with those of known standards and determined by peak areas from the chromatograms (Lee et al. 2012).
DPPH free-radical-scavenging activity
The DPPH activity was measured by the method of Shimada et al. (1992). Briefly, a sample and a methanolic solution of DPPH were mixed and kept in the dark for 60 min. The absorbance of the reaction mixture at 517 nm was determined. BHA and trolox (50 μg/mL) were used as the positive control.
Total antioxidant activity
The antioxidant capacity was determined by the method of Miller and Rice-Evans (1997) and Arnao et al. (2001). Peroxidase, H2O2, ABTS, and distilled water were mixed and stored in the dark for 1 h at 25 °C. A sample was subsequently added and the absorbance at 734 nm was determined. BHA and trolox (50 μg/mL) were used as the positive control.
Antiglycation activity
The antiglycation activity was determined by the method of Wang et al. (2011). Briefly, BSA (60 mg/mL, 100 μL), fructose (1.5 M, 100 μL) and sample (50–200 μg/mL final concentration, 100 μL) were prepared in 0.2 M potassium phosphate buffer (pH 7.4, containing 0.06 % sodium azide), and then were added to a 1.5-mL microfuge tube. A series of tubes containing these solutions were incubated at 50 °C and their fluorescence intensities were determined by withdrawing 100 μL of each reaction and addition to a 96-well plate. A spectrofluorometer (FLx 800, BioTek, Winooski, VT) was set to an excitation and emission wavelengths of 360 nm and 460 nm, respectively. For the determination of BSA-fructose glycation with a known glycation inhibitor, 100 μL of 10 mM aminoguanidine solution (in final concentration) was introduced in place of deionized water in each of the reactions containing BSA and fructose described above.
Formation of fructosamine (an Amadori product)
The reactants described above, namely, BSA/fructose/water and BSA/fructose/AG or sample, were incubated at 50 °C for fructosamine determination using a published procedure (Wells-Knecht et al. 1995). Briefly, after incubation, 20 μL of the each reaction mixture was mixed with 160 μL of deionized water and 0.8 mL 300 μM NBT (in 100 mM, pH 10.35, sodium carbonate buffer) and then incubated at ambient temperature (25 °C) for 15 min. The absorbance of the reaction mixtures at 530 nm was determined using a spectrophotometer (U-2001, Hitachi Co. Ltd., Tokyo, Japan).
Formation of α-dicarbonyl compounds
The determination of α-dicarbonyl compounds was performed using the Girard-T assay (Wang et al. 2011). Briefly, 20 μL of the incubated solution was mixed with 80 μL of deionized water, 50 μL of Girard-T reagent (500 mM in deionized water), and 850 μL of 500 mM sodium (pH 2.9) in a test tube and incubated at ambient temperature (25 °C) for 1 h. The absorbance of the solution at 290 nm was then determined.
α-Amylase inhibition assay
This assay was conducted according to Apostolidis and Lee (2010) with slight modification. Briefly, 200 μL of sample was mixed with 200 μL of α-amylase (13 U/mL, in 0.02 M phosphate buffer, pH 6.9, with 0.006 M NaCl) in 1.5 mL eppendorf tube. After incubating at 37 °C for 30 min, 200 μL of 0.25 % (w/v) starch (in 0.02 M phosphate buffer, pH 6.9) was added and the mixture was further incubated at 37 °C for 30 min, followed by adding 400 μL of DNS color reagent. The reaction was terminated by heating in boiling water bath for 5 min. After cooling to room temperature, the absorbance was measured at 540 nm using a spectrophotometer (U-2001, Hitachi Co. Ltd., Tokyo, Japan).
α-Glucosidase inhibition assay
This assay was conducted according to Apostolidis and Lee (2010) with slight modification. In brief, 50 μL of sample was mixed with 100 μL of α-glucosidase (2 U/mL, in 0.1 M phosphate buffer, pH 6.9) in 1.5 mL eppendorf tube, followed by incubating at 37 °C for 10 min. After adding 50 μL of 5 mM PNPG (in 0.1 M phosphate buffer, pH 6.9), the mixture was further incubated at 37 °C for 10 min. The reaction was terminated by heating at 100 °C and then diluted with 1 mL deionized water. The absorbance was measured at 405 nm by a spectrophotometer (U-2001, Hitachi Co. Ltd., Tokyo, Japan).
