Abstract
We report a lesion–symptom mapping analysis of visual speech production deficits in a large group (280) of stroke patients at the sub-acute stage (<120 days post-stroke). Performance on object naming was evaluated alongside three other tests of visual speech production, namely sentence production to a picture, sentence reading and nonword reading. A principal component analysis was performed on all these tests' scores and revealed a ‘shared’ component that loaded across all the visual speech production tasks and a ‘unique’ component that isolated object naming from the other three tasks. Regions for the shared component were observed in the left fronto-temporal cortices, fusiform gyrus and bilateral visual cortices. Lesions in these regions linked to both poor object naming and impairment in general visual–speech production. On the other hand, the unique naming component was potentially associated with the bilateral anterior temporal poles, hippocampus and cerebellar areas. This is in line with the models proposing that object naming relies on a left-lateralised language dominant system that interacts with a bilateral anterior temporal network. Neuropsychological deficits in object naming can reflect both the increased demands specific to the task and the more general difficulties in language processing.
Keywords: Stroke, Principal component analysis, VBM, Picture naming, Speech production, Sentence reading, Nonword reading, Sentence production, Picture description, BCoS, CT
Highlights
-
•
The shared and unique neural substrates of object naming were examined in 280 stroke patients.
-
•
Clinical CT scans and performance on clinical language tests were analysed using voxel-based morphometry.
-
•
Principal component analysis on the test scores identified a shared language component and a unique naming component.
-
•
The general language component recruits a widespread left-lateralised fronto-temporal network.
-
•
The unique object naming component is linked to the bi-anterior temporal poles and cerebellar areas.
1. Introduction
Recognising a specific object and saying aloud its name promptly are rather effortless for the most part. However, deficits in naming objects emerge as a frequent symptom of brain damage (Bayles and Tomoeda, 1983; Bell et al., 2001; Hodges et al., 2000; Hodges and Patterson, 2007) occurring, for instance, in at least 14% of stroke patients (e.g. Nøkleby et al., 2008; Tatemichi et al., 1994). In clinical practice, object naming is widely used as a test of language functions in bedside neuropsychological examination (e.g. in MoCA, MMSE). It is also common as a behavioural treatment approach for naming disorders, or aphasia at large, to train whole word naming to simple pictures (e.g. Conroy et al., 2009; Nickels, 2002). In this study, we examined the cognitive and neural relevance between object naming and other visual speech production tasks using a lesion–deficit mapping approach.
Deficits in object naming among neurological patients could arise at several levels of processing. Existing cognitive theories (Humphreys et al., 1999; Levelt et al., 1999) posit that naming an object requires at a minimum four processing steps to take place: 1) visual perception; 2) retrieval of semantic knowledge about the object; 3) access to the associated phonological representation; and 4) articulation. Likewise, a neuroanatomically-constrained model (Ueno et al., 2011; Ueno and Lambon Ralph, 2013) specifically highlights the interactive contribution of semantic and phonological pathways in supporting naming. Disruptions to various parts of these pathways, using computational stimulation, have been shown to affect naming and other spoken language abilities. In correspondence with the computational account, an elegant VBM study by Butler and collaborators (Butler et al., 2014) examined the common neuro-cognitive components that are shared across a number of language (including object naming) and executive function tasks. They identified three components: phonology, semantic and executive-cognition. In particular, object naming was loaded almost equally on both phonology and semantic. Also, as reported in this study, the phonological component was related to the left perisylvian regions encompassing the temporal, insula and inferior frontal cortices while the semantic component was related to the left anterior temporal area.
Evidence from neuropsychological reports suggests that object naming is supported by a large network of different brain regions along the Sylvian fissure with the left frontal and temporal lobes being particularly critical (Damasio et al., 2004, 1996; Hillis et al., 2006, 2001). Baldo et al. (2013) used voxel-based lesion symptom mapping to relate performance on a test of object naming to neural correlates based on the lesion maps of patients with left hemispheric stroke. Their results showed an association between naming deficits and lesions to significant portions of the left temporal cortex including the superior and middle sections and underlying white matter with an extension to the inferior parietal cortex. Similar patterns of extensive left perisylvian lesions were reported in studies using cortical electrical stimulation during neurosurgery (Corina et al., 2010) and perfusion-weighted magnetic resonance imaging (DeLeon et al., 2007). In particular, DeLeon and colleagues (2007) identified the lesions to the superior and middle temporal gyri and the anterior temporal pole to be most predictive of the lexical–semantic mapping deficits (i.e. a failure to linking concepts to phonological output) in naming. Additionally, a recently growing body of literature has emphasised the role of the anterior temporal lobe (ATL) in naming (e.g. Domoto-Reilly et al., 2012; Rogers et al., 2006). Notably patients with semantic dementia typically have prominent ATL atrophy and progressive anomia (i.e. naming impairment) (Bright et al., 2008; Jefferies and Lambon Ralph, 2006; Noppeney et al., 2007). According to Patterson and Roger (Patterson et al., 2007; Rogers et al., 2004), ATL serves as a central representation ‘hub’ of the brain, integrating modality-specific representations (e.g. smell, shape, colour, name) from different regions to constitute domain-general concepts (see also Lambon Ralph, 2014 for a review).
In many neuropsychological studies of object naming (e.g. Baldo et al., 2013; DeLeon et al., 2007), patients have been restricted to those only with left hemispheric damage. This limited the ability to draw inferences about potential contributions of particular regions in the rest of the brain to a given function. For example, Brambati et al. (2006) examined the anatomical organisation of object naming using voxel-based morphometry (VBM) in patients with a range of neurodegenerative diseases. They reported a link between overall naming performance and bilateral atrophy in the superior and inferior temporal gyri, anterior fusiforms and hippocampi, in additional to some left-sided atrophy. Similarly, studies using functional imaging show activations in extensive brain regions during object naming (Garn et al., 2009; Léger et al., 2002; Okada et al., 2000; Spitzer et al., 1998). Price and colleagues (2005) conducted a meta-analysis of the functional imaging studies on object naming in healthy individuals. This meta-analysis study identified regions primarily along the occipito-temporal cortices on the left; however, greater involvement of the right hemisphere was also noted when object naming was compared with baseline conditions controlling for perceptual processing and speech production. In the current study, we performed the whole brain correlation analysis using VBM.
Object naming is very similar to other speech production abilities such as reading as they both require speech response driven by visual inputs. Interestingly, however, there is limited comprehensive account of how object naming is distinguished from other visual speech production tasks at the neuronal level. Only a few fMRI studies have directly contrasted the neural activation of object naming to single word reading (Bookheimer et al., 1995; Moore and Price, 1999; Price et al., 2006). For example, Moore and Price's (1999) study found shared mechanism in the inferior temporal cortex (among other regions) which responded more to both words and objects relative to viewing meaningless visual stimuli. Compared with word reading, increased activation during object naming was observed in the anterior fusiform. The authors (Moore and Price, 1999) explained that the anterior part of fusiform has been linked to semantic processing, with object naming being more dependent on semantic processing than reading. Functional imaging studies of other speech production tasks alone such as sentence production in picture description (e.g. Grande et al., 2012) highlight the involvement of a large bilateral network which includes both the anterior (e.g. inferior frontal gyrus, anterior part of superior and middle temporal gyri) and posterior (e.g. temporo-parietal and occipital cortices) regions of the left hemisphere. However, there is a lack of neuropsychological data directly comparing performance on object naming with a series of visual speech production tasks using a common set of patients.
The present study used performance data from a stroke sample on a clinical cognitive screen (BCoS; Humphreys et al., 2012). The BCoS assesses language abilities including object naming as well as reading and picture description (see the Behavioural Measures subsection and the Supplementary material S1, for detailed description). All these tasks assess identification of visual stimuli and generation of spoken responses. Despite the similarities, each task potentially has its specific demands. To increase the demands on recognition and semantic processing, the object naming task in BCoS includes low frequency object items. In contrast, the sentence production (picture description) task is designed to assess primarily syntactic and morphological processing while demands on recognition and semantic/name retrieval of the target objects were made minimal (by using very frequent object items, e.g. ‘book’, and also by actually providing the name of the target objects alongside the picture stimulus to the participant). The sentence reading task requires the participant to read aloud a sentence containing some relatively low frequency and exception words (i.e.‘irregular words’ as described in Coltheart et al., 2001). This task would tap the lexical and non-lexical phonological processing. Finally, BCoS also assesses nonword reading, which can only be achieved by non-lexical phonological processing and not aided with semantic knowledge. Table 1 outlines the potential cognitive–language processes underlying these four visual speech production tasks. We speculate that the object naming task may have greater demands on recognition and semantic knowledge of objects relative to other tasks tested in the present study.
Table 1.
Outline of the cognitive processes underlying the visual speech production tasks in BCoS.
| Tasks | Object recognition | Word/letter recognition | Action recognition | Semantic | Syntax | Output lexical phonology | Output non-lexical phonology | Articulation |
|---|---|---|---|---|---|---|---|---|
| Object naming | ✓✓ | X | X | ✓✓ | X | ✓✓ | X | ✓✓ |
| Sentence production | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | X | ✓✓ |
| Sentence reading | X | ✓✓ | X | ✓ | ✓ | ✓ | ✓ | ✓✓ |
| Nonword reading | X | ✓✓ | X | X | X | X | ✓✓ | ✓✓ |
In a large sample of sub-acute stroke patients, we examined the lesions associated with impaired object naming and then in relation to other visual speech production tasks (in order to isolate regions specific to object naming). As another approach, we also performed a principal component analysis in order to identify the shared and unique mechanisms of object naming and the other language tasks. We applied a fully-automated voxel-based correlational method to assess the relationship between the performance on the language tasks (based on the raw and PCA scores) and the density of grey and white matters (based on patients' clinical CT scans).
