Abstract
Oats (Avena sativa L.) have received considerable attention for their high content of dietary fibres, phytochemicals and nutritional value. It is believed that consumption of oats possesses various health benefits such as hypocholesterolaemic and anticancerous properties. Oats have also recently been considered suitable in the diet of celiac patients. Owing to their high nutritional value, oat-based food products like breads, biscuits, cookies, probiotic drinks, breakfast cereals, flakes and infant food are gaining increasing consideration. Research and development on oat and its products may be helpful in combating various diseases known to mankind. This paper provides an overview of the nutritional and health benefits provided by oats as whole grains and its value added products. It is designed to provide an insight on the processing of oats and its effect on their functional properties. The manuscript also reviews various uses of oats and its fractions for clinical and industrial purposes and in development of value added food products.
Keywords: Oats, Phytochemicals, Speciality foods, Functional properties, Dietary fibre
Introduction
Cereal grains feed a large population around the world. They constitute a significant part of daily diet of the consumers. Wheat, rice and maize are the leading grains in terms of consumption (Bushuk 2001). These grains are consumed as whole or in fractionated forms. Oat remains an important cereal crop in the developing world and the most popularly cultivated species is Avena sativa L. and is trivially known as common covered white oat (White 1995). Oat requires lesser nutrients (N-Sodium, P-Phosphorus and K-Potassium) to cultivate than that required for wheat or maize. Since oat requires more moisture to produce a given unit of dry matter than all other cereals except rice, it grows well in cool and moist climate (Forsberg and Reeves 1995). Oat is predominantly grown in American and European countries, mainly Russia, Canada and United States of America. It is used mostly for animal feeding and to some extent as human food. The use of oat as animal feed has declined steadily owing to emerging use and interest in oats as human health food (Ahmad et al. 2010).
Studies reveal that oat possesses beneficial health effects against gastrointestinal problems (Anderson and Bridges 1993; Wrick 1993, 1994; Stark and Madar 1994), and also boasts of anti-cancerous effects (Oku 1994; Salminen et al. 1998; Gallaher 2000). Oat consumption in human diet has been increased because of health benefits associated with dietary fibres such as β-glucan, functional protein, lipid and starch components and phytochemicals present in the oat grain. Oats also contain a varied range of phenolic compounds including ester linked glycerol conjugates (Gray et al. 2002), ester linked alkyl conjugates (Daniels and Martin 1967), ether and ester linked glycerides (Collins 1986), anthranilic acids and avenanthramides (AVAs) (Dimberg et al. 1993). These compounds possess high level of antioxidant activity. These antioxidants are concentrated in the outer layer of the kernel in the bran fraction of the oat grain. The nutritional benefits of oat have attracted attention from researchers worldwide and have resulted in the increased interest of food industry in using oats as food ingredient in various food products including infant foods (Del Valle et al. 1981), bread (Zhang et al. 1998), oat milk (Onning et al. 1999), beverages (Gupta et al. 2010), breakfast cereals (Ryan et al. 2011) and biscuits (Ballabio et al. 2011).
Consumption of oat as whole grain cereal
Whole grain consumption has assured growing popularity owing to the health benefits they provide, opening various novel opportunities for consumption of flavourful cereal grains. American Association of Cereals Chemists (AACC) International defined whole grains as ‘the intact ground, cracked or flaked caryopsis, whose principal anatomical components, the starchy endosperm, germ and bran are present in substantially the same relative proportion as they exist in the intact caryopsis’ (AACC 1999).
Whole grain oat contains considerable amount of valuable nutrients such as proteins, starch, unsaturated fatty acids and dietary fibre as soluble and insoluble fractions. Oat also contains micronutrients such as vitamin E, folates, zinc, iron, selenium, copper, manganese, carotenoids, betaine, choline, sulphur containing amino acids, phytic acid, lignins, lignane and alkyl resorcinols (Flander et al. 2007). Although, wheat and rice are consumed in considerably higher quantities worldwide than oat, oat has the advantage that it is consumed as a whole grain cereal normally than its processed products (Peterson 2001). Increasing recognition is now being given to the consumption of whole grain cereals due to the prophylactic benefits they provide (Marquart et al. 2007).
Nutritional components of oats
Oat has a well-balanced nutritional composition. It is a good source of carbohydrates and quality protein with good amino acid balance. Oat contains high percentage of oat lipids especially unsaturated fatty acid, minerals, vitamins and phytochemicals (Head et al. 2010). Oats’ nutritional components and their percent availability are given in Table 1.
Table 1.
Nutritional components of oat
| Component of oat | Availability in oat (%) | References |
|---|---|---|
| Starch | 60 % | Berski et al. (2011) |
| Protein | Total: 11–15 % | Robert et al. (1985) |
| Globulins: 80 % of total protein | Lasztity (1996) | |
| Prolamins: 15 % of total protein | Klose et al. (2009) | |
| Glutelin: 5–66%of total protein | ||
| Albumin: 1–12 % of total protein | ||
| Lipid | 5–9 % | Flander et al. (2007) |
| Keying et al. (2009) | ||
| Dietary fibres | β-glucan: 2.3–8.5 % | Flander et al. (2007) |
| Phytochemicals | α-Tocotrienols and α-tocopherols: | Peterson (2001) |
| 86–91 % of total tocols | Matilla et al. (2005) | |
| Phenolic compounds: 5.7 % | ||
| Protocatechuic | ||
| p-hydroxy benzoic acid | ||
| Vanillic | ||
| Syringic | ||
| Ferulic | ||
| Caffeic | ||
| p-coumaric | ||
| Sinapic | ||
| Flavonoids (trace amounts) : | ||
| Apigenin | ||
| Glycosylvitexin | ||
| Isovitexin | ||
| Tricin | ||
| Vitexin | ||
| Avenanthramides: | ||
| AVA1: 2.1–4.3 % | ||
| AVA3: 2.8–6.2 % | ||
| AVA4: 2.5–4.7 % | ||
| Trace Minerals | Calcium : 0.54 % | Chavan and Kadam (1989) |
| Iron : 0.047 % | ||
| Vitamins | Thiamine : 0.002 % | Chavan and Kadam (1989) |
| Riboflavin : 0.001 % | ||
| Niacin : 0.032 % |
Oat starch
Starch constitutes about 60 % of oat grain. It is mainly a constituent of endosperm. There is considerable difference observed between the physicochemical properties of oat starch and other cereal starches. Differences in physicochemical properties are also observed in different cultivars of oat. These differences are probably due to differences in the magnitude of interaction between and among starch chains within the amorphous and crystalline regions of the native granules and by the chain length of amylose and amylopectin fractions of oat starch. Oat starch offers untypical properties such as small size of granules, well developed granule surface and high lipid content (Berski et al. 2011). Hoover and Vasanthan (1992) studied the characteristics of oat starches in light of their differences with other cereal starches. They reported that oat starches showed higher swelling factor, decreased amylose leaching, coleaching of a branched starch component and amylose during pasting process, higher peak viscosity and set back, low gel rigidity, greater susceptibility towards acid hydrolysis, greater resistance to α-amylase action and high free-thaw stability. However, wide range of differences has been observed among different cultivars of oats (Hoover and Senanayake 1996; Hoover et al. 2003). Starch has been classified into three fractions on the basis of digestion rate, rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch (RS). Slow rate starch digestibility is important for human health to maintain balances blood glucose levels. SDS is one of the most important fractions as it moderates the glycemic response and improves nutritional quality of the food (Ovando-Martinez et al. 2013). Resistant starch has been recognized as functional fibre. It is believed to perform an important role in digestive physiology. It escapes digestion and provides fermentable carbohydrates for colonic bacteria, similar to oligosaccharides such as fructo-oligosaccharides. They also provide benefits such as the production of desirable metabolites including short chain fatty acids in colon. Along with the therapeutic effects, resistant starch provides better appearance, texture and mouth feel than conventional fibres (Martinez Flores et al. 1999). Resistant starch is naturally found in cereal grains and in heated starch or starch containing foods, but is frequently destroyed during processing. RS doses of 20–30 g/day are required to observe physiological effects of RS consumption. However, this level is 3–4 times higher than the actual consumption reported human diet (5–10 g/day); estimated RS intake among the United States population is 3–8 g/day. Most foods have RS content less than 3 g per serving (Murphy et al. 2008). Oats contain significant amount of RS and other starch fractions. Approximately 7 % RDS, 22 % SDS and 25 % RS of the total starch has been reported in oats (Ovando-Martinez et al. 2013). Regular consumption of oat can be used to supplement these starches in diet.
Oat protein
Oat is considered to be a potential source of low cost protein with good nutritional value. Oat has a unique protein composition along with high protein content of 11–15 %. Cereal proteins have been classified into four types according to their solubility as follows: albumins (water soluble), globulins (salt water soluble), prolamins (soluble in dilute alcohol solution) and glutelins (soluble in acids or bases). Oat protein not only differs in the structural properties but also differs in distribution of protein fraction in comparison to other cereal grains. Other cereals such as wheat and barley have characteristic protein matrix which lacks in oat. In wheat and some other cereals, the storage protein is insoluble in salt solutions, while in oats, a large portion of salt water soluble globulins also belong to the storage proteins of the endosperm (Klose et al. 2009).