Statistical analysis
Experimental results were analyzed in triplicates and expressed as means ± standard deviation (SD). The results were subjected to one-way analysis of variance (ANOVA) and Duncan’s multiple range tests, and the significance of differences between sample means was calculated. P ≤ 0.05 was considered significant.
Results and discussion
Antioxidative activities of EEB and rutin
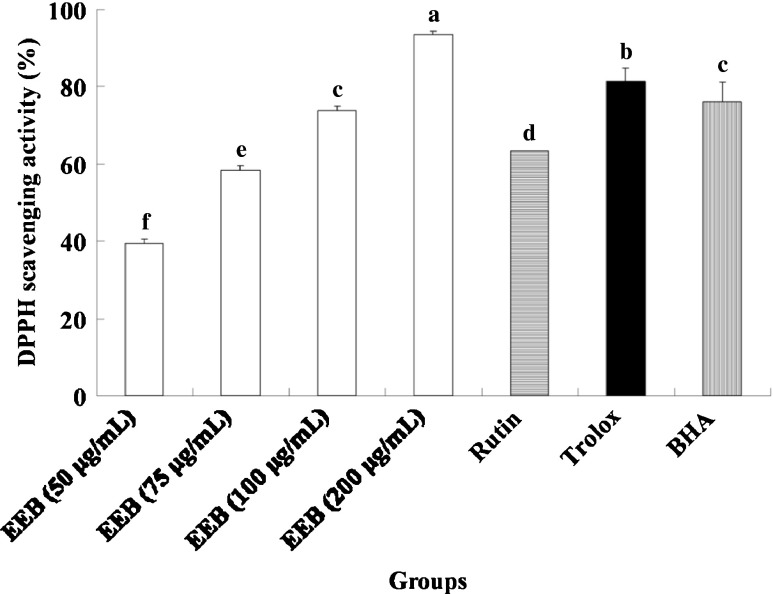
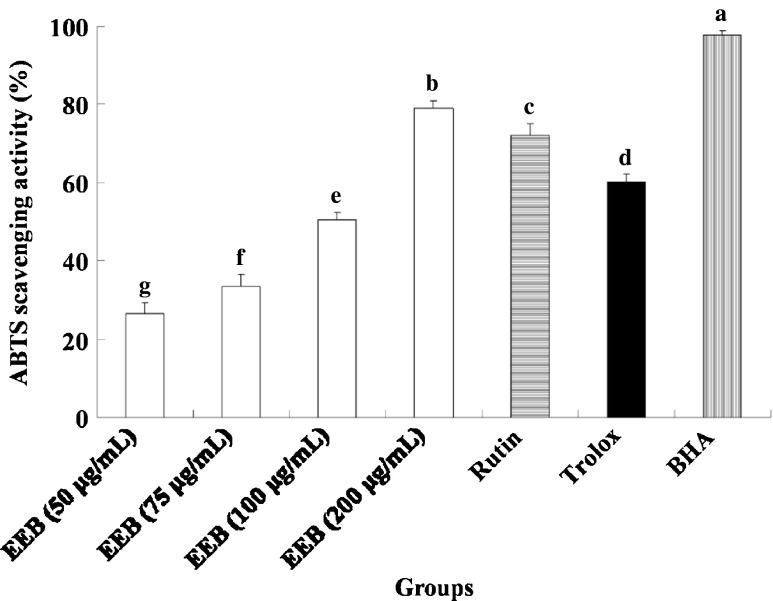
Recently, we found that rutin is the major compound in 75 % ethanol extract from buckwheat (EEB) (Shen et al. 2012; Lee et al. 2012). As shown in Table 1, EEB contained 208.2 mg/g of rutin and 36.0 mg/g of quercetin as determined by an HPLC assay. Using DPPH as a substrate, the radical-scavenging activities of EEB (50, 75, 100, and 200 μg/mL) and rutin (40 μg/mL) were shown to increase with increasing concentration (Fig. 1). However, the DPPH-scavenging activity of trolox and BHA standard both exhibited high scavenging ability for DPPH radicals. The effects of EEB and rutin on ABTS+ investigated using the TEAC assays are shown in Fig. 2. The total antioxidant activity of all samples increased with increasing concentrations. The ABTS+ radical-scavenging activity of trolox and BHA were also had great scavenging ability.