2. Methods
2.1. Subjects
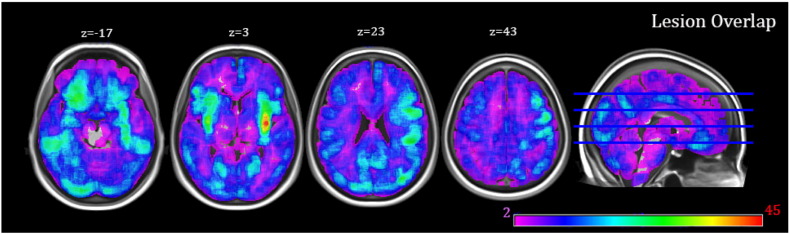
All patients were recruited from the stroke units of 12 hospitals in the West Midlands, UK, as part of the Birmingham University Cognitive Screen trial (BUCS; http://www.bucs.bham.ac.uk). The broad inclusion criteria of the trial were that the patient should be at the sub-acute stage (<120 days post-stroke), physically stable and well enough to maintain concentration for around an hour to complete the cognitive assessment (judged by a trained assessor of the multi-disciplinary stroke team). No restrictions were placed according to aphasic type or severity. The sample of this present study was made up of 280 patients (141 males, average age: 70.88 years ± 14.06std, ranging between 26 and 93 years) selected from the BUCS database of 532 cases with clinical CT scans available. As previously estimated in the patient group of the BUCS trial, 41.4% had middle cerebral artery (MCA) stroke, 10.4% posterior cerebral artery stroke and 13.4% due to other affected vascular territories (Chechlacz et al., 2014a). For the present study, we excluded patients whose CT scans were of poor quality (n = 37), or if the scans showed abnormally large ventricles (n = 4). To control for the potential confounding effect due to the presence of abrupt high intensity signals, we also eliminated cases with haemorrhage (n = 42). We further excluded patients who were non-right-handed (n = 54), or who were scanned more than 120 days post-stroke (n = 1) or on the same day (within 24 h) of their stroke (n = 114). This resulted to a final total of 280 patients. A lesion overlap map for our patients is presented in Fig. 1. As shown, lesions of the patients cover the entire brain in the two hemispheres with maximum overlaps in the right MCA territory (see Supplementary material S3 for the method used to create the individual lesion maps).
Fig. 1.
Stroke lesion overlap map showing the distribution of lesions (based only the patients who had an identified lesion in the brain, n = 237). Lesions were identified using an automated delineation method (outlined in Gillebert et al., 2014). Shared voxels (across patients) are shown on a heat map with the deepest red indicating that the most number of participants had that voxel included in their lesion.
All patients provided written informed consent conforming to the ethics protocols approved by the UK National Health Service ethics committee, the local NHS trusts and the Birmingham University ethics procedures.
2.2. Behavioural measures
The cognitive abilities of the patients were examined using the BCoS Cognitive Screen (Humphreys et al., 2012) (see also Bickerton et al., 2014; http://www.cognitionmatters.org.uk). The test battery was developed to examine five core ‘domains’ of daily cognitive functions: i) language, ii) attention and executive functions, iii) memory, iv) praxis and v) number processing. This is achieved by 27 paper and pencil tasks with each designed to tap into various cognitive processes under each domain (http://www.cognitionmatters.org.uk/bcos.php). The tests were designed to be aphasia and neglect friendly, to be as sensitive as possible to identify cognitive impairments (with validated age-matched cut-off scores) and to optimise time efficient test administration (i.e. the entire screen was developed to be completed within 60 min). During the study, experimenters were blind to the specific condition of the patient and the location of any lesion. On average, patients were tested 24 days post-stroke (with 65% tested within the first month after stroke).
2.2.1. Object naming
Object naming falls within the language domain of BCoS (also referred to as ‘picture naming’). The stimuli comprise 14 grey-level, shaded hand drawings. The items were chosen to cover a range of frequency according to the subjective familiarity ratings (469–543 out of 700) from the MRC psycholinguistic database (Wilson, 1988). In order to represent a variety of semantic categories, half of the items were living things (e.g. bat) and half non-living (e.g. spanner). Among the living items there were 2 animals, 3 fruits and 2 vegetables while the non-living category consisted of 2 tools, 1 kitchen implement and 4 other household implements. In order to detect word production problems sensitive to stimulus length, half of the items had a long name (being composed of 6–9 letters) and half a short name (3–5 letters). During the task, participants were presented with each drawing of an object printed centrally on an A4 sheet of paper. A maximum of 15 s was allowed per item for the patient to give a response. Each correct naming response carried one point and the maximum task score was 14.
2.2.2. Other visual speech production tasks
In addition to object naming, we assessed performance on other BCoS tests that also required speech output to visual stimuli. The tests included sentence production to a picture (to describe what a person was doing), reading a sentence containing some low frequency and exception words and reading some nonwords (for details, refer to the Supplementary material S1).
2.3. Neuroimaging assessment
2.3.1. Acquisition of brain images
For each patient, computed tomography (CT) images were collected as part of the standard clinical procedures. The scans were acquired using one of these scanners: Siemens Sensation 16; GE Medical System LightSpeed 16 or LightSpeed plus. The CT images were provided by the hospitals in digital DICOM format after they had been anonymised. These images covered the whole brain with an in-plane resolution of 0.5 × 0.5 mm2 and a slice thickness of 4–5 mm. The in-plane resolution (along the x–y plane) of a CT image was higher than that of a typical MR structural scan (1 × 1 mm2), but the resolution along the superior–inferior direction (z-axis) was poorer in CT compared to MR.
CT images depict the density of the tissue and as such have a clear biological interpretation. CT scans also provide an undistorted image of the tissue density. However, changes in tissue density, especially due to ischemic stroke, may be underestimated on a CT scan, at least when the scan is conducted within the first 24 h after a stroke (Mohr et al., 1995). Therefore, in the current study, we included only patients who had their CT taken at least 24 h post-stroke. Also, to account for possible changes in lesions following a stroke, the analysis models included as a covariate the interval (in days) between the stroke and the CT scan. On average the CT scans were taken 7.26 days after stroke, with 74% of cases within 1 week of the stroke.
2.3.2. Pre-processing of brain images
The CT images were pre-processed using SPM8 (Statistical Parametric Mapping, Welcome Department of Cognitive Neurology, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/~spm). The quality of the CT scans was first assessed visually to ensure that only good quality data (e.g. free from head movement and other image artefacts) were included in the analysis. This quality check was done on the raw CT images and also on the segmented, normalised images by comparing them to the a-priori tissue templates. This resulted in removal of around 7% of the patients. Pre-processing started with converting the images to NIfTI format and normalising (Ashburner and Friston, 2003) them to an in-house CT template. This initial normalisation stage was primarily based on skull shape and aimed to transform the images into MNI (Montreal Neurological Institute) space to optimise the following procedures. Next, the unified-segmentation algorithm implemented in SPM12 (i.e. Seg8 in SPM8; Ashburner and Friston, 2005) was employed. In the unified model, the priors of the tissue class, from which intensities are drawn, are encoded by deformable tissue probability maps. The a-priori tissue class maps indicate the probability of finding expected signal sources at each voxel: grey matter (GM), white matter (WM), cerebrospinal fluid (CSF), bone, fat and air in the brain. To account for the presence of damaged tissue due to stroke, a modified segmentation procedure similar to the approach of Seghier and colleagues (2008) was adopted to include a seventh tissue class. In creating an additional prior for abnormal tissue, we assumed that in each grey or white matter voxel there is a 10% chance of it belonging to an abnormal tissue class. This 10% estimation was computed based on the lesion volume size (versus the brain size) estimated in the BUCS database (for details, see Chechlacz et al., 2012). Furthermore, for the grey and white matter tissue classification, we assumed a single Gaussian distribution for the underlying intensities. To account for potential inhomogeneity of the abnormal tissue we used 2 different Gaussian distributions to model the intensities in this tissue class. What is more, CT images as opposed to MRI do not suffer field bias due to field strength inhomogeneity; therefore we did not correct for that in the model. Finally, the segmented white and grey matter images were normalised using the parameters estimated in the unified-segmentation algorithm again and smoothed using a 12-mm full-width-at-half-maximum (FWHM) Gaussian kernel to accommodate random field theory assumptions of continuity (Worsley and Friston, 1995). The preprocessed GM and WM maps were then used in our analyses to explore voxel by voxel the relationship between brain lesion and behavioural performance.
2.4. Data analysis
2.4.1. Behavioural measures
All the patients completed the object naming test. For the additional visual speech production tasks used in the analyses, we replaced missing data with the group average. Data from 2 patients were missing in sentence reading, 8 in sentence production and 8 in nonword reading. To examine the relationship between the performance on object naming and the covariates, a non-parametric Spearman-rank correlation (two-tailed) analysis was carried out.
Principal component analysis (PCA) was performed using Matlab 7.9 (The MathWorks, Natick, MA, USA) to identify shared and unique components of object naming in relation to other language tasks. Our sample consisted of 280 subjects, which is considered fairly adequate (N = 300) for PCA (Comrey and Lee, 1992). To increase the robustness of the analysis, especially for small to moderate sample size, a subject-to-variable ratio of at least 5:1 has also been suggested (Hatcher, 1994). With 280 cases and 4 variables in this study, a ratio of 70:1 is very adequate to justify the purposes of PCA. The use of PCA to rotate behavioural data which is then related to the distribution of brain lesions has been demonstrated in recent neuropsychological studies of stroke (Butler et al., 2014; Chechlacz et al., 2014b) and developmental prosopagnosia (Garrido et al., 2009).
To account for differences in the maximum scores of the language tests (object naming: 14; picture description: 8; sentence reading: 40; nonword reading: 6), we re-scaled the raw scores on each test linearly to range between 0 and 20. We used in the PCA the re-scaled scores of the 280 participants on the four language tests and then extracted component loadings (i.e. coefficients) and eigenvalues. No additional rotation was applied to the data. Individual performance scores on each principal component were also derived and used in the VBM analyses.
2.4.2. Voxel-based morphometry (VBM)
Using SPM8, all reported VBM analyses were performed on the scans from 280 patients to determine the neural correlates of object naming and its related cognitive components. Random effects analyses were conducted within the general linear model framework and correlations between the behavioural measures and the integrity of brain tissues were computed (Ashburner and Friston, 2001).
We created three separate general linear models. First, we determined the common lesions that were associated with object naming deficits in our sample using the participants' raw scores (Analysis 1). Second, we identified the lesions unique for object naming by controlling in the model for other language tasks including sentence production, sentence reading and nonword reading (Analysis 2). Third, we examined the neural correlates of the individual scores on the principal components derived from the PCA (Analysis 3). This model included all principal components though we focused primarily on the lesions associated with a shared language component and a component that was unique to object naming. Reduced integrity of grey and white matter was analysed separately for each model.
In all analyses, the following measures were included as covariates of no interest: age, gender, years of education, interval between stroke and CT scanning, interval between stroke and cognitive testing, and measures of general cognitive state (we used tests of each participant's overall orientation, see Supplementary material S2 for details). Inclusion of these covariates allowed us to control for various confounding factors that might have a potential impact on cognitive performance or the extent of lesion. We focused on results that were reliable at a FWE-corrected threshold of p < 0.05 at the cluster level, unless stated otherwise. For completeness we report in the tables all clusters with a voxel-wise threshold of Z = 2.32 and an extent threshold of at least 300 voxels (the expected cluster size by chance with the above Z threshold is 233 voxels). The brain co-ordinates throughout are presented in the standardised MNI space. Anatomical labelling was based on the Anatomical Automatic Labelling toolbox (Tzourio-Mazoyer et al., 2002), Duvernoy's (1999) Human Brain Atlas, the JHU White matter tractography atlas (Hua et al., 2008) and the MRI Atlas of Human White Matter by Mori (2005).