Oat contains lower quantity of prolamins (15 %) relative to the high amount of globulins (80 %) of the total oat protein. Prolamins (avenins) are low molecular weight (30 kDa) fractions of oat proteins. These prolamins are soluble in 50–70 % ethyl alcohol or 40 % 2-propyl alcohol. Prolamins have high percentage of glutamine and proline and are low in lysine as compared to the other protein fractions (Capouchova et al. 2004). Avenins, a type of prolamins, have storage function similar to that of other cereal prolamins. Glutelin values are reported to be varying from 5 to 66 % of the total protein as they are difficult to be completely solubilised and are dependent on the extraction solvent and solvent concentration (Robert et al. 1985). Of the total metabolically active proteins of oat, water soluble albumin accounts for most of the fraction. Albumins account for about 1–12 % of the total oat protein. In general, albumin and globulin have higher lysine content. Thus oats are rich in lysine content compared to other cereals while they have rather lower content of glutamic acid and prolamin (Lasztity 1996).
Celiac disease is triggered by the ingestion of gluten in gluten intolerant persons. Gluten is an alcohol soluble complex protein present mostly in wheat and other related cereals such as barley and rye. In individuals who are genetically susceptible, the ingestion of gluten causes an inappropriate small intestinal immune response characterized by villous atrophy and crypt hyperplasia (Fasano and Catassi 2001; Wahab et al. 2001), resulting in malabsorption of protein, fats, carbohydrates, soluble vitamins, folate and minerals especially, iron and calcium. The only therapy available at present is to completely exclude gluten from the diet of the individual. Oat contains comparatively more favourable and nutritionally more valuable composition of protein fractions (Capouchova et al. 2004). However, it has long been debated, whether oat can be considered safe for celiac patients (Ballabio et al. 2011). Dicke et al. (1953) and Baker and Read (1976) recommended complete elimination of oats; while, Ripsin et al. (1992), Janatuinen et al. (1995) and Storsrud et al. (1998) advocated the use of oats in celiac diet. The use of oats in gluten free diet depends on the composition of the protein fractions; albumins, globulins, prolamins (avenins) and glutelins. Prolamins together with glutelins forms the reserve protein located in the grain endosperm, which forms about 60–70 % of the grain proteins of cereals. The prolamin fractions are less susceptible to hydrolysis and hence are also difficult to digest. The prolamins content in oats (10–15 % of the total protein) is rather low as compared to wheat (40–50 %), rye (30–50 %) and barley (35–45 %) (Capouchova et al. 2004). Kumar and Farthing (1995) stated that avenins (oat prolamins) could be responsible for toxicity in the celiac patients only if oats are consumed in high amounts, as compared to rye and barley. Capouchova et al. (2004, 2006) reported that the amount of prolamins in oats varies with species, variety and time of cultivation and suggested that use of oats in celiac diet could be risky. However, recently European commission regulation (EC) No. 41/2009 has included oats amongst permitted ingredients, if the gluten content does not exceed 20 ppm (mg/kg) (European Commission 2009).
Lapvetelainen and Aro (1994) reported that oats contain 78 % alkali-soluble, 11 % alcohol- soluble, 2.9 % salt-soluble protein fraction, 4 % residual protein and non-protein nitrogen (NPN). To broaden application of oat protein as a food ingredient, chemical modification was performed to improve solubility and further increase already good emulsifying and binding properties. These properties were found desirable for possible application in different low fat food products (Mohamed et al. 2009). Acylation and succinylation of oat protein improve their solubility, fat binding and emulsifying properties whereas acylation adversely affects water holding capacity and foaming stability of oat proteins (Ma and Khanzada 1987).
Dietary fibres
Dietary fibres (DF) are an essential part of the human diet. They consist of many substances of plant origin that are not digested in the human upper gastrointestinal tract. They include polysaccharides such as cereal β-glucan, arabinoxylans and cellulose. Dietary fibres are located in the cell walls of the grain. The outer layers, the seed coat and the pericarp contribute significantly to the insoluble dietary fibre content of the grain. According to American Association of Cereal Chemists (AACC), a dietary fibre is defined as “the edible part of plant or analogous carbohydrates that are resistant to digestion and absorption in the human small intestine with complete or partial fermentation in the large intestine. It includes polysaccharides, oligosaccharides, lignin and associated plant substances. Dietary fibres promote beneficial physiological effects including laxation and/or blood cholesterol attenuation and/or blood glucose attenuation” (AACC 2001).
According to this definition, oat β-glucans are components of dietary fibre. As β-glucan is a plant polysaccharide resistant to digestion and absorption in the small intestine, it also attenuates both blood cholesterol and glucose. Schneeman (2001) suggested that dietary fibre regulates the rate of nutrient digestion and absorption and serves as a substrate for the microflora of the gut and promotes laxation.
The Codex Alimentarius Commission’s committee on nutrition and foods in 2008 adopted a new definition of dietary fibre as “carbohydrate polymers with 10 or more monomeric units, which are not hydrolysed by the endogenous enzymes in small intestine of humans” (Codex alimentarius 2010). Starch is not considered to be part of dietary fibre because it is hydrolysed by enzymes and is absorbed in the small intestine. Whole oats contain significant amount of dietary fibre, especially water soluble (1→ 3) (1→ 4) β-glucan (Peterson 2001). The β-glucan content in oat ranges from 2.3 to 8.5/100 g (Flander et al. 2007). The Food and Drug Administration (FDA) has accepted a health claim stating that a daily intake of 3 g of soluble oat β-glucan can lower the risk of coronary heart disease (FDA 1997; Amundsen et al. 2003; Berg et al. 2003). It is also known to reduce blood cholesterol level (Ripsin et al. 1992). Dietary fibres, particularly oat β-glucan has potential anti-cancerous property, as they reduce compounds which are causative agents of colon cancer (Sadiq Butt et al. 2008; Bode and Dong 2009; Hsueh et al. 2011), reduce blood cholesterol level (Ripsin et al. 1992; Amundsen et al. 2003; Chen et al. 2006) and reduce blood pressure (He et al. 2004). The recommended dose of β-glucan for a single food is 0.75 g/serving (Flander et al. 2007).
Lipids
Oat is a good source of lipids. It contains much higher levels of lipids than other cereals which are excellent sources of energy and unsaturated fatty acids. The majority of lipids of oats are in the endosperm. The fat content of oat ranges from 5.0 to 9.0 % of the total lipid content. The lipid content in an intact kernel of oat stored for 1 year at room temp was found to be stable (Keying et al. 2009), due to the protection from endogenous antioxidants such as tocopherols, L- ascorbic acid, thiols, phenolic amino acids and other phenolic compounds.
The lipids and other lipid associated compounds in the oat groat play an important role in the functionality of oat products. The high lipid content of oat provides an advantage when used for animal feed as it provides high energy along with good fatty acid composition. But when used as human food, this high lipid content provides fewer benefits, while leading to various processing problems such as poor flavour and excessive browning of toasted products. Along with lipids, oat contains considerable amount of lipases, which are capable of acting under low moisture condition. If not controlled, these lipases cause rancidity and short storage life for processed products of oat (Lehtinen et al. 2003).
Phytochemicals
Oat has been widely shown to provide a vast range of human health benefits such as reduced symptoms of diabetes (Tapola et al. 2005) and obesity (Zdunczyk et al. 2006). The primary component of oat responsible for these health benefits is considered to be β-glucan, however phenolic compounds of oat and other antioxidant compounds also provide health benefits. Oats possess antioxidant capacity mainly due to presence of tocopherols, tocotrienols, phytic acid, flavanoids and non flavanoid phenoilc compounds such as AVAs.
Vitamin E
Antioxidants such as vitamin E are known to protect the body from damaging free radicals and play an important role in prevention of diseases such as cancer (Packer 1991), arthritis (Yoshikawa et al. 1983), atherosclerosis (Srivastava 1986), cataract (Trevithick et al. 1981; Ross et al. 1981; Creigton et al. 1985) etc. Oat germ has high levels of tocopherols (a and c isomers), whereas tocotrienols are mainly concentrated in endosperm but, are absent in germ. The primary tocol of oat is α-tocotrienol but, small amount of tocopherols and their β homologs are also present. The total tocols ranged from 19 to 30.3 mg/kg. Out of the total tocols, α-tocotrienol & α-tocopherols combinedly account for 86 to 91 %. Tocols are found to be stable in unprocessed groats for over 7 months of storage at room temperature, while processing of oats result in degradation of these compounds within 1 to 2 months (Peterson 2001).
Phenolic compounds
Oat is a good source of phenolic compounds. These phenolic compounds may contribute to the functional and nutritional properties of the grain. Cereals account for phenolic compounds derived mainly from hydroxybenzoic and hydroxycinnamic acids. Early studies have shown that the phenolic acids in oat possess antioxidant properties both in vitro (Peterson 2001; Shewry et al. 2008) and in vivo (Ryan et al. 2007). The major phenolic acids in oats are ferulic, p-coumaric, caffeic, vanillic, hydroxybenzoic acid and their derivatives (Matilla et al. 2005; Kova cova and Malinova 2007). Traditionally, polyphenols are considered potent antioxidants. Emerging studies shows that polyphenols may have far more important effects in vivo such as enhancing endothelial function (Caton et al. 2010), cellular signalling and anti-inflammatory property (Ramos 2008). Oat hulls have not much uses in food but they contain significant amount of soluble ferulic acid; an avenanthramide antioxidant and also several other phenolic acids. The total free phenolic acid esters in oats are found to be low at about 8.7 mg/kg, whereas soluble phenolic acid esters account for 20.6 mg/kg and insoluble phenolic acids totalled to be about 57.7 mg/kg (Peterson 2001).