Table 1.
The contents of flavonoids, rutin and quercetin in EEB
| EEB | Concentration |
|---|---|
| (mg/g extract) | |
| Flavonoids | 301.0 ± 8.6 |
| Rutin | 208.2 ± 3.6 |
| Quercetin | 36.0 ± 3.0 |
Each value is expressed as mean ± SD (n = 3)
Fig. 1.
The DPPH scavenging activity of EEB. Data were shown as mean ± SD (n = 3). Trolox and BHA (50 μg/mL) were as positive control groups. Significantly difference was shown as various letters (p < 0.05)
Fig. 2.
The ABTS scavenging activity of EEB. Data were shown as mean ± SD (n = 3). Trolox and BHA (50 μg/mL) were as positive control groups. Significantly difference was shown as various letters (p < 0.05)
Antiglycation activities of EEB and rutin
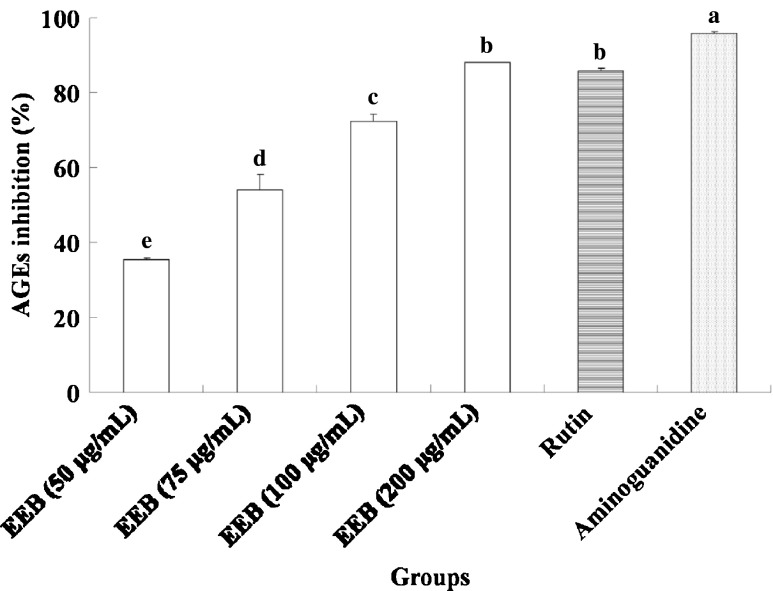
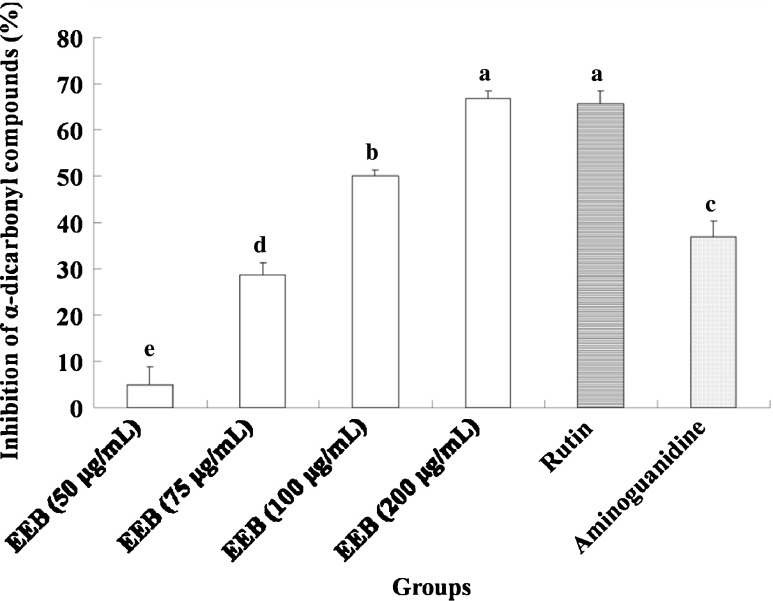
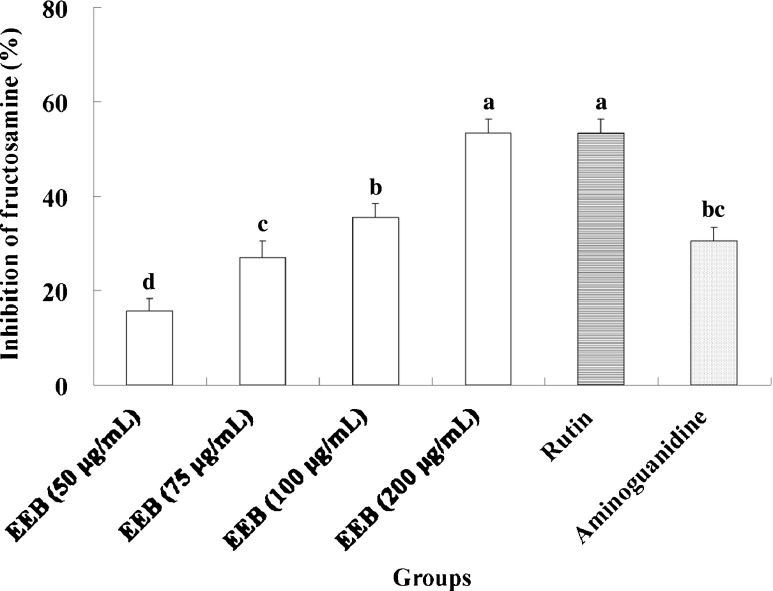
In this study, we demonstrated that EEB inhibits the generation of AGEs (Fig. 3). Moreover, this activity was mediated by suppressing α-dicarbonyl compounds (Fig. 4) and lowering the levels of fructosamine formation (Fig. 5). Furthermore, rutin was used to evaluate antiglycative capacity at doses equivalent to EEB. The results indicated that compared with rutin exerted stronger antiglycative activities for attenuating the generation of AGEs, fructosamine, and α-dicarbonyl compounds, suggesting that rutin may be the active compound in EEB. Aminoguanidine also showed antiglycation activity similar to EEB and rutin.
Fig. 3.
The inhibition of EEB on AGEs formaion. Data were shown as mean ± SD (n = 3). Aminoguanidine (10 mM final concentration) was as positive control group. Significantly difference was shown as various letters (p < 0.05)
Fig. 4.