3. Results
3.1. Behavioural results
On average, patients were able to name 10.02 (sd = 3.88) objects correctly. Compared to the cut-off points established from the age-matched healthy controls (http://www.bcos.bham.ac.uk), 108 patients scored lower than the cut-off points and were classified as impaired. Table 2 provides the descriptive data and average scores on the cognitive and language tasks.
Table 2.
Descriptive data and average test scores of the sample (N = 280). The scores reflect: Means (standard deviations in parentheses).
| Variables | Scores |
|---|---|
| Age in years | 70.88 (14.06) |
| Years of education | 10.89 (2.51) |
| Stroke–scan interval (in days) | 7.26 (13.78) |
| Stroke–cognitive screen interval (in days) | 24.07 (21.1) |
| BCoS cognitive screen tests | |
| i. Object naming (max = 14) | 10.02 (3.88) |
| ii. Sentence production (max = 8) | 6.35 (2.55) |
| iii. Sentence reading (max = 42) | 34.49 (13.08) |
| iv. Nonword reading (max = 6) | 4.01 (2.26) |
| v. Orientation (general cognitive state) | |
| - Personal info. (max. = 8) | 7.3 (1.63) |
| - Time & space (max. = 6) | 5.48 (0.99) |
Performance on object naming was significantly but weakly associated with age (rs = −0.164, p < 0.01) and years of education (rs = 0.218, p < 0.01). In addition, individuals who performed worse at object naming also had poorer overall ‘orientation’, measured in terms of their knowledge of personal information (rs = 0.548, p < 0.01) and time and space (rs = 0.328, p < 0.01). Assessment on the ‘time and space’ measure was based on multiple-choice tests and hence did not rely on speech production. Not surprisingly object naming was correlated significantly with all other language measures including sentence production (rs = 0.596, p < 0.01), sentence reading (rs = 0.636, p < 0.01) and nonword reading (rs = 0.566, p < 0.01).
As an attempt to dissociate processes underlying object naming and the other visual speech production tasks, we ran a PCA on the re-scaled raw data of four language tests (i.e. object naming, sentence production, sentence and non-word reading). The PCA revealed that the four tasks all loaded on the first component with loadings ranging between 0.4 and 0.6. This ‘shared’ component accounted for 77.9% of the variance. As all the tasks required both visual perception and generation of a verbal response, we assume that this component represents a process relating to the conversion of visual information into phonological outputs. Another component of interest accounted for 4.7% of the variability and had a dominant high loading from object naming, separating it from the other three visual speech production tasks. This suggests that there are processes that are uniquely involved in object naming but not in any other language tasks tested. Table 3 shows the loadings on these two components and the correlations between the raw scores on each language test and the individual scores on each of the two principal components. There were two other components from the PCA outputs: 1) one dissociating nonword reading from the other three language tasks (11.6% of the variance explained); and 2) one specifically dissociating sentence reading from sentence production (5.8% of the variance explained). As these two other components were not unique for object naming, they were not the central focus of the present study (although they were also included in the GLM model).
Table 3.
Component loadings and correlations between the behavioural scores on each of the BCoS language tests and on the principal components.
| The shared language component |
The unique object naming component |
|||
|---|---|---|---|---|
| Language tests | Loading | Correlation | Loading | Correlation |
| Object naming | 0.422 | 0.795** | 0.840 | 0.584** |
| Sentence production | 0.495 | 0.754** | −0.285 | −0.078 |
| Sentence reading | 0.489 | 0.798** | −0.461 | −0.003 |
| Nonword reading | 0.582 | 0.887** | 0.020 | 0.082 |
For each component, the first column shows the loadings from each of the language tests and the second column shows the correlation coefficients between the raw scores on each language test and the subject's scores on each component.
*Significant at p<0.05;
Significant at p<0.001.
To further explore the presence of a unique object naming component we counted the number of patients who were classified as impaired in object naming but showed normal performance on all the other language tasks. Only 7 patients fulfilled the criteria. On the other hand, there were 10 patients classified as impaired in all the three language tasks but retained normal functioning in object naming. This may suggest a potential double dissociation between object naming and other visual–speech language tasks although it appears to be a very rare phenomenon.
As lesion size is a factor potentially contributing to the severity of any deficit, each patient's lesion volume was calculated. We then examined its relationship with the performance on the four language tasks and the rotated scores for the shared and unique naming components. Lesion volume correlated weakly with object naming (rs = −0.167, p = 0.005), sentence reading (rs = −0.136, p = 0.023), sentence production (rs = −0.207, p < 0.001) and nonword reading (rs = −0.126, p = 0.035). Regarding the PCA components, lesion volume again correlated weakly with the general language component (rs = −0.175, p = 0.003) but not at all with the unique naming component (rs = −0.058, p = 0.337).
3.2. Neuroimaging results
Next, we related the behavioural measures to the neuroimaging data in order to determine the structural lesion correlates of object naming and their associations with underlying cognitive processes represented by the two principal components of interest. Here, we report the results of three analyses, and for each we computed separate probability maps for the grey and white matter (GM & WM, respectively): Analysis 1 — correlation with only the raw score of object naming; Analysis 2 — correlation with object naming after accounting for performances on the other visual speech production tasks; and Analysis 3 — (a) correlation with the individual scores on the ‘shared’ language component and (b) with the ‘unique’ object naming component.
3.2.1. GM analyses
3.2.1.1. Analysis 1 — overall performance on object naming
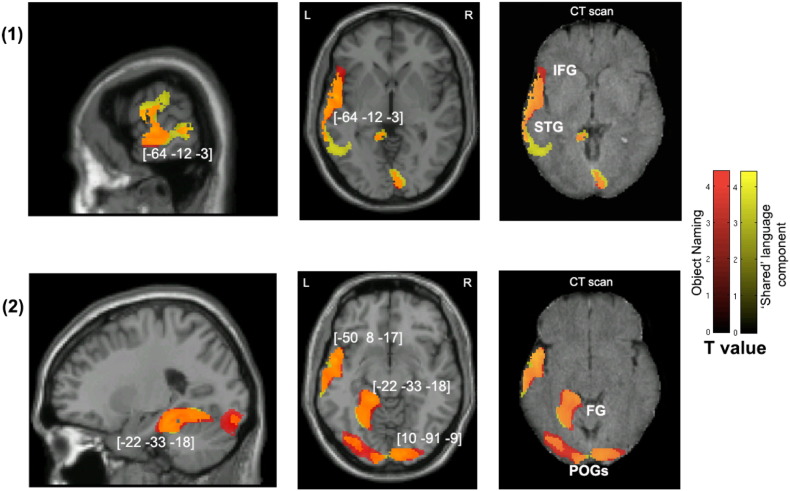
This VBM analysis based on the raw scores of object naming alone revealed a significant positive relationship with voxels in the left fronto-temporal and medial temporal regions and also the bilateral occipital cortices. In particular, impaired performance was significantly associated with GM damage in the bilateral posterior visual cortices, the left superior temporal gyrus extending to the insula and inferior frontal gyrus, and the left fusiform (Table 4, Analysis 1).
Table 4.
Grey matter correlates of Analysis 1 — overall object naming and Analysis 3a — a ‘shared’ language component. Age, gender, years of education, stroke–scan interval, stroke–screen interval and general cognitive state indices (orientation scores) were entered as covariates in both analyses.
| Analysis 1: overall object naming (NO control of other language tasks) |
Analysis 3a: shared language component |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Structures |
Cluster size |
MNI coordinates |
Z (peak) |
Cluster size |
MNI coordinates |
Z (peak) |
||||
| x | y | z | x | y | z | |||||
| Fronto-temporal region | ||||||||||
| Left STG (extending into insula and IFG) | 5523** | −64 | −12 | −3 | (4.23) | 6516** | −66 | −15 | −3 | (4.32) |
| Temporo-occipital region | ||||||||||
| Left fusiform | 6486** | −22 | −33 | −18 | (4.33) | 2861** | −22 | −33 | −18 | (4.22) |
| Left posterior occipital | −34 | −79 | −17 | (4.28) | 3135** | −34 | −79 | −17 | (3.42) | |
| Right posterior occipital | 3361** | 14 | −93 | 24 | (3.8) | 10 | −91 | −9 | (3.26) | |
Note: the first column under each analysis reports the size (number of voxels) of each of the clusters with amplitude of voxels surviving Z = 2.32 (uncorrected). In each cluster, the MNI coordinates (x, y, z) and the peak reliability in brackets (Z) are reported. Acronyms: IFG — inferior frontal gyrus; STG — superior temporal gyrus.
p < 0.05 FWE-corrected significant at cluster level.
3.2.1.2. Analysis 2 — performance on object naming in relation to the other language tasks
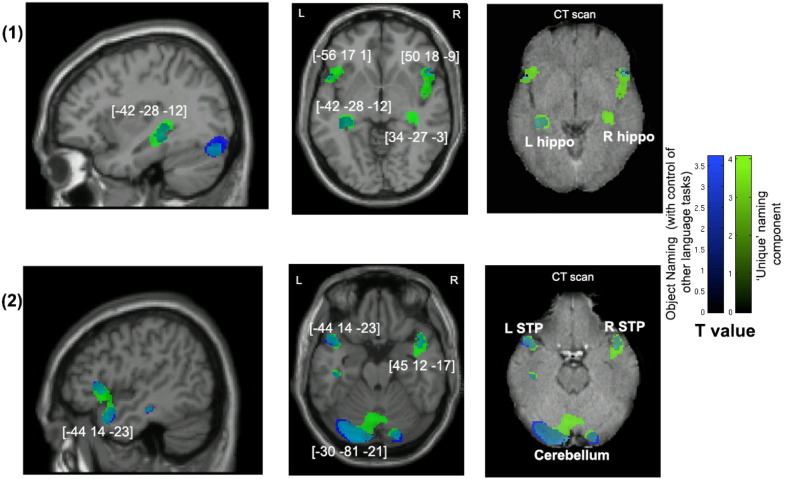
To identify the neural correlates specific to object naming but not to other visual speech production tasks, we included the scores of 3 other language tests (i.e. sentence production, sentence reading and non-word reading) as covariates in a separate model. The results of this analysis did not survive the cluster-level threshold of p < 0.05 with FWE correction. However, they do suggest that lesions to the bilateral anterior superior temporal and inferior frontal gyri, the left hippocampus and several regions in the cerebellum were associated uniquely with object naming (Table 5, Analysis 2). Notably, lesions to the left fusiform and lingual gyri were not found to be associated uniquely with object naming after we controlled for the other language tasks.
Table 5.