Avenanthramides (AVAs)
Oats are known for a unique group of antioxidants reported among cereals known as avenanthramide (AVA) (Dimberg et al. 1993; Meydani 2009). There are abundant AVAs in oat, namely 2c, 2p & 2f, number 2 indicates 5 hydroxyanthranilic acid and letter c, p and f indicates the kind of hydroxylcinnamic acids as p-caumaric, caffeic and ferulic acids, respectively. Dimberg et al. (1993) reported that AVAs have an antioxidant activity of 10–30 times greater than that of other phenolic antioxidants such as vanillin and caffeic acid. Preliminary studies indicated that the AVAs might possess anti-inflammatory and antiatherogenic properties, since they inhibit monocyte adhesion to human aortic endothelial cells and are presumed to inhibit release of proinflammatory compounds from macrophages (Liu et al. 2004). They are also involved in controlling the blood pressure, as they produce nitric oxide which dilates the blood vessels (Nie et al. 2006).
Processing of oats
Oats are processed in order to produce oat based food products with health-beneficial properties. Table 2 illustrates the effect of various processing steps associated with oats and their effects on functional and nutritional quality of oats.
Table 2.
Effects of processing on oats
| Processing | Effect (s) | References |
|---|---|---|
| Pearling | De-branning, Along with milling, improves flour yield; Microbial decontamination, Facilitates separation of β-glucan rich fractions | Laca et al. (2006) |
| Wang et al. (2007) | ||
| Heat processing: Kiln drying, steam processing, microwave processing | Maillard reaction, Prevention of lipid hydrolysis, Stabilize the oat flour, Enzyme deactivation, Increased phenolic content and antioxidant activity in oat bran extracts, Reduction of grain micro flora, Brings characteristic oat taste | Fors and Schlich (1989) Jiaxun et al. (1993) |
| Klensporf and Jelen (2008) | ||
| Keying et al. (2009) | ||
| Superheated steam processing | Acceptable moisture content, Acceptable colour, Increased viscosity, Deactivation of peroxidase, | Head et al. (2010) |
| Germination | Improve its nutritional value, Reduce anti nutritional factors, Improve the functionality of oat seed proteins, Increase in avananthramide content, Increased activity of β-glucanase (Degradation of β-glucan), Increased total protein content (increase in essential amino acids such as lysine and tryptophan) also | Peterson (1998) |
| Wilhelmson et al. (2001) | ||
| Kaukovirta-Norja et al. (2004) | ||
| Skoglund et al. (2008) | ||
| Extrusion cooking | Resistant starch can be possibly generated, Gelatinization of starch molecule, Cross linking of proteins, Generation of flavour in oats | Vasanthan et al. (2002) |
| Zhang et al. (2011) | ||
| Hydrothermal processing | Moisturizing, Seasoning, Heating and cooling of grains | Panasiewicz et al. (2009) |
| Supercritical fluid extraction | Better extraction of aromatic compounds from oats. | Morello (1994) |
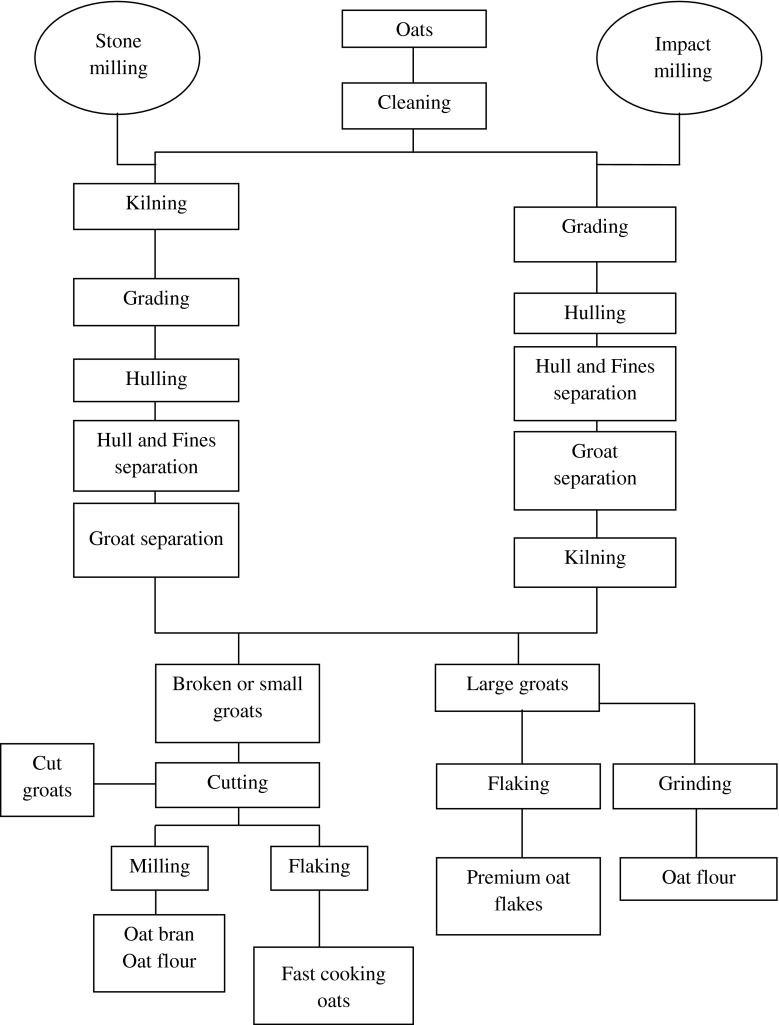
Milling
Prior to processing of oats into products, the oats are dehulled and groats are subsequently separated and decontaminated. Oat milling is performed to get good quality appearance and taste. The milling operations consist of cleaning, grading, hulling, ‘hull, fine and groat separation’ and kilning (Fig. 1). Oats are graded on the basis of groat length and thickness. The kernels are dehulled using either impact or stone hulling systems. However, impact hulling is more commonly used than stone hulling (Zwer 2004).
Fig. 1.
The milling process of oat kernel (Adapted from Zwer 2004)
Pearling
Pearling technology, also referred to as debranning and pre-processing, was originally used for polishing of rice and wheat. By integrating pearling with milling of wheat, improved flour yield was obtained (Bradshaw 2005). Laca et al. (2006) suggested that pearling could lead to substantial microbial decontamination of wheat grains. Pearling of oat has been studied to a limited extent. These studies demonstrate high potential of oat pearling for removal of trichomes that is found to be closely related to aluminium content in oats. Industrial application and control of oat pearling may be easier than in the case of wheat because of their softness and higher lipid content which reduce kernel breakage. Application of pearling technology to oat facilitates separation of β-glucan-rich fractions from pericarp, aleurone and sub-aleurone layers of oat (Wang et al. 2007).
Flaking
Oat groats are mainly flaked. Flaking process involves various unit operations such as cleaning, heat treatment, dehulling, cutting and flaking (or milling). These steps are mainly dependent upon the final oat product and also on the variety of oats (covered or naked) used. The oats are cleaned to remove coarse field trash, dust etc. which may interfere in further processing. Oats are rich in lipid content and hence oat flour shows high adhesiveness and is difficult to handle. Despite having this disadvantage, oat flakes are the most common whole grain oat product used in baking industry (Kaur et al. 2012). Owing to high amount of lipases, the lipids may be prone to hydrolysis leading to rancidity in the flaked oats. Thus oats for food purpose are heat treated in order to deactivate the enzymes responsible for changes in oat lipids (Deane and Commers 1986). Generally, during the heat treatment, the moisture is increased to approximately and the grains are kept at temperature above 100 °C for 90–120 min (Ganssmann and Vorwerck 1995). Additionally, heat treatment provides other benefits such as destruction of bacteria and fungi and also development of oat aroma. Oats are graded to have similar sized grains before they are dehulled. This improves the efficiency of dehulling process. Steam (99–104 °C) is used to increase the moisture content and soften the groats to obtain minimum breakage during the flaking process. Steamed oats develop characteristic oat flavour and steaming also results in deactivation of enzymes including lipases. In a study, flaking of intact oat groat produced rolled oats of 0.5–0.8 mm thickness. After flaking, the rolled oats were cooled with air to about 45 °C and the product had a moisture content of about 9–11.5 % (Deane and Commers 1986).
Heat processing
A typical heat processing operation of oats includes kiln drying and steam stabilization, while superheated steam processing and microwave heating are recent methods used for processing of oats. Moltenberg et al. (1986) reported that thermal treatment of oats may result in rancid and bitter flavour if processed with hulls. Fors and Schlich (1989) reported that the biggest influence on flavour composition was the lipid content and the processing of oats such as heat treatment and milling before or after roasting. Maillard reaction is often associated with heat treatment, which is involved in flavour development in oats (Klensporf and Jelen 2008).
Prevention of lipid hydrolysis in oats is the main goal in manufacture of oat based products. Though kiln drying and steam processing serve the purpose, novel processing techniques are been explored. Microwave heating is one such technique used to deactivate lipase and lipoxygenase in cereal bran, germ, soybean (Jiaxun et al. 1993), groundnuts (Ramesh et al. 1995), rapeseed (Ponne et al. 1996) and olive oil (Farag et al. 1997). Microwave heating is reported to stabilize the oat flour, by enzyme deactivation. Microwave heating at above 150 °C for 15 min, has shown increased phenolic content and antioxidant activity in oat bran extracts (Keying et al. 2009).