Inhibition of EEB on alpha-dicarbonyl compounds. Data were shown as mean ± SD (n = 3). Aminoguanidine (10 mM final concentration) was as positive control group. Significantly difference was shown as various letters (p < 0.05)
Fig. 5.
Inhibition of EEB on fructosamine. Data were shown as mean ± SD (n = 3). Aminoguanidine (10 mM final concentration) was as positive control group. Significantly difference was shown as various letters (p < 0.05)
Anti-α-amylase and α-glucosidase effects of EEB and rutin
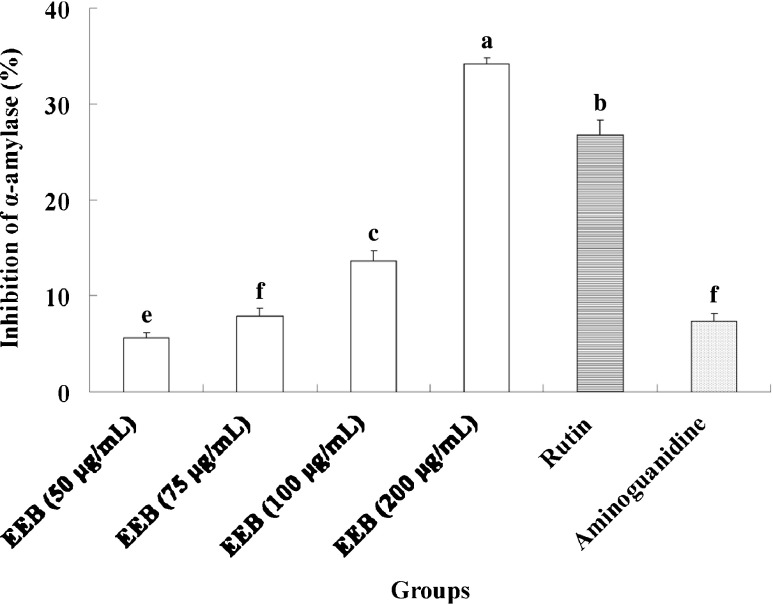
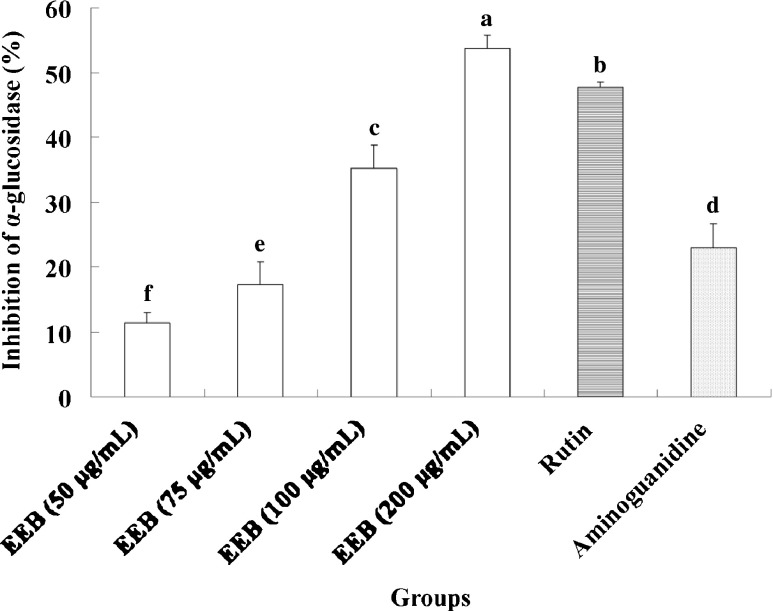
The discovery of specific, high-affinity inhibitors of α-amylase for the development of therapeutics has remained elusive (Krentz and Bailey 2005; Cheng and Fantus 2005). We found that EEB and rutin markedly attenuated α-amylase activity in vitro (Fig. 6). α-Glucosidase inhibitors are believed to act as anti-diabetic agents by impeding sugar degradation and attenuating postprandial hyperglycemia. Thus, inhibition of the activity of α-glucosidase and hydrolysis of carbohydrates would be beneficial for controlling blood glucose levels in diabetes patients. Small molecules that can interact with α-glucosidase have the potential to decrease postprandial hyperglycemia by inhibiting the activity of the α-glucosidase, and could be used to delay the absorption of carbohydrate (Lo and Wasser 2011; Takahashi and Miyazawa 2012). We found that EEB and rutin treatment resulted in a clear suppression of α-glucosidase activity (Fig. 7).
Fig. 6.
Inhibition of EEB on alpha-amylase activity. Data were shown as mean ± SD (n = 3). Significantly difference was shown as various letters (p < 0.05)
Fig. 7.