Grey matter correlates of Analysis 2 — object naming after controlling for other language tasks and Analysis 3b — a ‘unique’ naming component. Age, gender, years of education, stroke–scan interval, stroke–screen interval and general cognitive state indices (orientation scores) were entered as covariates in both analyses.
| Analysis 2: object naming (after controlling for other language tasks) |
Analysis 3b: unique object naming component |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Structures |
Cluster size | MNI coordinates |
Z (peak) |
Cluster size |
MNI coordinates |
Z (peak) |
||||
| x | y | z | x | y | z | |||||
| Fronto-temporal region | ||||||||||
| Left IFG | 668 | −56 | 17 | 1 | (3.06) | 1709 | −56 | 17 | 1 | (3.14) |
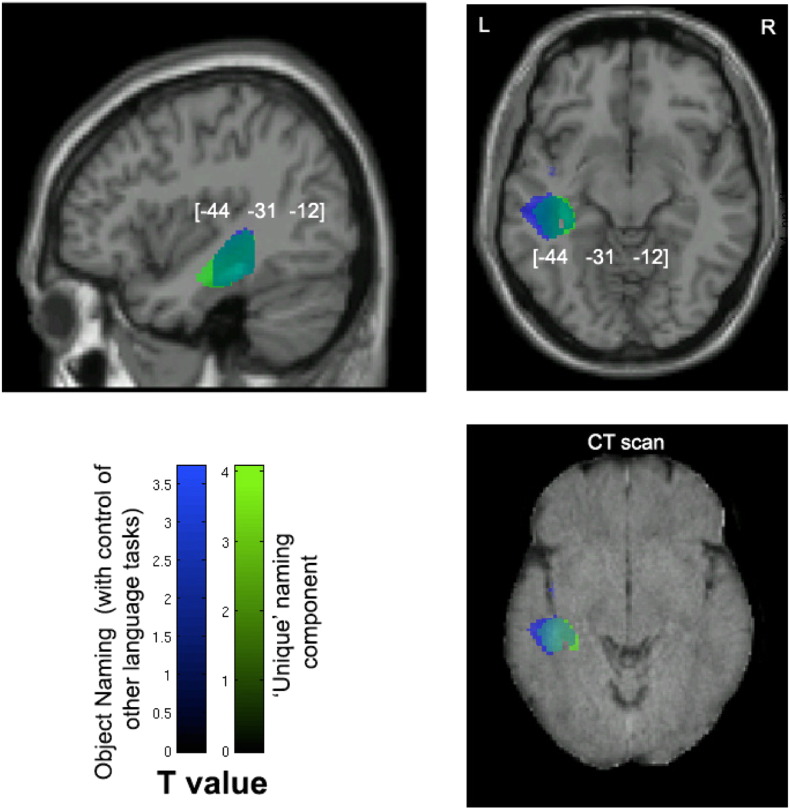
| Left ant. STG (covering STP) | 482 | −48 | 15 | −24 | (2.92) | −44 | 14 | −23 | (2.98) | |
| Right IFG | 499 | 51 | 20 | −8 | (2.91) | 2106 | 50 | 18 | −9 | (3.38) |
| Right ant. STG (covering STP) | 45 | 12 | −17 | (2.79) | 45 | 8 | −20 | (3.49) | ||
| Medial temporal region | ||||||||||
| Left hippocampus | 857 | −39 | −30 | −5 | (3.18) | 1387 | −42 | −28 | −12 | (4.00) |
| Right hippocampus | 488 | 34 | −27 | −3 | (3.14) | |||||
| Occipital region | ||||||||||
| Right posterior occipital | 1540 | 12 | −88 | 25 | (3.68) | 1156 | 10 | −90 | 30 | (3.36) |
| Cerebellum | ||||||||||
| Middle cerebellar cluster | 458 | −6 | −70 | −14 | (2.80) | 3431** | −3 | −69 | −14 | (3.41) |
| Left cerebellar cluster | 2635 | −30 | −81 | −21 | (3.46) | −15 | −87 | −27 | (3.37) | |
| Right cerebellar cluster | 970 | 14 | −87 | −33 | (3.58) | 1056 | 15 | −85 | −32 | (4.00) |
Note: the first column under each analysis reports the size (number of voxels) of each of the clusters with amplitude of voxels surviving Z = 2.32 (uncorrected). In each cluster, the MNI coordinates (x, y, z) and the peak reliability in brackets (Z) are reported. Acronyms: IFG — inferior frontal gyrus; ant. STG — anterior superior temporal gyrus; STP — superior temporal pole.
p < 0.05 FWE-corrected significant at cluster level.
3.2.1.3. Analysis 3 — the ‘shared’ and ‘unique’ naming components
This VBM analysis correlated each brain voxel with the subject's score on a principal component generated from the PCA procedure. From Analysis 3a (Table 4), the ‘share’ component was reliably correlated with lesions in the left lateral fronto-temporal and fusiform regions and the bilateral visual cortices. These were similar to the lesion pattern observed when performance on object naming was modelled alone (without controlling for any other language tasks, i.e. Table 4, Analysis 1; see also Fig. 2). On the other hand, the lesion pattern associated with the ‘unique’ naming component (Table 5, Analysis 3b) mirrored the results yielded in Analysis 2 (Table 5) where performances on the other three language tasks were partialled out from object naming (Fig. 3).
Fig. 2.
Voxel-wise statistical analysis of grey matter damage: overall object naming and the shared language component. Note: VBM results showing voxels corresponding to grey matter damage in (red) overall object naming, (yellow) the ‘shared’ language principal component and (orange) their overlay. Please note that the lesioned areas are coloured according to their significance level in the VBM analysis, where brighter colours mean higher t-values. The numbers in brackets indicate peak MNI coordinates. Rows (1) & (2) show the neural correlates of the results in different views of the brain. Across each row, the first two images are T1-weighted MR images overlaid with statistical parametric maps (SPMs) generated from the VBM analyses. In addition, to further illustrate the possible use of CT scans in lesion–function mapping analysis, SPMs are plotted on CT images (the right-most image across each row) of the same axial views. IFG, inferior frontal gyrus; STG, superior temporal gyrus; FG, fusiform gyrus; POG, posterior occipital gyrus.
Fig. 3.
Voxel-wise statistical analysis of grey matter damage: object naming with control of the performance on other visual speech production tasks and the unique naming component. Note: VBM results showing voxels corresponding to grey matter damage in (blue) object naming after controlling for the other visual speech production tasks, (green) the ‘unique’ naming principal component and (cyan) their overlay. Please note that the lesioned areas are coloured according to their significance level in the VBM analysis, where brighter colours mean higher t-values. The numbers in brackets indicate peak MNI coordinates. Also refer to the notes in Fig 2 for further guidelines on viewing the images. L, left; R, right; hippo, hippocampus; STP, superior temporal pole.
3.2.2. WM analyses
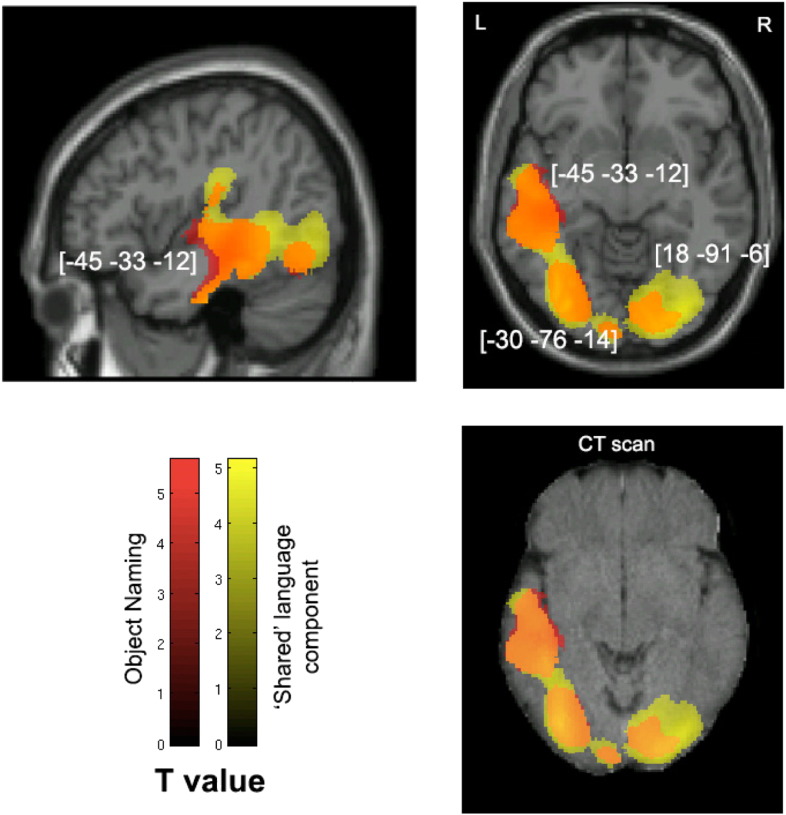
3.2.2.1. Analysis 1 — overall performance on object naming
VBM analysis of WM maps and object naming scores alone revealed a significant relationship between object naming deficits and reduced white matter density in an extensive region encompassing the temporal and inferior parietal lobes in the left hemisphere and the bilateral visual cortices (Table 6, Analysis 1). Damage to WM in temporo-parietal areas is likely to disconnect the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, parts of the superior longitudinal fasciculus including the arcuate fasciculus (Hua et al., 2008; Mori et al., 2005). Lesions in the bilateral occipital cortices, on the other hand, are linked to damage of the posterior end of the inferior fronto-occipital fasciculi (Hua et al., 2008; Mori et al., 2005).
Table 6.
White matter correlates of Analysis 1 — object naming and Analysis 3a — a ‘shared’ language component. Age, gender, years of education, stroke–scan interval, stroke–screen interval and general cognitive state indices (orientation scores) were entered as covariates in both analyses.
| Analysis 1: overall object naming (NO control of other language tasks) |
Analysis 3a: shared language component |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster size |
MNI coordinates |
Z (peak) |
Cluster size |
MNI coordinates |
Z (peak) |
Affected fibres |
||||
| x |
y |
z |
x |
y |
z |
|||||
| Temporo-parieto-occipital region | ||||||||||
| 4533** | −30 | −76 | −14 | (4.41) | 29,887** | −32 | −76 | −12 | (5.02) | Left IFOF, ILF, SLF (including AF), right IFOF |
| ≫ Left occipital | −28 | −27 | 33 | (4.75) | ||||||
| 4105** | 15 | −88 | 15 | (3.97) | >> A large cluster of bi-occipital, left temporal and inferior parietal cortices | |||||
| ≫ Right occipital | ||||||||||
| 11,114** | −45 | −33 | −12 | (5.5) | ||||||
| ≫ Left temporal extending into parietal | ||||||||||
Note: the first column under each analysis reports the size (number of voxels) of each of the clusters with amplitude of voxels surviving Z = 2.32 (uncorrected). In each cluster, the MNI coordinates (x, y, z) and the peak reliability in brackets (Z) are reported. Acronyms: IFOF — inferior frontal–occipital fasciculus; ILF — inferior longitudinal fasciculus; SLF — superior longitudinal fasciculus; AF — arcuate fasciculus.
p < 0.05 FWE-corrected significant at cluster level.