Superheated steam processing
Commercial oat processing involves conditioning with saturated (wet) steam followed by kiln drying (upto 100 min at 88–98 °C) (Cenkowski et al. 2006). Kiln drying develops the characteristic oat taste, brings about starch gelatinization to a certain degree and helps in reduction of grain microflora. Although widely used, kiln drying is difficult to control and not an energy efficient process. In addition, there is a risk of grain cross contamination with microorganisms present in the air used for cooling of the grain after the kilning process. Superheated steam processing is an alternative method for drying of food products (Uengkimbuan et al. 2006). The superheated steam is generated by addition of sensible heat to saturated steam. The addition of heat increases steam temperature above corresponding saturation or boiling point at a given pressure.
Oat groats processed with superheated steam at low temperature (110–130 °C) showed acceptable moisture content (9–10 g/100 g wet basis) and colour. These also exhibited higher values of viscosity than oats processed commercially. Superheated steam processing of oat is found to be effective in deactivation of peroxidase in oat groats (Head et al. 2010).
Germination
Germination of cereal seed has been used for centuries to soften the kernel structure, improve its nutritional value, reduce antinutritional effects and improve the functionality of oat seed proteins (Kaukovirta-Norja et al. 2004). Skoglund et al. (2008) reported an increase in avananthramide content of oat during germination. The activity of β-glucanase was found to be increased during germination of oat resulting in almost total degradation of β-glucan (Peterson 1998). β-glucan is known to have health benefits in humans. Thus, the degradation is not desired if the oats are intended for use in food products rather than brewing purpose. A shorter germination process (72 h) at low temp (15 °C) showed 55–60 % of β-glucan content can be retained (Wilhelmson et al. 2001). Also total protein content was found to be increased during germination, leading to increase in essential amino acids such as lysine and tryptophan. This improves nutritional value of oat protein (Peterson 1998).
Fermentation
Fermentation is an ancient and economical process for producing and preserving food products. It is found to provide health benefits by reducing the non-nutritive compounds such as phytates in cereals. This reduction in phytate results in increase in amount of soluble iron, zinc and calcium (Blandino et al. 2003). Apart from the extension of shelf life, fermentation also caused improvement in texture and flavour (Chavan and Kadam 1989). Martensson et al. (2001) reported a non-dairy fermented product based on oat. The product named Adavena M40 was comparable to yoghurt and had high acceptability. It also retained high β-glucan content after fermentation.
Extrusion cooking
Extrusion is a thermal processing that involves the application of high heat, high pressure and shear forces to an uncooked mass such as cereal foods (Kim et al. 2006). Extrusion cooking has advantages over other common processing methods because of low cost, speed, high productivity, versatility, unique product shapes and energy savings. Extrusion cooking is used extensively in the food industry for production of breakfast cereals and snacks from cereals such as oats and its fractions (Meuser and Wiedmann 1989; Kahlon et al. 1998; Zhang et al. 2011). It leads to change in starch components, in solubility of dietary fibre and enhances functional properties of cereal products (Vasanthan et al. 2002). Resistant starch can possibly be generated by extrusion of starch-rich materials such as whole grain meal from oats and barley (Huth et al. 2000). Extrusion cooking is involved in various physical, chemical and textural changes including gelatinization of starch molecule, cross linking of proteins and the generation of flavour in oats (Harper and Clark 1979; Linko and Mercier 1981; Yao et al. 2006; Zhang et al. 2011).
Supercritical fluid extraction
Supercritical fluid extraction provides an excellent alternative to chemical solvent extraction method. It has been used to extract and isolate various valuable natural compounds from various sources (Mansoori et al. 1988; Martinelli et al. 1991; Del Valle and Aguilera 1999; Hartono et al. 2001). Supercritical fluid extraction of aromatic compounds from extruded oat ready-to-eat cereal was studied by Morello (1994). The study reported increased efficiency and reduced time of extraction of aromatic compounds from the product. This technique has been reported to affect various physical and chemical properties of oat and its products (Stevenson et al. 2007).
Value added oat based products
In recent years, demand of oat based product has been increased due to increased knowledge about the many nutritional benefits of oats. Increased consumer awareness towards health has emphasized on intake of high fibre diet. Oat is an excellent source of dietary fibre. Thus promoting its use in functional food products based on oat such as porridge, oatmeal, muesli, granola bars, oat flour, oat bread, biscuits and cookies, oat milk, oatrim, oat based probiotic drink, oat based breakfast cereals, flakes and infant food. Oat β-glucan can be used to stabilize ice creams. Oat antioxidants are useful to stabilize milk and meat products sensitive to fat oxidation during storage. Oat proteins have been used in food products including heat resistant chocolates owing to their viscous and emulsifying properties (Zwer 2004). The incorporation of oat has been found to improve the overall quality of the foodstuffs. Sanchez-Pardo et al. (2010) reported enhanced textural characteristics for pound cake made with 25 % (w/w) of oat fibre than the conventional product. Table 3 illustrates various uses of oat for clinical, industrial and food purpose.
Table 3.
Utilization of oats for food clinical and industrial purposes
| Use (s) | Component of oat | References |
|---|---|---|
| Food uses: | ||
| Bread | Oat flour | Zhang et al. (1998) |
| Oat starch | Flander et al. (2007) | |
| Oat lecithin | ||
| Beverage | Whole oat | Gupta et al. (2010) |
| Biscuits and cookies | Oat flour | Ballabio et al. (2011) |
| Breakfast cereal | Whole oat | Ryan et al. (2011) |
| Pasta products | Oat starch | Chillo et al. (2009) |
| Hager et al. (2013) | ||
| Granola bars and cereals | Whole oat and resistant starch fractions | Aigster et al. (2011) |
| Infant Food | Whole oat | Del Valle et al. (1981) |
| Oat milk | Whole oat extract | Onning et al. (1999) |
| Oat based non-dairy fermented yoghurt, Adavena M40 | Whole oat | Martensson et al. (2001) |
| Fat substitute in meat balls, dairy and bakery products | Oat bran | Yilmaz and Daglioglu (2003) |
| Lee et al. (2005) | ||
| Liu and Wang (2006) | ||
| Fat substitute | Oat dextrin | Crehan et al. (2000) |
| Soluble β-glucan | Sun et al. (2008) | |
| Shen et al. (2011) | ||
| Stabilizer in ice creams | β-glucan | Zwer (2004) |
| Clinical Uses: | ||
| Gluten-free diet | Whole oat | Ballabio et al. (2011) |
| Cholesterol lowering effect | β-glucan | Wang et al. (1992) |
| Kahlon et al. (1993) | ||
| Hallfrisch et al. (1997) | ||
| Anticancerous effect | β-glucan | Hsueh et al. (2011) |
| Short chain fatty acids | Murphy et al. (2004) | |
| Industrial Uses: | ||
| Methane production for Biogas | Oat husk | Kusch et al. (2011) |
Bread is an important part of daily diet for a vast population throughout the world. Flander et al. (2007) has reported an oat based bread with nutty, mild and pleasant flavour. Oats have an excellent moisture retention property that keeps bread fresh for longer period (McKechnie 1983). Incorporation of oat starch or oat lecithin to wheat bread was found to retard the staling rate of the bread (Zhang et al. 1998). Oat proteins are susceptible to denaturation by heat treatment resulting in poor baking properties of oat. Oats also lack in gluten essential for visco-elastic property of flour used for bread making.
Yilmaz and Daglioglu (2003) have reported use of oat bran in meat balls as fat substitute. Meat balls prepared with 20 % oat bran is reported to have highest protein, salt and ash content. These meat balls with oat bran showed high sensory acceptability. Oat based breakfast cereals have received considerable attention in recent times. These are rich in functional ingredients such as β-glucan and bioactive components which are known to reduce serum and plasma cholesterol levels and reducing postprandial glycemic response (Ryan et al. 2011).
Oat starches and their modified products have been reported to be used in pasta products and were found to be organoleptically comparable to conventional product (Chillo et al. 2009; Hager et al. 2013). Healthy foods such as low calorie, low fat and high fibre granola bars and cereals are developed using oats and its resistant starches (Aigster et al. 2011). Oat dextrin is hydrolysed product of oat starch consisting of α (1→ 4) and α (1→ 6) linked D glucose polymer and/or oligomers with a dextrose equivalent (DE) value less than 20. Oat dextrines possess different physicochemical properties including solubility and viscosity (Sun et al. 2008). Oatrim, a powder consisting of oat dextrines and soluble β-g1ucan is a non-sweet starch hydrolysate fat substitute. It stabilises substantial amount of water in gel like matrix, resulting in lubricant and flow properties similar to that of fats (Inglett et al. 1994; Shen et al. 2011). Along with oat bran, these are also been reported to be used in food industry as fat substitutes such as in meat products (Crehan et al. 2000), dairy products (Liu and Wang 2006) and bakery products such as cakes (Lee et al. 2005).
Other uses
Consumption of oats, oatmeal and oat bran provides various clinical and industrial usages. It is known to reduce total plasma cholesterol and low density lipoprotein cholesterol level, postprandial blood glucose and insulin response, occurrence of coronary heart disease, chronic inflammation of arteries and development of cancer and atherosclerosis.