Inhibition of EEB on alpha-glucosidase activity. Data were shown as mean ± SD (n = 3). Significantly difference was shown as various letters (p < 0.05)
Diabetes is a life-long disease characterized by high levels of blood sugar. The resulting nonenzymatic glycation of proteins that promotes the irreversible formation of AGEs is a significant factor associated with hyperglycemia (Negre-Salvayre et al. 2009). For example, the importance of the glycation reaction was investigated following the discovery of HbA1c in diabetes patients (Koenig et al. 1976). Recent evidence suggests that AGEs have a propensity to generate reactive oxygen species (Bonnefont-Rousselot 2002). Furthermore, glucose and other aldehydes, free or protein-bound, can undergo autooxidation reactions and generate radicals and other reactive intermediates, such as H2O2 and other peroxides, which contribute to the formation of AGEs. This process is often termed glycoxidation (Wolff and Dean 1987). Cardiovascular disease is the major cause of premature death in individuals with diabetes and is mainly marked by increased arterial atherosclerosis. Approaches to reduce AGE-induced damage in cardiovascular tissue have been considered (Susic et al. 2004a, b).
Glycation occurs in vivo by the covalent binding of aldehyde or ketone groups of reducing sugars to the free amino groups of proteins, forming AGE structures that can be identified by their yellow-brown color and/or fluorescence. Free amino groups react with α-dicarbonyls by oxidation, dehydration, and cycling reactions to produce fluorescent AGEs. Moreover, the generation of fructosamine (an Amadori product) and α-dicarbonyl compounds can be determined at 530 and 290 nm, respectively (Wang et al.2011).