3.2.2.2. Analysis 2 — performance on object naming in relation to the other language tasks
After controlling for performances on the three other visual speech production tasks, object naming correlated with WM integrity of a smaller area in the left temporal lobe and a small cluster of voxels in the cerebellum (Table 7, Analysis 2). However, these relationships were only weakly reliable at the cluster level. Lesion to this temporal area affects most likely the temporal tail of the arcuate fasciculus. Damage to the cerebellum, on the other hand, is likely to impede transfer of information along the middle cerebellar peduncle.
Table 7.
White matter correlates of Analysis 2 — object naming after controlling for other language tasks and Analysis 3b — a ‘unique’ naming component. Age, gender, years of education, stroke–scan interval, stroke–screen interval and general cognitive state indices (orientation scores) were entered as covariates in both analyses.
| Analysis 2: object naming (after controlling for other language tasks) |
Analysis 3b: unique object naming component |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster size |
MNI coordinates |
Z (peak) |
Cluster size |
MNI coordinates |
Z (peak) |
Affected fibres | ||||
| x | y | z | x | y | z | |||||
| Temporal region | ||||||||||
| 2782* | −44 | −31 | −12 | (4.81) | 3123* | −42 | −30 | −11 | (4.76) | Temporal end of AF |
| Cerebellum | ||||||||||
| 490 | −8 | −55 | −35 | (3.05) | 749 | −4 | −52 | −35 | (3.28) | MCP |
Note: the first column under each analysis reports the size (number of voxels) of each of the clusters with amplitude of voxels surviving Z = 2.32 (uncorrected). In each cluster, the MNI coordinates (x, y, z) and the peak reliability in brackets (Z) are reported. Acronyms: AF — arcuate fasciculus; MCP — middle cerebellar peduncle.
p < 0.1; **p < 0.05 FWE-corrected significant at cluster level.
3.2.2.3. Analysis 3 — the ‘shared’ and ‘unique’ naming components
A significant relationship was found between the subject's score on the ‘shared’ component and WM density of an extensive region in the left temporal and parietal cortices and the bilateral occipital cortices (Table 6, Analysis 3a). There is a high degree of similarities between these results and those of Analysis 1, which modelled object naming alone (Fig. 4). In contrast, the white matters associated with the ‘unique’ naming component (Table 7, Analysis 3b) greatly overlap with the outputs of Analysis 2 (Table 7), the one that looked at object naming after partialling out the performances on other language tasks (Fig. 5).
Fig. 4.
Voxel-wise statistical analysis of white matter damage: overall object naming and the shared language component. Note: VBM results showing voxels corresponding to white matter damage in (red) overall object naming, (yellow) the ‘shared’ language principal component and (orange) their overlay. Please note that the lesioned areas are coloured according to their significance level in the VBM analysis, where brighter colours mean higher t-values. The numbers in brackets indicate peak MNI coordinates. On the top row are two T1-weighted MR images overlaid with statistical parametric maps (SPMs) generated from the VBM analyses. In addition, to further illustrate the possible use of CT scans in lesion–function mapping analysis, SPMs are plotted on a CT image (bottom right) of the same axial view as the T1-weighted image above it.
Fig. 5.
Voxel-wise statistical analysis of white matter damage: object naming with control of the performance on other visual speech production tasks and the unique naming component. Note: VBM results showing voxels corresponding to white matter damage in (blue) object naming after controlling for the other visual speech production tasks, (green) the ‘unique’ naming principal component and (cyan) their overlay. The numbers in brackets indicate peak MNI coordinates. Also refer to the notes in Fig. 4 for further guidelines on viewing the images.
Finally, we included lesion volume as an additional covariate in all the VBM analyses and the pattern of results did not change.
4. Discussion
To examine the relationship between object naming and other visual speech production tasks, we carried out a principal component analysis across the language tasks. This analysis revealed a ‘shared’ component that loaded across all the tasks. This component was linked to damage to the bilateral posterior occipital cortices and left-lateralised regions including the fusiform and the superior temporal gyrus (STG) extending into the insula and the inferior frontal gyrus (IFG). Similarly, the white matter damage associated with this ‘shared’ language component was left lateralised. These regions were also related to poor object naming when it was assessed alone without controlling for performances on other visual speech production tasks. In contrast to these analyses of common language processes, we also evaluated the processes particularly stressed in object naming by (i) including the other visual speech production tasks as regressors in the VBM analysis and (ii) examining the ‘unique’ naming component that dissociates object naming from the other tasks. These analyses indicated particular involvement in object naming of the two anterior superior temporal poles (extending to IFG), as well as the hippocampus and cerebellum.
4.1. Shared neural substrates of object naming
PCA across object naming, sentence production, and sentence and nonword reading produced a component that accounted for more than 75% of the total variance and the loadings from the four tasks on this component ranged between 0.4 and 0.6. This ‘shared’ component is likely to stress more visual recognition, phonological retrieval and articulation because all the tasks rely on speech response to a visual input and the nonword reading task would not require semantic processing. When analysed alone, object naming (Analysis 1) was linked to the same left lateralised network as the ‘shared’ component (Analysis 3a). This indicates that without additional care being taken to isolate factors stressed by object naming, lesion–symptom analyses of object naming tend to highlight visual and phonological processing found in a number of language tasks.
The four language tasks employed in the present study all potentially demand high-level processing of visual inputs. This may be why the shared component was linked to the lateral occipital cortex and the fusiform gyrus. In agreement with our findings, these two regions have often been associated with processing of complex visual inputs such as faces, objects and words (Bar et al., 2006, 2001; Dien, 2009; Grill-Spector et al., 2001; Herbster et al., 1997; Kanwisher and Yovel, 2006; Malach et al., 2002; McCarthy et al., 1997), even though each type of stimulus may recruit slightly different segments of the striate and extrastriate cortices (see Dehaene et al., 2002; McCandliss et al., 2003, but also Price and Devlin, 2003; Starrfelt and Gerlach, 2007). Yet, it is likely that stroke which typically affects a large area in the brain impairs various types of high-level visual processing simultaneously (as in our case) due to the spatial proximity of the corresponding loci within the occipito-temporal regions.
The involvement of the left inferolateral frontal gyrus (extending to the insula) and the superior temporal gyrus in shared language processes is not surprising. These results accord with a recent study showing links between these temporo-frontal areas and a phonological factor of language (Butler et al., 2014). The IFG has long been held to play an important role in the production of meaningful speech (Broca, 1861). Infarction to this frontal area has been related to a number of speech impediments including apraxia of speech and expressive aphasia (for a detailed review, see Caplan, 1987). Besides, a body of evidence implicates also the left insular gyrus (a neighbouring brain structure of the IFG) in speech production (Dronkers, 1996; Kleist, 1934; Mazzocchi and Vignolo, 1979; Mohr et al., 1978). A recent review of the clinical and functional imaging literature suggests that the insula participates in articulatory planning and control processes (Ackermann and Riecker, 2010). Along with STG these areas constitute part of the dorsal pathway that has been proposed to specialise in phonological processing (Hickok and Poeppel, 2007; Rauschecker and Scott, 2009; Saur et al., 2008).
The present study also observed associations between white matter lesions in the left temporal and bilateral ventral occipital lobes and impairment in a general visual-to-speech language function, represented by the shared component. Putting together the previously proposed functions of various parts of the visual-to-speech network, here we suggest that impairment in the general language function requiring visual-to-speech interaction can also be the results of disconnection between the occipital and ventral temporal lobes and/or also between the occipital and inferior frontal lobes via the temporal regions.
4.2. Unique neural substrates of object naming
We also attempted to dissociate the brain regions recruited especially by object naming. This was done by analysing the neural correlates of object naming with the other three language tasks included as regressors, and also by assessing the ‘unique’ PCA component that isolated object naming from the other tasks. The ‘unique’ object naming component was associated with the bilateral anterior temporal lobes, the hippocampi and several cerebellar areas (Analysis 3b). Corroborating evidence was provided by an additional analysis (Analysis 2) using object naming as the variable of interest while controlling for other visual speech production tasks. However, these ‘unique’ associations did not reach family-wise significance (apart from a cerebellar area). This is probably because the occurrence of cases of deficits at object naming only in the absence of more general language impairment is rather rare.
Nevertheless, we note that the observation of a potential bi-anterior temporal association with object naming is in agreement with previous studies testing semantic dementia (Jefferies and Lambon Ralph, 2006; Lambon Ralph et al., 2007) and temporal lobe epilepsy (Hodges and Patterson, 2007; Lambon Ralph et al., 2012; Seidenberg et al., 2002). These past studies concur that the anterior temporal lobes contribute to semantic representation (see also Gough et al., 2005; Pobric et al., 2007; Schwartz et al., 2009; Tranel et al., 1997; Woollams, 2012) necessary for accurate object naming. Putting this together with the results of left-lateralised lesions associated with the shared language component (discussed earlier), our findings are complementary to a proposed model of naming that suggests a left-localised phonological representation system which connects strongly to a bilaterally distributed conceptualisation network (Lambon Ralph et al., 2001; Schapiro et al., 2013). The unique involvement of cerebellar areas and the hippocampus in object processing is less clear. Recent literature has implicated the hippocampus in some forms of semantic processing (Bonelli et al., 2011; Holdstock et al., 2002; Manns et al., 2003; Ryan et al., 2008; Schacter et al., 1996) or processing of complex visual stimuli (Sawrie et al., 2000; Sehatpour et al., 2010; Vannucci et al., 2003) and the cerebellum in high-level cognitive operations (Desmond and Fiez, 1998; Molinari et al., 1997). Still, further investigation is needed to clarify the unique contribution of these neural substrates to object naming deficits following stroke.
4.3. Methodological consideration
Our study provides additional evidence for the use of the clinically acquired behavioural and structural imaging data in voxel-based correlation analysis (refer also to Chechlacz et al., 2012). Specifically, the behavioural data were derived from a large-scale clinical trial of cognitive testing and the CT scans were collected from clinics and hospitals as part of the standard everyday medical practice. Despite being the preferred modality in clinical stroke units (e.g. Karnath et al. (2004) reported that CT was used for 72 out of 140 stroke patients at admission), CT images are not usually used in statistical anatomical research. Only recently the first high-resolution CT template (to aid normalisation of the images) was published (Rorden et al., 2012) and image-processing algorithms were improved to make statistical analysis of large CT datasets more feasible (Gillebert et al., 2014). This article reported the use of CT scans in automated lesion–symptom mapping to answer a psycholinguistic question and the findings accord with past studies based on other high resolution structural scans.