Gluten allergy
Oats are officially concluded as gluten free by European commission regulation (EC) No. 41/2009 and thus found to be suitable for the celiac patients. Earlier studies on the suitability of oats for patients with celiac disease (CD) showed contradictory results (Dissanayake et al. 1974; Baker and Read 1976). It may be because of lack of sensitive and reliable diagnostic tests and of suitable clinical trials. Even probable contamination of oat with other cereals may occur in the field, during transportation, storage, milling or food processing (Kanerva et al. 2006). Owing to this, oats were primarily excluded from gluten free diet. Subsequent clinical studies have proved that consumption of moderate or even large amounts of oat can be tolerated by the majority of adult CD patients. Various reports suggest safety of oats to be included in the gluten free diet in children suffering from celiac disease (Hoffenberg et al. 2000; Hogberg et al. 2004; Holm et al. 2006). Oats are considered as suitable in celiac disease. Hence, gluten free products such as pasta, biscuit and snacks have been developed for celiac patients from oats (Ballabio et al. 2011).
Cholesterol lowering effect
Soluble fibres have been known to lower blood cholesterol levels. Oat fibre has been associated with reduced risk of heart diseases. A number of mechanisms have been proposed which includes reduced rate of absorption because of increased viscosity of the gut contents, binding of bile salts to increase excretion and production of short chain fatty acids in the large intestine from fermentation of undigested carbohydrates which then inhibit cholesterol synthesis. Consumption of 1–10 % of β-glucan was found to be successful in lowering cholesterol, glucose and insulin response in moderately hypercholesterolemic patients (Hallfrisch et al. 1997). Oat bran has been found to lower total serum cholesterol in hypercholesterolemic patients by 23 % with no change in high density lipoprotein (HDL) cholesterol level (Anderson et al. 1991). Upon consumption of 140 g of rolled oats, an average reduction of 11 % in the plasma total was observed (Poinerou et al. 2001). The hypocholesterolemic effect of oats is attributed to β-glucan; two hypotheses for possible mechanisms are suggested; first, the intestinal viscosity effect of β-glucan which is believed to increase the thickness of the unstirred layer of the small intestine, slowing and inhibiting the absorption of lipids and cholesterol (Wang et al. 1992). The second mechanism of action hypothesized is that β-glucan causes binding of bile acids in the intestine causing them to be excreted in faecal waste. The body cholesterol is broken down to replace them, thus changes in bile acid metabolism in response to β-glucan have been implicated in their hypocholesterolemic action (Kahlon et al. 1993). Oats have been studied to contain lunasin peptide (approximately 0.197 mg/g of grain) known to possesses cholesterol reducing properties (Nakurte et al. 2013).
Anticancerous effect of oats
The high amount of short chain fatty acids among dietary fibre fractions from oat is believed to possess potent anticancerous activity. In vitro studies imply butyrate exerts multiple effects to modulate gene expression and regulatory effect of apoptosis and cell cycle. This is involved in countering colon cancer (Hsueh et al. 2011). Short chain fatty acids such as butyric acid are used by colonic mucosa as a source of energy (Roediger 1982). Butyric acid, acetic acid and propionic acid stimulate cell proliferation in normal colonic epithelium. These acids retard the growth of carcinoma cell lines and also induce apoptosis (programmed cell death) in carcinoma cells. Though limited data is available, some beneficial effects of β-glucan on carcinoma cells are reported (Murphy et al. 2004). Fung Chan et al. (2009) reported no direct cytotoxic reports of β-glucan on a panel of common cancer cell lines tested including carcinoma, sarcoma and blastoma. Contradicting the health benefits, the report suggests that the β-glucan stimulates the proliferation of monocytic lineage leukaemic cells in vitro and facilitates the maturation of dendritic cells derived from leukemic cells. Lunasin peptides isolated form oats are believed to have anti-inflammatory and anticancerous properties (Nakurte et al. 2013).
Industrial usage
Oat husk is lignocellulosic biomass and it is a by-product of mills. Kusch et al. (2011) reported use of oat husk for production of methane for biogas purpose. An innovative continuous two phase, two stage prototype biogas plant at Yettereneby Farm in Jarna, Sweden (2003) used oat husk for production of biogas in solid phase slow process. The biogas production by this method is slow but it is a steady process.
Future opportunities
Oat compounds provide various opportunities for incorporating oats in functional food products. There is a great need to determine the bioavailability of antioxidants from oat and other food sources and to determine various effects on human and animal health. Oats contain very interesting components including antioxidants and β-glucan. Oat, being a convenience food material consumed by humans irrespective of the age, requires more scientific attention to justify and modify its nutraceutical status in geriatric as well as paediatric diets. Research and development is further needed to determine novel functional compounds in oat to extract these components in fractions that can be incorporated in food products. Food security being envisaged as a global concern, process upgradation of oat and oat derived products need to be worked on so as to ensure their proper utilization and thus contributing to the growing nutritional demands. Another area for research may include alteration of specific enzyme or enzyme system in oats by genetic engineering to alter the pathways to yield high production of the desired constituents.
Acknowledgement
First author acknowledges the financial support from the INSPIRE Fellowship of the Department of Science and Technology, Government of India.
References
- AACC (1999) Definition of whole grain. Published online www.aaccnet.org/definations/wholegrain.asp. American Association of Cereal Chemists International, St. Paul, Minnesota, USA
- AACC The definition of dietary fibre. Report of the dietary fibre definition committee to the board of directors of the American association of cereal Chemists. Cereal Foods World. 2001;46:112–129. [Google Scholar]
- Ahmad A, Anjum FM, Zahoor T, Nawaz H, Ahmed Z. Extraction and characterization of β-glucan from oat for industrial utilization. Int J Biol Macromol. 2010;46:304–309. doi: 10.1016/j.ijbiomac.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Aigster A, Duncan SE, Conforti FD, Barbeau WE. Physicochemical properties and sensory attribute of resistant starch-supplemented granola bars and cereals. LWT-Food Sci Technol. 2011;44:2159–2165. [Google Scholar]
- Amundsen AL, Haugum B, Andersson H. Changes in serum cholesterol and sterol metabolites after intake of products enriched with an oat bran concentrate within a controlled diet. Scand J Food Nutr. 2003;47(2):68–74. [Google Scholar]
- Anderson JW, Bridges SR. Hypocholesterolemic effects of oat bran in humans. In: Wood PJ, editor. Oat bran. St. Paul, Minnesota, USA: American Association of Cereal Chemists International; 1993. pp. 139–157. [Google Scholar]
- Anderson JW, Spencer DB, Hamilton CC, Smith SF, Tietyen J, Bryant CA, Oeltgen P. Oat-bran cereal lowers serum total and LDL cholesterol in hypercholesterolemic men. Am J Clin Nutr. 1991;52:495–499. doi: 10.1093/ajcn/52.3.495. [DOI] [PubMed] [Google Scholar]
- Baker PG, Read AE. Oats and barley toxicity in celiac patients. Postgrad Med J. 1976;52:264–268. doi: 10.1136/pgmj.52.607.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabio C, Uberti F, Manferdelli S, Vacca E, Boggini G, Redaelli R, Catassi C, Lionetti E, Penas E, Restani P. Molecular characterisation of 36 oat varieties and in vitro assessment of their suitability for celiac’s diet. J Cereal Sci. 2011;54:110–115. [Google Scholar]
- Berg A, Konig D, Deibert P, Grathwohl D, Berg A, Baumstark MW. Effect of an oat bran enriched diet on the atherogenic lipid profile in patients with an increased coronary heart disease risk. Ann Nutr Metab. 2003;47:306–311. doi: 10.1159/000072404. [DOI] [PubMed] [Google Scholar]
- Berski W, Ptaszek A, Ptaszek P, Ziobro R, Kowalski G, Grzesik M, Achremowicz B. Pasting and rheological properties of oat starch and its derivatives. Carbohydr Polym. 2011;83:665–671. [Google Scholar]