In a background study, a series of classical polyphenol antioxidants, including 10 flavonoids (Wu et al. 2009a, b), 12 phenolic acids (Wu et al. 2010a, b), curcumin, and silymarin (Wu et al. 2011b), were screened in vitro to evaluate their effects on protein glycation, crosslinking, and the formation of AGEs. Notably, 2 of these polyphenolic compounds showed consistent inhibition of AGEs formation in experimental animals (Wu et al. 2011b). Rutin and its metabolite have been reported to inhibit AGEs (Pashikanti et al. 2010). In this study, we found that EEB and rutin markedly attenuated the generation of AGEs in vitro. Recent pharmacokinetic studies of rutin and its aglycone derivative quercetin in human volunteers reported blood concentrations of up to 3.5 μmol/L for quercetin and monophenolic rutin metabolites when doses of 50 mg of either rutin or its metabolite quercetin were given in the diet (Erlund et al. 2000). Most orally consumed rutin is probably broken down into low molecular weight phenolic acids by the colonic microflora. Furthermore, flavonoid intake has been estimated to be as high as 1 g/day (Kuhnau 1976), and plasma levels of such flavonoids and their metabolites are markedly increased after consumption (Conquer et al. 1998). Thus, the dietary intake of flavonoids may offer effective protection against glyoxal- and methylglyoxal-induced protein damage. Our study demonstrates that equimolar concentrations of rutin metabolites are effective inhibitors of reactive dicarbonyl species-induced protein damage and AGE formation.
Abundant evidence exists suggesting that the excessive production of reactive oxygen species and reactive nitrogen species may lead to oxidative stress and loss of cell function resulting in an increased risk of various diseases and diabetes (Halliwell and Gutteridge 1998). Oxidative stress is defined as an imbalance between the status of the antioxidant defense system and the production of oxygen-derived species. We found that EEB and rutin effectively scavenged free radicals, including DPPH and ABTS.
Although several digestive enzymes are responsible for breaking down starch, α-amylase and α-glucosidase are the enzymes that are commonly used in the established enzymatic models for screening potential agents for the management of hyperglycemia. In the intestinal tract, starch is broken down into oligosaccharides and disaccharides, followed by hydrolysis by α-glucosidase to glucose before being absorbed into the blood circulatory system (Sun et al. 2008). Therefore, the use of a α-glucosidase inhibitor has been proposed as a good strategy for preventing hyperglycemia. The reason for this is that such a compound can prolong the processes of carbohydrate digestion in the intestinal tract and lengthen the duration of glucose absorption, consequently leading to a lower postprandial blood glucose level (Gao et al. 2008). Studies have shown that certain phenolic phytochemicals from plant sources are effective α-glucosidase inhibitors (Kwon et al. 2006). Phytochemicals such as stigmasterol, β-sitosterol, lupeol, as well as ursolic and oleanolic acids were reported to possess hypoglycemic activity via α-glucosidase inhibition (Ortiz-Andrade et al. 2007; Yoshikawa et al. 1996; Ivorra et al. 1988; Matsuda et al. 1998; Perez and Vargas 2002). The main adverse effects of currently available anti-hyperglycemic drugs (e.g., acarbose) are due to their excessive inhibition of α-amylase activity, which leads to abnormal bacterial fermentation of undigested carbohydrates in the colon (Dehghan-Kooshkghazi and Mathers 2004). Similar findings have been reported for plant extract such as cranberry, mulberry, rhodiola, oregano, and rosemary (Kim et al. 2011). In the current study, we found that EEB and rutin both inhibited α-glucosidase and α-amylase activity in a dose-dependent manner.
In conclusion, we confirmed EEB acted as a glycation inhibitor, and thus, as an anti-amylase and anti-glucosidase agent, is clearly important and may be useful for the treatment of diabetes.
Acknowledgments
Disclosure
No competing financial interests exist.