CT scanning measures tissue density and provides meaningful biological signals, making them relatively easy for comparison across scanner sites. However, CT scans, similar to T1- and T2-weighted MR images, fail to detect cortical dysfunction arising from inadequate cortical perfusion within a region that is structurally intact. Abnormal reduction in perfusion may contribute to cognitive deficits (for example see Hillis et al., 2000; Karnath et al., 2005; Ticini et al., 2010). Besides, it has been shown that lesions caused by ischemic stroke may not be immediately identified when CT scans are acquired too early (Wardlaw and Farrall, 2004), especially within the first 24 h post-stroke (Mohr et al., 1995). The current study, therefore, excluded scans that were taken less than a day after a stroke. As tissue loss continues for a few weeks to several months after stroke, signals arising from lesioned tissues may vary with time. To control for that, we added the interval between the stroke and the scan acquisition as a covariate measure.
The use of clinical data in general also has a few shortfalls. Behavioural and imaging measurements may not be as accurate as those acquired in a laboratory environment. This is mostly because under the time pressure in clinical settings measurements are designed to capture the most essential diagnostic information at the expense of the reliability gained through trial repetition. However, the advantage we have had here is a much larger sample size compared to a typical lesion–symptom mapping study, as well as the reduced burden imposed on patients wishing to contribute to research. After all, we believe that this represents a reasonable trade-off (for more information about this clinical trial, see also Bickerton et al., 2014). Moreover, in many past studies patients were pre-selected based on specific lesion locations (e.g. Baldo et al., 2013; DeLeon et al., 2007; looking at left hemispheric damage only) or cognitive impairment (e.g. Mesulam et al., 2009; Schwartz et al., 2009; focusing only on specific aphasic patients). Here, we used minimal exclusion criteria, meaning that our results can be generalised to a broad population of patients with sub-acute stroke at large. As another point to note, the four tests included in our comparison all require at least visual and phonological (/motor) processing and both types of processing were captured by the shared PCA component. This restricted the ability to make interpretation on the cognitive processes underlying object naming.
The one-to-one lesion–symptom mapping correlational approach used in VBM is another potential limitation. Object naming is a complex cognitive task and damage to any parts of the cortical network sustaining the underlying cognitive processes would lead to deficits at object naming. The fact that lesions are usually sampled unevenly across the brain in stroke patients (Ng et al., 2007) may be a limitation for mass-univariate (voxel-wise) analysis since this is likely to reduce the statistical power of identifying brain–function (lesion–deficit) relationship contributed by less frequent lesions (see also Chechlacz et al., 2013). Yet, it is worth mentioning that the lesion coverage of our patients encompassed the entire brain.
As a final note, the current study used parametric analysis, with both the brain signal and the behavioural measures represented as continuous variables. Compared to having lesions manually delineated by the researchers or any human staff, the brain tissue density was automatically assessed using a unified-segmentation algorithm. This procedure has several advantages. First, it is user independent and hence replicable. Also, it is blind to the cause of tissue reduction and would note any abnormal tissue change. In other words, results may not be attributed solely to ‘lesion’ per se but there could be other conditions causing neural abnormality. Be that as it may, we argue that overlooking tissue loss that is not primarily caused by a stroke insult (it would be the case in manual lesion delineation procedures) may result in misinformed function–lesion mapping because any abnormal tissue change, whatever the cause, can impair cognitive functioning.
4.4. Implications of the study
The clinical and scientific values of the present study are threefold. Firstly, we tested the relevance between object naming and other common spoken language abilities because in many bedside neuropsychological assessments object naming is often tested to indicate retained language function (e.g. in MoCa, MMSE). Our results suggest that deficit at object naming in the majority of patients can be a good predictor for more general language impairment, which is evidenced by the great extent of the shared lesions contributing to a ‘shared’ language component in a typical clinical population. Secondly, our study provides evidence for the possibility of using data collected primarily for everyday clinical assessment to address a scientific question in the psycholinguistic context. Particularly, clinical CT scans were analysed with the use of the most up-to-date statistical tools that were originally developed for handling high resolution imaging data. As discussed above, our findings correspond with the past literature based on other neuroscience techniques. Finally, to date there are various treatment approaches (and their variations) for naming impairment, or aphasia at large, including explicitly training individuals in whole word naming (with or without provision of particular cues) (for review see Nickels, 2002). Another approach to the problem specifically directs at the level of phonologic processor through training in phoneme production and comprehension of phonological sequence knowledge (Kendall et al., 2008). Our data posit that naming deficits are very likely to occur with more generic language impairment in converting visual inputs to speech production. As a result, training tapping into more general language–cognitive processes such as phoneme production and/or visual form recognition may be more beneficial.
4.5. Conclusions
The current study used VBM in a large sample of sub-acute stroke patients to determine the common and dissociable neural substrates of object naming in relation to various language tasks that require visually-driven speech production. We showed a distinction between a large neural network commonly engaged across various language tasks (within the left temporal cortex and its surrounding areas) and a number of potentially specific brain regions (particularly the bilateral anterior temporal lobes) required to support object naming. These findings are in line with the hypothesis that object naming relies on a left-lateralised language dominant system that interacts closely with a bi-anterior temporal network. Beyond this, our work also highlights the value of examining patient performance in object naming in relation to other language tasks, as is done by screens such as the BCoS which provide an overall profile of cognition in patients.
Acknowledgements
This work was supported by the Stroke Association (TSA 2010/2) and the Oxford NIHR Cognitive Health Clinical Research Facility (RP-DG-0610-10046). The authors are grateful to all the participants who took part in the research and the UK West Midlands Stroke Research Network for their generous support and advice in patient recruitment. We thank the support of the local principal investigators and collaborators who facilitated data collection in the respective research sites and also Dr Magdelena Chechlacz of University of Oxford for providing statistical advice and assistance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.01.015.
Appendix A. Supplementary data
Supplementary methods
References
- Ackermann H., Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct. Funct. 2010;214(5–6):419–433. doi: 10.1007/s00429-010-0257-x. 20512374 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Why voxel-based morphometry should be used. NeuroImage. 2001;14(6):1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Spatial normalization using basis functions. Hum. Brain Funct. 2003;2:1–26. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. 15955494 [DOI] [PubMed] [Google Scholar]
- Baldo J.V., Arévalo A., Patterson J.P., Dronkers N.F. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex. 2013;49(3):658–667. doi: 10.1016/j.cortex.2012.03.001. 22482693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M., Kassam K.S., Ghuman A.S., Boshyan J., Schmid A.M., Schmidt A.M., Dale A.M., Hämäläinen M.S., Marinkovic K., Schacter D.L., Rosen B.R., Halgren E. Top-down facilitation of visual recognition. Proc. Natl. Acad. Sci. U. S. A. 2006;103(2):449–454. doi: 10.1073/pnas.0507062103. 16407167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M., Tootell R.B., Schacter D.L., Greve D.N., Fischl B., Mendola J.D., Rosen B.R., Dale A.M. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29(2):529–535. doi: 10.1016/s0896-6273(01)00224-0. 11239441 [DOI] [PubMed] [Google Scholar]
- Bayles K.A., Tomoeda C.K. Confrontation naming impairment in dementia. Brain Lang. 1983;19(1):98–114. doi: 10.1016/0093-934x(83)90057-3. 6222782 [DOI] [PubMed] [Google Scholar]
- Bell B.D., Hermann B.P., Woodard A.R., Jones J.E., Rutecki P.A., Sheth R., Dow C.C., Seidenberg M. Object naming and semantic knowledge in temporal lobe epilepsy. Neuropsychol. 2001;15(4):434–443. doi: 10.1037//0894-4105.15.4.434. 11761032 [DOI] [PubMed] [Google Scholar]
- Bickerton W., Demeyere N., Francis D., Kumar V., Remoundou M., Balani A., Harris L., Williamson J., Lau J.K., Samson D., Riddoch M.J., Humphreys G.W. The BCoS cognitive profile screen: utility and predictive value for stroke. Neuropsychology. 2014 doi: 10.1037/neu0000160. [DOI] [PubMed] [Google Scholar]
- Bookheimer S.Y., Zeffiro T.A., Blaxton T., Gaillard W., Theodore W. Regional cerebral blood flow during object naming and word reading. Hum. Brain Mapp. 1995;3(2):93–106. [Google Scholar]
- Bonelli S.B., Powell R., Thompson P.J., Yogarajah M., Focke N.K., Stretton J. Hippocampal activation correlates with visual confrontation naming: fMRI findings in controls and patients with temporal lobe epilepsy. Epilepsy Research. 2011;95(3):246–254. doi: 10.1016/j.eplepsyres.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati S.M., Myers D., Wilson A., Rankin K.P., Allison S.C., Rosen H.J., Miller B.L., Gorno-Tempini M.L. The anatomy of category-specific object naming in neurodegenerative diseases. J. Cogn. Neurosci. 2006;18(10):1644–1653. doi: 10.1162/jocn.2006.18.10.1644. 17014369 [DOI] [PubMed] [Google Scholar]
- Bright P., Moss H.E., Stamatakis E.A., Tyler L.K. Longitudinal studies of semantic dementia: the relationship between structural and functional changes over time. Neuropsychologia. 2008;46(8):2177–2188. doi: 10.1016/j.neuropsychologia.2008.02.019. 18395761 [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siege de la faculte du langage articule suivies d'une observation d'aphemie. Bull. Soc. Anat. 1861;6:330–357. [Google Scholar]