- Blandino A, Al-Aseeri ME, Pandiella SS, Cantero D, Webb C. Cereal based fermented foods and beverages. Food Res Int. 2003;36:527–543. [Google Scholar]
- Bode AM, Dong Z. Cancer prevention research - then and now. Nat Rev Cancer. 2009;9:508–516. doi: 10.1038/nrc2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J (2005) Developments in semolina milling. Grain Feed Mill Tech 14–17
- Bushuk W. Rye prosuction and uses worldwide. Cereal Foods World. 2001;2:70–73. [Google Scholar]
- Capouchova I, Petr J, Tlaskalova Hogenova H, Michalik I, Famera O, Urminska D, Tuckova L, Knoblochova H, Borovska D. Protein fractions of oats and possibilities of oat utilization for patients with celiac disease. Czech J Food Sci. 2004;22(4):151–162. [Google Scholar]
- Capouchova I, Petr J, Krejcirova L. Protein composition of sorghum and oat grain and their suitability for gluten-free diet. Agriculture. 2006;93(4):271–284. [Google Scholar]
- Caton PW, Pothecary MR, Lees DM, Khan NQ, Wood EG, Shoji T, Kanda T, Rull G, Corder R. Regulation of vascular endothelial function by procyanidin-rich foods and beverages. J Agric Food Chem. 2010;58:4008–4113. doi: 10.1021/jf9031876. [DOI] [PubMed] [Google Scholar]
- Cenkowski S, Ames N, Muir WE. Infrared processing of oat groats in a laboratory-scale micronizer. Can Biosyst Eng. 2006;48:3.17–3.25. [Google Scholar]
- Chavan JK, Kadam SS. Nutritional improvement of cereals by fermentation. Crit Rev Food Sci Nutr. 1989;28:349–400. doi: 10.1080/10408398909527507. [DOI] [PubMed] [Google Scholar]
- Chen J, He J, Wildman RP, Reynolds K, Streiffer W. A randomized controlled trial of dietary fiber intake on serum lipids. Eur J Clin Nutr. 2006;60:62–68. doi: 10.1038/sj.ejcn.1602268. [DOI] [PubMed] [Google Scholar]
- Chillo S, Civica V, Lannetti M, Suriano N, Mastromatteo M, Del Nobile MA. Properties of quinoa and oat spaghetti loaded with carboxymethylcellulose sodium salt and pregelatinized starch as structuring agents. Carb Polym. 2009;78:932–937. [Google Scholar]
- Codex alimentarius (2010) 25 new or revised codex standards or related texts or amendments to these texts and may new revisions. http://www.ift.org/public-policy-and-regulations/~/media/Public Policy/International Advocacy/33rd Session of the Codex Alimentarius Commission.pdf (Assessed on December 2010)
- Collins FW. Oat phenolics: structure, occurrence and function. In: Webster FH, editor. Oats: chemistry and technology. St. Paul: American Association of Cereal Chemists International; 1986. pp. 227–295. [Google Scholar]
- Crehan CM, Hughes E, Troy DJ, Buckley DJ. Effects of fat level and maltodextrin on the functional properties of frankfurters formulated with 5 %, 12 %, and 30 % fat. Meat Sci. 2000;55:463–469. doi: 10.1016/s0309-1740(00)00006-1. [DOI] [PubMed] [Google Scholar]
- Creigton MO, Ross WM, Stewart DeHaan PJ, Trevithick JR. Modelling cortical cataractogenesis VII. Effects of vitamin E treatment on galactose induced cataracts. Exp Eye Res. 1985;40:213–222. doi: 10.1016/0014-4835(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Daniels DG, Martin HR. Antioxidants in oats: monoesters of caffeic and ferulic acids. J Sci Food Agric. 1967;18:589–595. [Google Scholar]
- Deane D, Commers E. Oat cleaning and processing. In: Webster FH, editor. Oats: chemistry and technology. St. Paul, Minnesota, USA: American Association of Cereal Chemists International; 1986. pp. 371–412. [Google Scholar]
- Del Valle JM, Aguilera JM. High pressure CO2 extraction: fundamental as and applications in the food industry. Food Sci Technol Int. 1999;5:1–24. [Google Scholar]
- Del Valle FR, Villanueva H, Reyes-govea J, Escobedo M, Bourges H, Ponce J, Munoz MJ. Development, evaluation and industrial production of a powdered soy-oats infant formula using a low-cost extruder. J Food Sci. 1981;46(1):192–197. [Google Scholar]
- Dicke WK, Weijers HA, Kamer JH. Celiac disease presence in wheat of a factor having a deleterious effect in cases of celiac disease. Acta Paediatr (Stockholm) 1953;12:32–42. doi: 10.1111/j.1651-2227.1953.tb05563.x. [DOI] [PubMed] [Google Scholar]
- Dimberg LH, Theander O, Lingnert H. Avenanthramides da group of phenolic antioxidants in oats. Cereal Chem. 1993;70:637–641. [Google Scholar]
- Dissanayake AS, Truelove SC, Whitehead R. Lack of harmful effect of oats on small-intestinal mucosa in celiac disease. Br Med J. 1974;4:189–191. doi: 10.1136/bmj.4.5938.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission (2009) Regulation (EC) No 41/2009 of 20 January 2009, 21. Concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten. Off Journal Eur Union L 16: 3
- Farag RS, El-Baroty G, Abd-El-Aziz N, Basuny AM. Stabilization of olive oil by microwave heating. Int J Food Sci Nutr. 1997;48(6):365–371. [Google Scholar]
- Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–651. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- FDA (1997) FDA allows whole oat foods to make health claim on reducing the risk of heart disease. Food and Drug Administration. U.S. Department of Health and Human Services, USA, Talk Paper 22 January 1997
- Flander L, Salmenkallio-Marttila M, Suortti T, Autio K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT - Food Sci Technol. 2007;40:860–870. [Google Scholar]
- Fors SM, Schlich P. Flavor composition of oil obtained from crude and roasted oats. In: Parliament TH, McGorrin RJ, Ho CT, editors. Thermal generation of aromas. Washington, USA: American Chemical Society; 1989. pp. 121–131. [Google Scholar]
- Forsberg RA, Reeves DL. Agronomy of oats. In: Welch RW, editor. The oat crop: production and utilization. London, UK: Chapman and Hall; 1995. pp. 223–251. [Google Scholar]
- Fung Chan GC, Chan WK, Yuen Sze DM (2009) The effects of β-glucan on human immune and cancer cells. J Hematol Oncol 2–25, doi:10.1186/1756-8722-2-25 [DOI] [PMC free article] [PubMed]
- Gallaher DD. Dietary fiber and its physiological effects. In: Schmidt M, Labuza TP, editors. Essentials of functional foods. Gaithersburg: Aspen Publishers, Inc.; 2000. pp. 271–292. [Google Scholar]
- Ganssmann W, Vorwerck K. Oat milling, processing, and storage. In: Welch RW, editor. The oat crop: production and utilization. London, UK: Chapman and Hall; 1995. pp. 369–408. [Google Scholar]
- Gray DA, Clarke MJ, Baux C, Bunting JP, Salter AM. Antioxidant activity of oat extracts added to human LDL particles and in free radical trapping assays. J Cereal Sci. 2002;36:209–218. [Google Scholar]
- Gupta S, Cox S, Abu-Ghannam N. Process optimization for the development of a functional beverage based on lactic acid fermentation of oats. Biochem Eng J. 2010;52:199–204. [Google Scholar]
- Hager AS, Czerny M, Bez J, Zannini E, Arendt EK. Starch properties, in vitro digestibility and sensory evaluation of fresh egg pasta produced from oat, teff and wheat flour. J Cereal Sci. 2013 [Google Scholar]
- Hallfrisch J, Scholfield DJ, Behall KM. Diets containing soluble oat extracts reduce urinary malondialdehyde in moderately hypercholesterolemic men and women. Nutr Biochem. 1997;8:497–501. doi: 10.1093/ajcn/61.2.379. [DOI] [PubMed] [Google Scholar]
- Harper JM, Clark JP. Food extrusion. Crit Rev Food Sci Nutr. 1979;11:155–215. doi: 10.1080/10408397909527262. [DOI] [PubMed] [Google Scholar]
- Hartono R, Mansoori GA, Suwono A. Prediction of solubility of biomolecules in supercritical solvents. Chem Eng Sci. 2001;56:6949–6958. [Google Scholar]
- He J, Streiffer RH, Muntner P, Krousel-Wood MA, Whelton PK. Effect of dietary fiber intake on blood pressure: a randomized, double-blind, placebo-controlled trial. J Hypertens. 2004;22:73–80. doi: 10.1097/00004872-200401000-00015. [DOI] [PubMed] [Google Scholar]
- Head DS, Cenkowski S, Arntfield S, Henderson K. Superheated steam processing of oat groats. LWT - Food Sci Technol. 2010;43:690–694. [Google Scholar]
- Hoffenberg EJ, Haas J, Drescher A, Bamhurst R, Osberg I, Bao F. A trial of oats in children with newly diagnosed celiac disease. J Pediatr. 2000;137:361–366. doi: 10.1067/mpd.2000.109003. [DOI] [PubMed] [Google Scholar]
- Hogberg L, Laurin P, Falth-Magnusson K, Grant C, Grodzinsky E, Jansson G, Ascher H, Browaldh L, Hammersjo JA, Lindberg E, Myrdal U, Stenhammar L. Oats to children with newly diagnosed coeliac disease: a randomised double blind study. Gut. 2004;53:649–654. doi: 10.1136/gut.2003.026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm K, Maki M, Vuolteenaho N, Mustalahti K, Ashorn M, Ruuska T, Kaukinen K. Oats in the treatment of childhood celiac disease: a 2-year controlled trial and a long-term clinical follow-up study. Aliment Pharm Therap. 2006;23:1463–1472. doi: 10.1111/j.1365-2036.2006.02908.x. [DOI] [PubMed] [Google Scholar]
- Hoover R, Senanayake PJN. Composition and physicochemical properties of oat starches. Food Res Int. 1996;29(1):15–26. [Google Scholar]
- Hoover R, Vasanthan T. Studies on isolation and characterization of starch from oat (Avena nuda) grains. Carb Polym. 1992;19:285–297. [Google Scholar]
- Hoover R, Smith C, Zhou Y, Ratnayake RMWS. Physicochemical properties of Canadian oat starches. Carb Polym. 2003;52:253–261. [Google Scholar]
- Hsueh CW, Chia HH, Jeng DH, Mon YY, Shing JW, Chau JW. Inhibitory effect of whole oat on aberrant crypt foci formation and colon tumor growth in ICR and BALB/c mice. J Cereal Sci. 2011;53:73–77. [Google Scholar]
- Huth M, Dongowski G, Gebhardt E, Flamme W. Functional properties of dietary fibre enriched extrudates from barley. J Cereal Sci. 2000;32:115–128. [Google Scholar]
- Inglett GE, Warner K, Newman RK. Sensory and nutritional evaluations of oatrim. Cereals Foods World. 1994;39(10):755–759. [Google Scholar]
- Janatuinen EK, Pikkarainen PH, Kemppainen TA. A comparison of diets with and without oats in adults with celiac disease. N Engl J Med. 1995;333:1033–1037. doi: 10.1056/NEJM199510193331602. [DOI] [PubMed] [Google Scholar]
- Jiaxun T, Rao R, Liuzzo J. Microwave heating for rice bran stabilization. J Microwave Power Electromagn Energy. 1993;28(3):156–164. [Google Scholar]
- Kahlon TS, Chow FL, Knuckles BE, Chiu MM. Cholesterol-lowering effects in hamsters of β-glucan-enriched barley fractions, dehulled whole barley, rice bran, and oat bran and their combinations. Cereal Chem. 1993;70:435–439. [Google Scholar]
- Kahlon TS, Edwards RH, Chow FI. Effect of Extrusion on Hypocholesterolemic Properties of Rice, Oat, Corn, and Wheat Bran Diets in Hamsters. Cereal Chem. 1998;75(6):897–903. [Google Scholar]
- Kanerva PM, Sontag-Strohm TS, Ryoppy PH, Alho-Lehto P, Salovaara HO. Analysis of barley contamination in oats using R5 and ω-gliadin antibodies. J Cereal Sci. 2006;44:347–352. [Google Scholar]
- Kaukovirta-Norja A, Wilhemson A, Poutanen K. Germination: a means to improve the functionality of oat. Agr Food Sci. 2004;13:100–112. [Google Scholar]
- Kaur KD, Jha A, Sabikhi L, Singh AK. Significance of coarse cereals in health and nutrition : a review. J Food Sci Technol doi. 2012 doi: 10.1007/s13197-011-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keying Q, Changzhong R, Zaigui L. An investigation on pretreatments for inactivation of lipase in naked oat kernels using microwave heating. J Food Eng. 2009;95:280–284. [Google Scholar]
- Kim JH, Tanhehco EJ, Ng PKW. Effect of extrusion conditions on resistant starch formation from pastry wheat flour. Food Chem. 2006;99:718–723. [Google Scholar]
- Klensporf D, Jelen HH. Effect of heat treatment on the flavor of oat flakes. J Cereal Sci. 2008;48:656–661. [Google Scholar]
- Klose C, Schehl BD, Arendt EK. Fundamental study on protein changes taking place during malting of oats. J Cereal Sci. 2009;49:83–91. [Google Scholar]
- Kova cova M, Malinova E. Ferulic and coumaric acids, total phenolic compounds and their correlation in selected oat genotypes. Czech J Food Sci. 2007;25:325–332. [Google Scholar]
- Kumar PJ, Farthing MGJ. Oats and celiac disease. N Engl J Med. 1995;333:1075–1076. doi: 10.1056/NEJM199510193331610. [DOI] [PubMed] [Google Scholar]
- Kusch S, Schumacher B, Oechsner H, Schafer W. Methane yield of oat husks. Biomass Bioenergy. 2011;35:2627–2633. [Google Scholar]
- Laca A, Mousia Z, Dıaz M, Webb C, Pandiella SS. Distribution of microbial contamination within cereal grains. J Food Eng. 2006;72(4):332–338. [Google Scholar]
- Lapvetelainen A, Aro T. Protein composition and functionality of high protein oats flour derived from integrated starch–ethanol process. Cereal Chem. 1994;71(2):133–139. [Google Scholar]
- Lasztity R. The chemistry of cereal proteins. Boca Raton, Florida, USA: CRC Press; 1996. [Google Scholar]
- Lee S, Kim S, Inglett GE. Effect of shortening replacement with oatrim on the physical and rheological properties of cakes. Cereal Chem. 2005;82(2):120–124. [Google Scholar]
- Lehtinen P, Kiiliaeinen K, Lehtomaeki I, Laakso S. Effect of heat treatment on lipid stability in processed oats. J Cereal Sci. 2003;37:215–221. [Google Scholar]
- Linko P, Mercier PCC. High-temperature, short-time extrusion cooking. In: Pomeranz Y, editor. Vol. IV. Advances in cereal science and technology. St. Paul: American Association of Cereal Chemists; 1981. pp. 145–235. [Google Scholar]
- Liu TT, Wang DW. Study on function of thickening and stabilization of maltdextrin in ice cream. Food Sci. 2006;27:233–236. [Google Scholar]
- Liu L, Zubik L, Collins FW, Marko M, Meydani M. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis. 2004;175:39–49. doi: 10.1016/j.atherosclerosis.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Ma CY, Khanzada G. Functional properties of deamidated oats protein isolate. J Food Sci. 1987;52(6):1583–1587. [Google Scholar]
- Mansoori GA, Schulz K, Martinelli E. Bioseparation using supercritical fluid extraction/retrograde condensation. Biotechnology. 1988;6:393–396. [Google Scholar]
- Marquart L, Jones JM, Cohen EA, Poutanen K. The future of whole grains. In: Jacobs ML Jr, McIntosh GH, Poutanen K, Reicks M, editors. Whole grains and health. Oxford: Blackwell publishing; 2007. pp. 3–15. [Google Scholar]
- Martensson O, Andersson C, Andersson K, Oste R, Holst O. Formulation of an oat based fermented product and its comparison with yoghurt. J Sci Food Agric. 2001;81:1314–1321. [Google Scholar]
- Martinelli E, Schulz K, Mansoori GA. Supercritical fluid extraction/retrograde condensation with applications in biotechnology. In: Bruno TJ, Ely JF, editors. Supercritical fluid technology. Boca Raton, Florida, USA: CRC Press; 1991. pp. 451–478. [Google Scholar]
- Martinez Flores HE, Chang YK, Bustos FM, Sinencio FS (1999) Extrusion-cooking of cassava starch with different fiber sources: effect of fibers on expansion and physicochemical properties. Adv Extr 271–278
- Matilla P, Pihlava JM, Hellstrom J. Contents of phenolic acids, alkyl and alkylresorcinol and avenanthramides in commercial grain products. J Agric Food Chem. 2005;53:8290–8295. doi: 10.1021/jf051437z. [DOI] [PubMed] [Google Scholar]
- McKechnie R. Oat products in bakery foods. Cereal Foods World. 1983;28:635–637. [Google Scholar]
- Meuser F, Wiedmann W. Extrusion plant design. In: Mercier C, Linko P, Harper JM, editors. Extrusion cooking. St. Paul, Minnesota, USA: American Association of Cereal Chemists; 1989. pp. 91–154. [Google Scholar]
- Meydani M. Potential health benefits of avenanthramides of oats. Nutr Rev. 2009;67:731–735. doi: 10.1111/j.1753-4887.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Biresaw G, Xu J, Hojilla-Evangelista MP, Rayas-Duarte P. Oats protein isolate: thermal, rheological, surface and functional properties. Food Res Int. 2009;42:107–114. [Google Scholar]
- Moltenberg EL, Magnus EM, Bjorge JM, Nilsson A. Sensory and chemical studies of lipid oxidation in raw and heat treated oat flours. Cereal Chem. 1986;73:579–587. [Google Scholar]
- Morello MJ. Isolation of aroma volatiles from an extruded oat ready-to-eat cereal: comparison of distillation: extraction and supercritical fluid extraction. ACS Symp Ser. 1994;543:95–101. [Google Scholar]
- Murphy EA, Davis JM, Brown AS, Carmichael MD, Mayer EP, Ghaffar A. Effects of moderate exercise and oat β-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J Appl Physiol. 2004;97:955–959. doi: 10.1152/japplphysiol.00252.2004. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Douglas JS, Birkett A. Resistant starch intakes in the United States. J American Diet Assoc. 2008;108:67–78. doi: 10.1016/j.jada.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Nakurte I, Kirhnere I, Namniece J, Saleniece K, Krigere L, Mekss P, Vicupe Z, Bleidere M, Legzdina L, Muceniece R. Detection of the lunasin peptide in oats (Avena sativa L) J Cereal Sci. 2013 [Google Scholar]
- Nie L, Wise ML, Peterson DM, Meydani M. Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis. 2006;186:260–266. doi: 10.1016/j.atherosclerosis.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Oku T. Special physiological functions of newly developed mono- and oligosaccharides. In: Goldberg I, editor. Functional foods: designer foods, pharmafoods, nutraceuticals. New York, USA: Chapman & Hall; 1994. pp. 202–218. [Google Scholar]
- Onning G, Wallmark A, Persson M, Akesson B, Elmstahl S, Oste R. Consumption of oat milk for 5 weeks lower serum cholesterol and LDL cholesterol in free living men with moderate hypercholesterolemia. Ann Nutr Metab. 1999;43:301–309. doi: 10.1159/000012798. [DOI] [PubMed] [Google Scholar]