References
- Abu Bakar MF, Mohamed M, Rahmat A, Fry J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus) Food Chem. 2009;113:479–483. doi: 10.1016/j.foodchem.2008.07.081. [DOI] [Google Scholar]
- Apostolidis E, Lee CM. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated α-glucosidase and α-amylase inhibition. J Food Sci. 2010;75:H97–H102. doi: 10.1111/j.1750-3841.2010.01544.x. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005;172:213–226. doi: 10.1503/cmaj.1031414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr. 1998;128:593–597. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- Dávalos A, de la Peña G, Sánchez-Martín CC, Teresa Guerra M, Bartolomé B, Lasunción MA. Effects of red grape juice polyphenols in NADPH oxidase subunit expression in human neutrophils and mononuclear blood cells. Br J Nutr. 2009;102:1125–1135. doi: 10.1017/S0007114509382148. [DOI] [PubMed] [Google Scholar]
- Dehghan-Kooshkghazi M, Mathers JC. Starch digestion, large bowel fermentation and intestinal mucosal cell proliferation in rats treated with the α-glucosidase inhibitor acarbose. Br J Nutr. 2004;91:357–365. doi: 10.1079/BJN20031063. [DOI] [PubMed] [Google Scholar]
- Dhar A, Desai KM, Wu L. Alagebrium attenuates acute methylglyoxal-induced glucose intolerance in Sprague–Dawley rats. Br J Pharmacol. 2010;159:166–175. doi: 10.1111/j.1476-5381.2009.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlund I, Kosonen T, Alfthan G, Maenpaa J, Perttunen K, Kenraali J, Parantainen J, Aro A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur J Clin Pharmacol. 2000;56:545–553. doi: 10.1007/s002280000197. [DOI] [PubMed] [Google Scholar]
- Gao H, Huang YN, Gao B, Xu PY, Inagaki C, Kawabata J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008;106:1195–1201. doi: 10.1016/j.foodchem.2007.07.064. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3. New York: Oxford University Press; 1998. pp. 7–30. [Google Scholar]
- Ivorra MD, D’Ocon MP, Paya M, Villar A. Anti-hyperglycemic and insulin-releasing effects of beta-sitosterol 3-beta-D-glucoside and its aglycone, beta-sitosterol. Arch Int Pharmacodyn Ther. 1988;296:224–231. [PubMed] [Google Scholar]
- Kim GN, Kwon YI, Jang HD. Mulberry leaf extract reduces postprandial hyperglycemia with few side effects by inhibiting α-glucosidase in normal rats. J Med Food. 2011;14:712–717. doi: 10.1089/jmf.2010.1368. [DOI] [PubMed] [Google Scholar]
- Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- Kwon YI, Vattem DA, Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac J Clin Nutr. 2006;15:107–118. [PubMed] [Google Scholar]
- Lee CC, Hsu WH, Shen SR, Cheng YH, Wu SC (2012) Fagopyrum tataricum (buckwheat) improved high-glucose-induced insulin resistance in mouse hepatocytes and diabetes in fructose-rich diet-induced mice. Exp Diabetes Res ID375673 [DOI] [PMC free article] [PubMed]
- Liu CL, Chen YS, Yang JH, Chiang BH. Antioxidant activity of tartary (Fagopyrum tataricum (L.) gaertn.) and common (Fagopyrum esculentum moench) buckwheat sprouts. J Agric Food Chem. 2008;56:173–178. doi: 10.1021/jf072347s. [DOI] [PubMed] [Google Scholar]
- Lo HC, Wasser SP. Medicinal mushrooms for glycemic control in diabetes mellitus: history, current status, future perspectives, and unsolved problems. Int J Med Mushrooms. 2011;13:401–426. doi: 10.1615/IntJMedMushr.v13.i5.10. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Li Y, Murakami T, Matsumura N, Yamahara J, Yoshikawa M. Antidiabetic principles of natural medicines. III. Structure-related inhibitory activity and action mode of oleanolic acid glycosides on hypoglycemic activity. Chem Pharm Bull. 1998;46:1399–1403. doi: 10.1248/cpb.46.1399. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Rice-Evans CA. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and black currant drink. Food Chem. 1997;60:331–337. doi: 10.1016/S0308-8146(96)00339-1. [DOI] [Google Scholar]
- Negre-Salvayre A, Salvayre R, Auge N, Pamplona R, Portero-Otin M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–3109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- Ortiz-Andrade RR, Garcia-Jimenez S, Castillo-Espana P, Ramirez-Avila G, Villalobos-Molina R, Estrada-Soto S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: An anti-hyperglycemic agent. J Ethnopharmacol. 2007;109:48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pashikanti S, de Alba DR, Boissonneault GA, Cervantes-Laurean D. Rutin metabolites: novel inhibitors of nonoxidative advanced glycation end products. Free Radic Biol Med. 2010;48:656–663. doi: 10.1016/j.freeradbiomed.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Perez GRM, Vargas SR. Triterpenes for Agarista mexicana as potential antidiabetic agents. Phytother Res. 2002;16:55–58. doi: 10.1002/ptr.966. [DOI] [PubMed] [Google Scholar]
- Shen SR, Hsu WH, Lee CC, Chang WC, Wu SC. Buckwheat extracts (Fagopyrum tataricum) and rutin attenuate Th2 cytokines production and cellular allergic effects in vitro and in vivo. J Funct Foods. 2012;4:793–799. doi: 10.1016/j.jff.2012.05.007. [DOI] [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Sun JE, Ao ZH, Lu ZM, Xu HY, Zhang XM, Dou WF, Xu ZH. Antihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J Ethnopharmacol. 2008;118:7–13. doi: 10.1016/j.jep.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Susic D, Varagic J, Ahn J, Frohlich ED. Crosslink breakers: a new approach to cardiovascular therapy. Curr Opin Cardiol. 2004;19:336–340. doi: 10.1097/01.hco.0000127135.73849.4f. [DOI] [PubMed] [Google Scholar]
- Susic D, Varagic J, Ahn J, Frohlich ED. Collagen cross-link breakers: a beginning of a new era in the treatment of cardiovascular changes associated with aging, diabetes, and hypertension. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:97–101. doi: 10.2174/1568006043481347. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Miyazawa M. Potent α-glucosidase inhibitors from safflower (Carthamus tinctorius L.) seed. Phytother Res. 2012;26:722–726. doi: 10.1002/ptr.3622. [DOI] [PubMed] [Google Scholar]
- Wang SH, Chang JC, Pokkaew R, Lee JF, Chiou RY. Modified fast procedure for the detection and screening of antiglycative phytochemicals. J Agric Food Chem. 2011;59:6906–6912. doi: 10.1021/jf201103t. [DOI] [PubMed] [Google Scholar]
- Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J. 1987;245:243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Yen GC. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem. 2005;53:3167–3173. doi: 10.1021/jf048550u. [DOI] [PubMed] [Google Scholar]
- Wu CH, Huang SM, Lin JA, Yen GC. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011;2:224–234. doi: 10.1039/c1fo10026b. [DOI] [PubMed] [Google Scholar]
- Wu CH, Huang SM, Yen GC. Silymarin: a novel antioxidant with antiglycation and anti-inflammatory properties in vitro and in vivo. Antioxid Redox Signal. 2011;14:353–366. doi: 10.1089/ars.2010.3134. [DOI] [PubMed] [Google Scholar]
- Wu CH, Wu CF, Huang HW, Jao YC, Yen GC. Naturally occurring flavonoids attenuate high glucose-induced expression of proinflammatory cytokines in human monocytic THP-1 cells. Mol Nutr Food Res. 2009;53:984–995. doi: 10.1002/mnfr.200800495. [DOI] [PubMed] [Google Scholar]
- Wu CH, Lin JA, Hsieh WC, Yen GC. Low-density-lipoprotein (LDL)-bound flavonoids increase the resistance of LDL to oxidation and glycation under pathophysiological concentrations of glucose in vitro. J Agric Food Chem. 2009;57:5058–5064. doi: 10.1021/jf9001445. [DOI] [PubMed] [Google Scholar]
- Wu CH, Yeh CT, Shih PH, Yen GC. Dietary phenolic acids attenuate multiple stages of protein glycation and high-glucose-stimulated proinflammatory IL-1beta activation by interfering with chromatin remodeling and transcription in monocytes. Mol Nutr Food Res. 2010;54:S127–S140. doi: 10.1002/mnfr.200900395. [DOI] [PubMed] [Google Scholar]
- Wu CH, Yeh CT, Yen GC. Epigallocatechin gallate (EGCG) binds to low-density lipoproteins (LDL) and protects them from oxidation and glycation under high-glucose conditions mimicking diabetes. Food Chem. 2010;121:639–644. doi: 10.1016/j.foodchem.2010.02.008. [DOI] [Google Scholar]
- Yoshikawa M, Murakami T, Harada E, Murakami N, Yamahara J, Matsuda H. Bioactive saponins and glycosides. VII. On the hypoglycemic principles from the root cortex of Aralia elata Seem: structure related hypoglycemic activity of oleanolic acid oligoglycoside. Chem Pharm Bull. 1996;44:1923–1927. doi: 10.1248/cpb.44.1923. [DOI] [PubMed] [Google Scholar]