- Butler R.A., Lambon Ralph M.A., Woollams A.M. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. Brain. 2014;137(12):3248–3266. doi: 10.1093/brain/awu286. 25348632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D. Neurolinguistics and Linguistic Aphasiology: An Introduction, Cambridge Studies in Speech Science and Communication. Cambridge University Press; Cambridge: 1987. [Google Scholar]
- Chechlacz M., Rotshtein P., Demeyere N., Bickerton W.L., Humphreys G.W. The frequency and severity of extinction after stroke affecting different vascular territories. Neuropsychologia. 2014;54:11–17. doi: 10.1016/j.neuropsychologia.2013.12.016. 24378715 [DOI] [PubMed] [Google Scholar]
- Chechlacz M., Rotshtein P., Humphreys G.W. Neuronal substrates of Corsi Block span: lesion symptom mapping analyses in relation to attentional competition and spatial bias. Neuropsychologia. 2014;64C:240–251. doi: 10.1016/j.neuropsychologia.2014.09.038. 25281309 [DOI] [PubMed] [Google Scholar]
- Chechlacz M., Rotshtein P., Roberts K.L., Bickerton W.-L., Lau J.K., Humphreys G.W. The prognosis of allocentric and egocentric neglect: evidence from clinical scans. PLOS One. 2012;7(11):e47821. doi: 10.1371/journal.pone.0047821. 23133604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechlacz M., Terry A., Demeyere N., Douis H., Bickerton W.L., Rotshtein P., Humphreys G.W. Common and distinct neural mechanisms of visual and tactile extinction: a large scale VBM study in sub-acute stroke. Neuroimage Clin. 2013;2:291–302. doi: 10.1016/j.nicl.2013.01.013. 24179784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M., Rastle K., Perry C., Langdon R., Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol. Rev. 2001;108(1):204–256. doi: 10.1037/0033-295x.108.1.204. 11212628 [DOI] [PubMed] [Google Scholar]
- Comrey A.L., Lee H.B. A First Course in Factor Analysis. second edition. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- Conroy P., Sage K., Lambon Ralph M. Improved vocabulary production after naming therapy in aphasia: can gains in picture naming generalize to connected speech? Int. J. Lang. Commun. Disord. 2009;44(6):1036–1062. doi: 10.1080/13682820802585975. 19294554 [DOI] [PubMed] [Google Scholar]
- Corina D.P., Loudermilk B.C., Detwiler L., Martin R.F., Brinkley J.F., Ojemann G. Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain Lang. 2010;115(2):101–112. doi: 10.1016/j.bandl.2010.04.001. 20452661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H., Grabowski T.J., Tranel D., Hichwa R.D., Damasio A.R. A neural basis for lexical retrieval. Nature. 1996;380(6574):499–505. doi: 10.1038/380499a0. 8606767 [DOI] [PubMed] [Google Scholar]
- Damasio H., Tranel D., Grabowski T., Adolphs R., Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. 15037130 [DOI] [PubMed] [Google Scholar]
- Dehaene S., Le Clec'H G., Poline J.-B., Le Bihan D., Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13(3):321–325. doi: 10.1097/00001756-200203040-00015. 11930131 [DOI] [PubMed] [Google Scholar]
- DeLeon J., Gottesman R.F., Kleinman J.T., Newhart M., Davis C., Heidler-Gary J., Lee A., Hillis A.E. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130(5):1408–1422. doi: 10.1093/brain/awm011. 17337482 [DOI] [PubMed] [Google Scholar]
- Desmond J.E., Fiez J.A. Neuroimaging studies of the cerebellum: language, learning and memory. Trends Cogn. Sci. 1998;2(9):355–362. doi: 10.1016/s1364-6613(98)01211-x. 21227232 [DOI] [PubMed] [Google Scholar]
- Dien J. A tale of two recognition systems: implications of the fusiform face area and the visual word form area for lateralized object recognition models. Neuropsychologia. 2009;47(1):1–16. doi: 10.1016/j.neuropsychologia.2008.08.024. 18805434 [DOI] [PubMed] [Google Scholar]
- Domoto-Reilly K., Sapolsky D., Brickhouse M., Dickerson B.C., Alzheimer's Disease Neuroimaging Initiative Naming impairment in Alzheimer's disease is associated with left anterior temporal lobe atrophy. Neuroimage. 2012;63(1):348–355. doi: 10.1016/j.neuroimage.2012.06.018. 22728617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers N.F. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. 8906789 [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply. second edition. Springer; Wien: 1999. [Google Scholar]
- Garn C.L., Allen M.D., Larsen J.D. An fMRI study of sex differences in brain activation during object naming. Cortex. 2009;45(5):610–618. doi: 10.1016/j.cortex.2008.02.004. 18639870 [DOI] [PubMed] [Google Scholar]
- Garrido L., Furl N., Draganski B., Weiskopf N., Stevens J., Tan G.C., Driver J., Dolan R.J., Duchaine B. Voxel-based morphometry reveals reduced grey matter volume in the temporal cortex of developmental prosopagnosics. Brain. 2009;132(12):3443–3455. doi: 10.1093/brain/awp271. 19887506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillebert C.R., Humphreys G.W., Mantini D. Automated delineation of stroke lesions using brain CT images. Neuroimage Clin. 2014;4:540–548. doi: 10.1016/j.nicl.2014.03.009. 24818079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough P.M., Nobre A.C., Devlin J.T. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J. Neurosci. 2005;25(35):8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. 16135758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande M., Meffert E., Schoenberger E., Jung S., Frauenrath T., Huber W., Hussmann K., Moormann M., Heim S. From a concept to a word in a syntactically complete sentence: an fMRI study on spontaneous language production in an overt picture description task. Neuroimage. 2012;61(3):702–714. doi: 10.1016/j.neuroimage.2012.03.087. 22504766 [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Kourtzi Z., Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41(10–11):1409–1422. doi: 10.1016/s0042-6989(01)00073-6. 11322983 [DOI] [PubMed] [Google Scholar]
- Hatcher L. A Step-by-step Approach to Using the SAS System for Factor Analysis and Structural Equation Modeling. SAS Institute Inc.; Cary, NC: 1994. [Google Scholar]
- Herbster A.N., Mintun M.A., Nebes R.D., Becker J.T. Regional cerebral blood flow during word and nonword reading. Hum. Brain Mapp. 1997;5(2):84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. 10096413 [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. 17431404 [DOI] [PubMed] [Google Scholar]
- Hillis A.E., Barker P.B., Beauchamp N.J., Gordon B., Wityk R.J. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology. 2000;55(6):782–788. doi: 10.1212/wnl.55.6.782. 10993996 [DOI] [PubMed] [Google Scholar]
- Hillis A.E., Kleinman J.T., Newhart M., Heidler-Gary J., Gottesman R., Barker P.B., Aldrich E., Llinas R., Wityk R., Chaudhry P. Restoring cerebral blood flow reveals neural regions critical for naming. J. Neurosci. 2006;26(31):8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. 16885220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A.E., Wityk R.J., Tuffiash E., Beauchamp N.J., Jacobs M.A., Barker P.B., Selnes O.A. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann. Neurol. 2001;50(5):561–566. doi: 10.1002/ana.1265. 11706960 [DOI] [PubMed] [Google Scholar]
- Hodges J.R., Bozeat S., Lambon Ralph M.A., Patterson K., Spatt J. The role of conceptual knowledge in object use evidence from semantic dementia. Brain. 2000;123(9):1913–1925. doi: 10.1093/brain/123.9.1913. 10960055 [DOI] [PubMed] [Google Scholar]
- Hodges J.R., Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6(11):1004–1014. doi: 10.1016/S1474-4422(07)70266-1. 17945154 [DOI] [PubMed] [Google Scholar]
- Holdstock J.S., Mayes A.R., Isaac C.L., Gong Q., Roberts N. Differential involvement of the hippocampus and temporal lobe cortices in rapid and slow learning of new semantic information. Neuropsychologia. 2002;40(7):748–768. doi: 10.1016/s0028-3932(01)00192-0. 11900726 [DOI] [PubMed] [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S., Calabresi P.A., Pekar J.J., Van Zijl P.C., Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. 17931890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G.W., Bickerton W.-L., Samson D., Riddoch M.J. BCoS Cognitive Screen. Psychology Press; London, UK: 2012. [Google Scholar]
- Humphreys G.W., Price C.J., Riddoch M.J. From objects to names: a cognitive neuroscience approach. Psychol. Res. 1999;62(2–3):118–130. doi: 10.1007/s004260050046. 10472198 [DOI] [PubMed] [Google Scholar]
- Jefferies E., Lambon Ralph M.A. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129(8):2132–2147. doi: 10.1093/brain/awl153. 16815878 [DOI] [PubMed] [Google Scholar]
- Kanwisher N., Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2006;361(1476):2109–2128. doi: 10.1098/rstb.2006.1934. 17118927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H.O., Fruhmann Berger M., Küker W., Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb. Cortex. 2004;14(10):1164–1172. doi: 10.1093/cercor/bhh076. 15142954 [DOI] [PubMed] [Google Scholar]
- Karnath H.O., Zopf R., Johannsen L., Fruhmann Berger M., Nägele T., Klose U. Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain. 2005;128(10):2462–2469. doi: 10.1093/brain/awh629. 16150848 [DOI] [PubMed] [Google Scholar]
- Kendall D.L., Rosenbek J.C., Heilman K.M., Conway T., Klenberg K., Gonzalez Rothi L.J., Nadeau S.E. Phoneme-based rehabilitation of anomia in aphasia. Brain Lang. 2008;105(1):1–17. doi: 10.1016/j.bandl.2007.11.007. 18237773 [DOI] [PubMed] [Google Scholar]
- Kleist K. Gehirnpathologie. Johann Ambrosius Barth; Leipzig: 1934. [Google Scholar]
- Lambon Ralph M.A. Neurocognitive insights on conceptual knowledge and its breakdown. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2014;369(1634) doi: 10.1098/rstb.2012.0392. 20120392 24324236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Ehsan S., Baker G.A., Rogers T.T. Semantic memory is impaired in patients with unilateral anterior temporal lobe resection for temporal lobe epilepsy. Brain. 2012;135(1):242–258. doi: 10.1093/brain/awr325. 22287382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Lowe C., Rogers T.T. Neural basis of category-specific semantic deficits for living things: evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130(4):1127–1137. doi: 10.1093/brain/awm025. 17438021 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M.A., McClelland J.L., Patterson K., Galton C.J., Hodges J.R. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J. Cogn. Neurosci. 2001;13(3):341–356. doi: 10.1162/08989290151137395. 11371312 [DOI] [PubMed] [Google Scholar]
- Léger A., Démonet J.-F., Ruff S., Aithamon B., Touyeras B., Puel M., Boulanouar K., Cardebat D. Neural substrates of spoken language rehabilitation in an aphasic patient: an fMRI study. Neuroimage. 2002;17(1):174–183. doi: 10.1006/nimg.2002.1238. 12482075 [DOI] [PubMed] [Google Scholar]
- Levelt W.J., Roelofs A., Meyer A.S. A theory of lexical access in speech production. Behav. Brain Sci. 1999;22(1):1–38. doi: 10.1017/s0140525x99001776. 11301520 [DOI] [PubMed] [Google Scholar]