- Ovando-Martinez M, Whitney K, Reuhs BL, Doehlert AC, Simsek S (2013) Effect of hydrothermal treatment on physicochemical and digestibility properties of oat starch. 52: 17–25
- Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S–1053S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- Panasiewicz M, Grochowicz J, Sobczak P. Influence of hydrothermal processes on selected physical properties of oat grain. J Food Eng. 2009;90:81–89. [Google Scholar]
- Peterson DM. Malting oats: effects on chemical composition of hull-less and hulled genotypes. Cereal Chem. 1998;75:230–234. [Google Scholar]
- Peterson DM. Oat antioxidants. J Cereal Sci. 2001;33:115–129. [Google Scholar]
- Poinerou S, Lupper R, Adess M, Nestel P. Oat β-glucan lowers total and LDL-cholesterol. Aust J Nutr Diet. 2001;58:51–55. [Google Scholar]
- Ponne CT, Moeller AC, Tijskens LMM, Bartels PV, Meijer MMT. Influence of microwave and steam heating on lipase activity and microstructure of rapeseed (Brassica napus) J Agric Food Chem. 1996;44(9):2818–2824. [Google Scholar]
- Ramesh M, Rao HP, Ramadoss CS. Microwave treatment of groundnut (Arachis hypogaea): extractability and quality of oil and its relation to lipase and lipoxygenase activity. LWT - Food Sci Technol. 1995;28(1):96–99. [Google Scholar]
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Ripsin CM, Keenan JM, Jacobs DR, Elmer PJ, Welch RR, Van Horn L. Oat products and lipid lowering. meta-analysis. J Am Med Assoc. 1992;267:3317–3325. [PubMed] [Google Scholar]
- Robert LS, Nozzolillo C, Altosaar I. Characterization of oat (Avena sativa L.) residual proteins. Cereal Chem. 1985;62:276–279. [Google Scholar]
- Roediger WEW. Utilization of nutrients by isolated epithelial cells of rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- Ross WM, Creighton MO, Stuart DeHaan PJ, Trevithick JR. Modelling cortical catarctogenesis. 3. In vitro effects of vitamin E on cataractogenesis in diabetic rat. Can J Ophthalmol. 1981;71:61–66. [PubMed] [Google Scholar]
- Ryan D, Kendall M, Robards K. Bioactivity of oats as it relates to cardiovascular disease. Nutr Res Rev. 2007;20:147–162. doi: 10.1017/S0954422407782884. [DOI] [PubMed] [Google Scholar]
- Ryan L, Thondre PS, Henry CJK. Oat-based breakfast cereals are a rich source of polyphenols and high in antioxidant potential. J Food Compos Anal. 2011;24:929–934. [Google Scholar]
- Sadiq Butt M, Tahir-Nadeem M, Khan MK, Shabir R, Butt MS. Oat: unique among the cereals. Eur J Nutr. 2008;47:68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function. Brit J Nutr. 1998;80(1):147–171. doi: 10.1079/bjn19980108. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pardo ME, Jimenez G, Gonzalic-Gracia I. Study about the addition of chemically modified starches (cross-linked corn starches), dextrins, and oat fibers in pound cake. Special Abstracts. J Biotechnol. 2010;150:316–319. [Google Scholar]
- Schneeman BO. Dietary fibre and gastrointestinal function. In: McCleary BV, Prosky L, editors. Advanced dietary fibre technology. Oxford, UK: Blackwell Science; 2001. pp. 168–173. [Google Scholar]
- Shen R, Luo S, Dong J. Application of oat dextrine for fat substitute in mayonnaise. Food Chem. 2011;126:65–71. [Google Scholar]
- Shewry PR, Piironen V, Lampi AM, Nystrom L, Li L, Rakszegi M, Fras A, Boros D, Gebruers K, Courtin CM, Delcour JA, Andersson AAM, Dimberg L, Bedo Z, Ward JL. Phytochemical and fiber components in oat varieties in the health grain diversity screen. J Agric Food Chem. 2008;56:9777–9784. doi: 10.1021/jf801880d. [DOI] [PubMed] [Google Scholar]
- Skoglund M, Peterson DM, Andersson R, Nilsson J, Dimberg LH. Avenanthramide content and related enzyme activities in oats as affected by steeping and germination. J Cereal Sci. 2008;48:294–303. [Google Scholar]
- Srivastava KC. Vitamin E exerts antiaggregatory effects without inhibiting the enzyme of the arachidonic acid cascade in platelets. Prostaglandins Leukot Med. 1986;21:177–185. doi: 10.1016/0262-1746(86)90151-4. [DOI] [PubMed] [Google Scholar]
- Stark A, Madar Z. Dietary fiber. In: Goldberg I, editor. Functional foods: designer foods, pharma foods, nutraceuticals. New York, USA: Chapman & Hall; 1994. pp. 183–201. [Google Scholar]
- Stevenson DG, Eller FJ, Radosavljevic M, Jane JL, Inglett GE. Characterisation of oat bran products with and without supercritical carbon dioxide extraction. Int J Food Sci Technol. 2007;42(12):1489–1496. [Google Scholar]
- Storsrud S, Lenner RA, Kilander A. The oat celiac study in Gothenburg. In: Lohinieni S, Collin P, Maki M, editors. Changing features of celiac disease. Tampere-celiac disease study group: University of Tampere; 1998. [Google Scholar]
- Sun JL, Li XH, Zeng J, Li GL, Zhao RX. Study on preparation technology of dextrin using medium and high temperature α-amylases. Food Sci. 2008;29:312–315. [Google Scholar]
- Tapola N, Karvonen H, Niskanen L, Mikola M, Sarkkinen E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr Metab Cardiovas. 2005;15:255–261. doi: 10.1016/j.numecd.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Trevithick JR, Creighton MO, Ross WM, Creighton MO. Modelling cortical catarctogenesis. 2. In vitro effects on the lens of agents preveting glucose and sorbitol induced cataracts. Can J Ophthalmol. 1981;16:32–38. [PubMed] [Google Scholar]
- Uengkimbuan N, Soponronnarit S, Prachayawarakorn S, Nathkaranakule A. A comparative study of pork drying using superheated steam and hot air. Dry Technol. 2006;24(12):1665–1672. [Google Scholar]
- Vasanthan T, Gaosong J, Yeung J, Li J. Dietary fiber profile of barley flour as affected by extrusion cooking. Food Chem. 2002;77:35–40. [Google Scholar]
- Wahab PJ, Crusius JBA, Meijer JWR, Goerres MS, Mulder CJJ. Gluten challenge in borderline gluten sensitive enteropathy. Am J Gastroenterol. 2001;96:1464–1469. doi: 10.1111/j.1572-0241.2001.03812.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Newman RK, Newman CW, Hofer PJ. Barley β-glucan alters intestinal viscosity and reduces plasma cholesterol concentration in chicks. J Nutr. 1992;122:2292–2297. doi: 10.1093/jn/122.11.2292. [DOI] [PubMed] [Google Scholar]
- Wang R, Koutinas AA, Campbell GM. Effect of pearling on dry processing of oats. J Food Eng. 2007;82:369–376. [Google Scholar]
- White EM. Structure and development of oats. In: Welch RW, editor. The oat crop: production and utilization. London, UK: Chapman and Hall; 1995. pp. 88–119. [Google Scholar]
- Wilhelmson A, Oksman-Caldentey KM, Laitila A, Suortti T, Kaukovirta-Norja A, Poutanen K. Development of a germination process for producing high β-glucan, whole grain food ingredients from oat. Cereal Chem. 2001;78:715–720. [Google Scholar]
- Wrick KL. Functional foods: cereal products at the food–drug interface. Cereal Foods World. 1993;38(4):205–214. [Google Scholar]
- Wrick KL. The potential role of functional foods in medicine and public health. In: Goldberg I, editor. Functional foods: designer foods, pharmafoods, nutraceuticals. New York, USA: Chapman & Hall; 1994. pp. 480–494. [Google Scholar]
- Yao N, Jannink JL, Alavi S, White PJ. Physical and sensory characteristics of extruded products made from two oat lines with different β-glucan concentrations. Cereal Chem. 2006;83(6):692–699. [Google Scholar]
- Yilmaz I, Daglioglu O. The effect of replacing fat with oat bran on fatty acid composition and physicochemical properties of meatballs. Meat Sci. 2003;65:819–823. doi: 10.1016/S0309-1740(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Tanaka H, Kondo M. Effect of vitamin E on ajuvant arthritis in rats. Biochem Med. 1983;29:227–234. doi: 10.1016/0006-2944(83)90043-1. [DOI] [PubMed] [Google Scholar]
- Zdunczyk Z, Flis M, Zielinski H, Wroblewska M, Antoszkiewicz Z, Juskiewicz J. In vitro antioxidant activities of barley, husked oat, naked oat, triticale, and buckwheat wastes and their influence on the growth and biomarkers of antioxidant status in rats. J Agric Food Chem. 2006;54:4168–4175. doi: 10.1021/jf060224m. [DOI] [PubMed] [Google Scholar]
- Zhang DC, Moore WR, Doehlert DC. Effects of oat grain hydrothermal treatments on wheat–oat flour dough properties and bread baking quality. Cereal Chem. 1998;75:602–605. [Google Scholar]
- Zhang M, Bai X, Zhang Z. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J Cereal Sci. 2011;54:98–103. [Google Scholar]
- Zwer PK. Oats. In: Wrigley C, Corke H, Walker CE, editors. Encyclopedia of grain science. Waltham, Massachusetts, USA: Elsevier Academic Press; 2004. pp. 365–375. [Google Scholar]