- Malach R., Levy I., Hasson U. The topography of high-order human object areas. Trends Cogn. Sci. 2002;6(4):176–184. doi: 10.1016/s1364-6613(02)01870-3. 11912041 [DOI] [PubMed] [Google Scholar]
- Manns J.R., Hopkins R.O., Squire L.R. Semantic memory and the human hippocampus. Neuron. 2003;38(1):127–133. doi: 10.1016/s0896-6273(03)00146-6. 12691670 [DOI] [PubMed] [Google Scholar]
- Mazzocchi F., Vignolo L.A. Localisation of lesions in aphasia: clinical-CT scan correlations in stroke patients. Cortex. 1979;15(4):627–653. doi: 10.1016/s0010-9452(79)80051-9. 95004 [DOI] [PubMed] [Google Scholar]
- McCandliss B.D., Cohen L., Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. 12860187 [DOI] [PubMed] [Google Scholar]
- McCarthy G., Puce A., Gore J.C., Allison T. Face-specific processing in the human fusiform gyrus. J. Cogn. Neurosci. 1997;9(5):605–610. doi: 10.1162/jocn.1997.9.5.605. 23965119 [DOI] [PubMed] [Google Scholar]
- Mesulam M., Rogalski E., Wieneke C., Cobia D., Rademaker A., Thompson C., Weintraub S. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain J. Neurol. 2009;132(9):2553–2565. doi: 10.1093/brain/awp138. 19506067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr J.P., Biller J., Hilal S.K., Yuh W.T., Tatemichi T.K., Hedges S., Tali E., Nguyen H., Mun I., Adams H.P. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke. 1995;26(5):807–812. doi: 10.1161/01.str.26.5.807. 7740571 [DOI] [PubMed] [Google Scholar]
- Mohr J.P., Pessin M.S., Finkelstein S., Funkenstein H.H., Duncan G.W., Davis K.R. Broca aphasia: pathologic and clinical. Neurol. 1978;28(4):311–324. doi: 10.1212/wnl.28.4.311. 565019 [DOI] [PubMed] [Google Scholar]
- Molinari M., Leggio M.G., Silveri M.C. Verbal fluency and agrammatism. Int. Rev. Neurobiol. 1997;41:325–339. doi: 10.1016/s0074-7742(08)60358-x. 9378596 [DOI] [PubMed] [Google Scholar]
- Moore C.J., Price C.J. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;10(2):181–192. doi: 10.1006/nimg.1999.0450. 10417250 [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., Nagae-Poetscher L.M., van Zijl P.C.M. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Ng Y.S., Stein J., Ning M., Black-Schaffer R.M. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38(8):2309–2314. doi: 10.1161/STROKEAHA.106.475483. 17615368 [DOI] [PubMed] [Google Scholar]
- Nickels L. Therapy for naming disorders: revisiting, revising, and reviewing. Aphasiology. 2002;16(10–11):935–979. [Google Scholar]
- Nøkleby K., Boland E., Bergersen H., Schanke A.-K., Farner L., Wagle J., Wyller T.B. Screening for cognitive deficits after stroke: a comparison of three screening tools. Clin. Rehabil. 2008;22(12):1095–1104. doi: 10.1177/0269215508094711. 19052248 [DOI] [PubMed] [Google Scholar]
- Noppeney U., Patterson K., Tyler L.K., Moss H., Stamatakis E.A., Bright P., Mummery C., Price C.J. Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain J. Neurol. 2007;130(4):1138–1147. doi: 10.1093/brain/awl344. 17251241 [DOI] [PubMed] [Google Scholar]
- Okada T., Tanaka S., Nakai T., Nishizawa S., Inui T., Sadato N., Yonekura Y., Konishi J. Naming of animals and tools: a functional magnetic resonance imaging study of categorical differences in the human brain areas commonly used for naming visually presented objects. Neurosci. Lett. 2000;296(1):33–36. doi: 10.1016/s0304-3940(00)01612-8. 11099827 [DOI] [PubMed] [Google Scholar]
- Patterson K., Nestor P.J., Rogers T.T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007;8(12):976–987. doi: 10.1038/nrn2277. 18026167 [DOI] [PubMed] [Google Scholar]
- Pobric G., Jefferies E., Ralph M.A. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc. Natl. Acad. Sci. U. S. A. 2007;104(50):20137–20141. doi: 10.1073/pnas.0707383104. 18056637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., Devlin J.T. The myth of the visual word form area. Neuroimage. 2003;19(3):473–481. doi: 10.1016/s1053-8119(03)00084-3. 12880781 [DOI] [PubMed] [Google Scholar]
- Price C.J., Devlin J.T., Moore C.J., Morton C., Laird A.R. Meta-analyses of object naming: effect of baseline. Hum. Brain Mapp. 2005;25(1):70–82. doi: 10.1002/hbm.20132. 15846820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., McCrory E., Noppeney U., Mechelli A., Moore C.J., Biggio N., Devlin J.T. How reading differs from object naming at the neuronal level. Neuroimage. 2006;29(2):643–648. doi: 10.1016/j.neuroimage.2005.07.044. 16137894 [DOI] [PubMed] [Google Scholar]
- Rauschecker J.P., Scott S.K. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat. Neurosci. 2009;12(6):718–724. doi: 10.1038/nn.2331. 19471271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.T., Hocking J., Noppeney U., Mechelli A., Gorno-Tempini M.L., Patterson K., Price C.J. Anterior temporal cortex and semantic memory: reconciling findings from neuropsychology and functional imaging. Cogn. Affect. Behav. Neurosci. 2006;6(3):201–213. doi: 10.3758/cabn.6.3.201. 17243356 [DOI] [PubMed] [Google Scholar]
- Rogers T.T., Lambon Ralph M.A., Garrard P., Bozeat S., McClelland J.L., Hodges J.R., Patterson K. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol. Rev. 2004;111(1):205–234. doi: 10.1037/0033-295X.111.1.205. 14756594 [DOI] [PubMed] [Google Scholar]
- Rorden C., Bonilha L., Fridriksson J., Bender B., Karnath H.O. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. 22440645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L., Cox C., Hayes S.M., Nadel L. Hippocampal activation during episodic and semantic memory retrieval: comparing category production and category cued recall. Neuropsychologia. 2008;46(8):2109–2121. doi: 10.1016/j.neuropsychologia.2008.02.030. 18420234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D., Kreher B.W., Schnell S., Kümmerer D., Kellmeyer P., Vry M.-S., Umarova R., Musso M., Glauche V., Abel S., Huber W., Rijntjes M., Hennig J., Weiller C. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. U. S. A. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. 19004769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawrie S.M., Martin R.C., Gilliam F.G., Faught R.E., Maton B., Hugg J.W., Bush N., Sinclair K., Kuzniecky R.I. Visual confrontation naming and hippocampal function: a neural network study using quantitative (1)H magnetic resonance spectroscopy. Brain. 2000;123(4):770–780. doi: 10.1093/brain/123.4.770. 10734008 [DOI] [PubMed] [Google Scholar]
- Schacter D.L., Alpert N.M., Savage C.R., Rauch S.L., Albert M.S. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc. Natl. Acad. Sci. U. S. A. 1996;93(1):321–325. doi: 10.1073/pnas.93.1.321. 8552630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro A.C., McClelland J.L., Welbourne S.R., Rogers T.T., Lambon Ralph M.A. Why bilateral damage is worse than unilateral damage to the brain. J. Cogn. Neurosci. 2013;25(12):2107–2123. doi: 10.1162/jocn_a_00441. 23806177 [DOI] [PubMed] [Google Scholar]
- Schwartz M.F., Kimberg D.Y., Walker G.M., Faseyitan O., Brecher A., Dell G.S., Coslett H.B. Anterior temporal involvement in semantic word retrieval: voxel-based lesion–symptom mapping evidence from aphasia. Brain. 2009;132(12):3411–3427. doi: 10.1093/brain/awp284. 19942676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Ramlackhansingh A., Crinion J., Leff A.P., Price C.J. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. Neuroimage. 2008;41(4):1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. 18482850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P., Dias E.C., Butler P.D., Revheim N., Guilfoyle D.N., Foxe J.J., Javitt D.C. Impaired visual object processing across an occipital–frontal–hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch. Gen. Psychiatry. 2010;67(8):772–782. doi: 10.1001/archgenpsychiatry.2010.85. 20679585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M., Griffith R., Sabsevitz D., Moran M., Haltiner A., Bell B., Swanson S., Hammeke T., Hermann B. Recognition and identification of famous faces in patients with unilateral temporal lobe epilepsy. Neuropsychologia. 2002;40(4):446–456. doi: 10.1016/s0028-3932(01)00096-3. 11684177 [DOI] [PubMed] [Google Scholar]
- Spitzer M., Kischka U., Gückel F., Bellemann M.E., Kammer T., Seyyedi S., Weisbrod M., Schwartz A., Brix G. Functional magnetic resonance imaging of category-specific cortical activation: evidence for semantic maps. Brain Res Cogn Brain Res. 1998;6(4):309–319. doi: 10.1016/s0926-6410(97)00020-7. 9593960 [DOI] [PubMed] [Google Scholar]
- Starrfelt R., Gerlach C. The visual what for area: words and pictures in the left fusiform gyrus. Neuroimage. 2007;35(1):334–342. doi: 10.1016/j.neuroimage.2006.12.003. 17239621 [DOI] [PubMed] [Google Scholar]
- Tatemichi T.K., Desmond D.W., Stern Y., Paik M., Sano M., Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J. Neurol. Neurosurg. Psychiatry. 1994;57(2):202–207. doi: 10.1136/jnnp.57.2.202. 8126506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticini L.F., de Haan B., Klose U., Nägele T., Karnath H.-O. The role of temporo-parietal cortex in subcortical visual extinction. J. Cogn. Neurosci. 2010;22(9):2141–2150. doi: 10.1162/jocn.2009.21315. 19642885 [DOI] [PubMed] [Google Scholar]
- Tranel D., Damasio H., Damasio A.R. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35(10):1319–1327. doi: 10.1016/s0028-3932(97)00085-7. 9347478 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. 11771995 [DOI] [PubMed] [Google Scholar]
- Ueno T., Lambon Ralph M.A. The roles of the “ventral” semantic and “dorsal” pathways in conduite d'approche: a neuroanatomically-constrained computational modeling investigation. Front. Hum. Neurosci. 2013;7:422. doi: 10.3389/fnhum.2013.00422. 23986670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T., Saito S., Rogers T.T., Lambon Ralph M.A. Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal–ventral language pathways. Neuron. 2011;72(2):385–396. doi: 10.1016/j.neuron.2011.09.013. 22017995 [DOI] [PubMed] [Google Scholar]
- Vannucci M., Dietl T., Pezer N., Viggiano M.P., Helmstaedter C., Schaller C., Elger C.E., Grunwald T. Hippocampal function and visual object processing in temporal lobe epilepsy. Neuroreport. 2003;14(11):1489–1492. doi: 10.1097/00001756-200308060-00017. 12960770 [DOI] [PubMed] [Google Scholar]
- Wardlaw J.M., Farrall A.J. Diagnosis of stroke on neuroimaging. BMJ. 2004;328(7441):655–656. doi: 10.1136/bmj.328.7441.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. MRC psycholinguistic database: machine-usable dictionary, version 2.00. Behav. Res. Methods Instrum. Comput. 1988;20(1):6–11. [Google Scholar]
- Woollams A.M. Apples are not the only fruit: the effects of concept typicality on semantic representation in the anterior temporal lobe. Front. Hum. Neurosci. 2012;6:85. doi: 10.3389/fnhum.2012.00085. 22529789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K.J., Friston K.J. Analysis of fMRI time-series revisited — again. Neuroimage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. 9343600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods