Abstract
Omega-3 polyunsaturated fatty acids (n-3 PUFA) have been implicated in the alleviation of asthma. Recent studies have demonstrated that the n-3 PUFA derived lipid mediators, protectin D1 and resolvin E1, may act as potent resolution agonists in airway inflammation. The effects of the n-3 PUFA tissue status itself on asthma pathogenesis remains to be further investigated.
In this study allergic airway inflammation induced by allergen sensitization and aerosol challenge in Fat-1 and wild-type (WT) mice was investigated. Fat-1 transgenic mice displayed increased endogenous lung n-3 PUFA. When allergen-sensitized and aerosol-challenged, these animals had decreased airway inflammation with decreased leukocyte accumulation in bronchoalveolar lavage fluid (BALF) and lung parenchyma. The Fat-1 mice had a shift to the right in the dose-response relationship for methacholine induced bronchoconstriction with a significant increase in the log ED200. The Fat-1 mice had lower BALF concentrations of the pro-inflammatory cytokines IL-1α, IL-2, IL-5, IL-9, IL- 13, G-CSF, KC and RANTES. Furthermore, increased lung tissue amounts of the counter-regulatory mediators protectin D1 and resolvin E1 were found in Fat-1 mice after bronchoprovocative challenge.
These results therefore demonstrate a direct protective role for lung n-3 PUFA in allergic airway responses and an increased generation of protectin D1 and resolvin E1 in this context.
Keywords: Omega-3, resolvins, protectins, inflammation, asthma, Fat-1 mice
1. Introduction
Asthma is a disorder of the airways characterized by airflow obstruction, bronchial hyperresponsiveness, and chronic inflammation accompanied by eosinophil and T-cell infiltration in the lungs [1]. The incidence and prevalence of asthma have been increasing in the last decades and there is no treatment currently available that can cure asthma.
Lipid mediators derived from omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids (PUFA) are important regulators of inflammation and may play key roles in the pathogenesis of asthma. Studies have indicated potent pro-phlogistic roles for the n-6 arachidonic acid (AA) derived lipid mediators in asthmatic airways. Pro-inflammatory cytokines and lipid mediators, such as 5-lipoxygenase derived leukotrienes correlate with asthma disease severity [2]; however, lipoxygenases do not only metabolize AA, but also metabolize the n-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) to generate bioactive mediators [3]. Protectins and Resolvins are pro-resolving mediators that are enzymatically derived from n-3 PUFA. Protectins and Resolvins of the D-series are formed from DHA, while resolvins of the E-series are derived from EPA [4]. Protectin D1 (PD1) and Resolvin E1 (RvE1) were previously shown to have potent anti-inflammatory and pro-resolving effects [5–7], including in the airway [8, 9]. PD1 is present in exhaled breath condensates of healthy and asthmatic individuals, and decreases in those suffering an acute asthma exacerbation [8].
Studies involving Greenland Inuit populations, who have a diet high in n-3 PUFA due to the intake of marine oils from cold water fish, have demonstrated an extremely low incidence of asthma [10]. Further epidemiological studies have confirmed that diets rich in n-3 PUFA are associated with a lower asthma prevalence [11]. In an even more direct line of evidence for the protective effect of n-3 PUFA in the context of airway inflammation, DHA was found to be protective in chronic airway inflammation when administered endobronchially [8, 12]. However, a recent meta-study failed to confirm a definitive protective effect of n-3 PUFA supplementation in asthma [13]. Inconsistent results in human nutritional studies may be due to confounding factors of diet or conversion of the parent n-6 and n-3 PUFA to multiple bioactive compounds with competing actions. A well-controlled model for increased n-3 PUFA tissue status, which would eliminate confounding factors of diet, would provide an experimental window onto the effects of increased tissue n-3 PUFA on asthmatic airway inflammation.
In this study, we used transgenic Fat-1 mice as a model of increased n-3 tissue status to test the effect of increased EPA and DHA tissue levels on the development of allergen-induced allergic airway inflammation. Fat-1 transgenic mice express a Caenorhabditis elegans desaturase (Fat-1 gene), leading to the endogenous formation of n-3 PUFA from n-6 PUFA. Due to this capability, Fat-1 mice have a higher tissue content of n-3 PUFA compared to WT littermates [14], and increased anti-inflammatory effects in models of mucosal organ injury, including acute lung injury, chemically induced colitis, hepatitis, and pancreatitis [15–18]. In colitis, endogenous formation of biologically relevant concentrations of resolvins and protectins were detected in the colons of Fat-1 mice rich in n-3 PUFA [16]. Here, the Fat-1 mouse model was used to directly assess the effects of n-3 PUFA on allergic airway responses in a mouse model of asthma.
2. Methods
2.1. Mice
Transgenic Fat-1 mice were created as described previously [14]. Heterozygous Fat-1 male mice were mated with wild type (WT) female mice to obtain offspring, with half being heterozygous Fat-1 mice and half being WT littermates. Animals were fed a special diet (10% corn oil) high in n-6 and low in n-3 fatty acids. Tail tissue fatty acid profiles were used for pre-experimental phenotyping of the animals.
2.2. Induction of allergic airway inflammation
For induction of allergic airway inflammation, 6–8 weeks old male Fat-1 and WT mice were injected intraperitoneally with 10 mg ovalbumin (Sigma, St. Louis, MO) plus 1 mg aluminium hydroxide as adjuvant in 0.2 ml normal saline on days 0 and 7 of the protocol, essentially as described previously [9].. On days 14, 15, 16 and 17, mice received aerosol challenges with 6% (wt/vol) OVA in PBS for 25 min per day. Mice were sacrificed on protocol day 18 to analyze lipid profiles of the lungs and the extent of airway inflammation.
2.3. Bronchoalveolar lavage preparation and cell counts
Bronchoalveolar lavage fluid (BALF) was collected using PBS with 0.6 mM EDTA (2 × 1 ml). The fluids and tissues collected were used for cytological and histological analysis as well as biochemical measurements. Cells were stained with a Wright-Giemsa stain (Sigma) to quantify leukocyte subsets and ≥ 200 cells were counted on each slide. As controls, two animals in each group with vehicle-only i. p. injection and PBS-only inhalation were added. The supernatants of BALF samples were analyzed for the presence of cytokines using a 23-panel of murine cytokine/chemokines in a Multiplex Suspension Array System (Bio-Rad, Hercules, CA).
2.4. Measurement of lung resistance
For measurement of airway hyperresponsiveness, anesthetized mice were mechanically ventilated with a flexiVent (SciReq, Chandler, AZ) and aerosolized methacholine (0, 1, 3, 10, 30 and 100 mg/ml) was delivered in-line through the inhalation port for 10 s. Airway resistance was determined as the mean of ten measurements obtained for each concentration of methacholine and is reported as the percent increase from baseline (PBS nebulization).
2.5. Analysis of polyunsaturated fatty acids and lipid mediators
Fatty acid profiles were analyzed by using gas chromatography as described previously using a fully automated HP6890 system equipped with a flame-ionization detector [19].. Peaks of resolved fatty acids were identified by comparison with fatty acid standards (NuChek Prep, Elysian, MN). Resolvin (Rv) E1 and protectin D1 (PD1) was analyzed by liquid chromatography–tandem mass spectrometry, essentially as described previously [15]. In short, the supernatant of a lung tissue homogenate in acetonitrile with d4-PGE2 as internal standard was dried under nitrogen, resuspended in 5 mM ammonium acetate in water, and injected into a Linear Ion Trap Quadrupole LC/MS/MS Mass Spectrometer.
2.6. Statistical Analysis
For data analysis Prism 5.00 software (GraphPad, San Diego, CA) was used. Comparison was made by using the Student t-test and one-way ANOVA. All values are presented as the mean ± SEM or as indicated. Level of significance was set at P <0.05.
3. Results
3.1. Expression of the Fat-1 transgene increases lung tissue n-3 PUFA
To determine the impact of Fat-1 expression on lung PUFA levels, lung tissue from Fat-1 and WT animals used in this study was extracted and analyzed by gas chromatography (GC). Analysis of lung fatty acid composition indicated that the amounts of the n-3 PUFA EPA (C20:5n-3) and DHA (C22:6n-3) in fat-1 heterozygote mice were significantly higher and levels of the n-6 PUFA AA trended to be lower in Fat-1 transgenic mice than in WT littermates (Fig. 1 and Table 1), leading to a marked decrease in the AA/(EPA+DHA) ratio from 6.3 to 1.6 (*** p < 0.001). These findings indicate that expression of Fat-1 effectively increased n-3 PUFA levels in lung tissue, resulting in a significant difference in lung fatty acid composition between Fat-1 and WT littermates despite the same diet for both groups.
Figure 1. Content of EPA and DHA is increased in the lung tissue of Fat-1 mice.
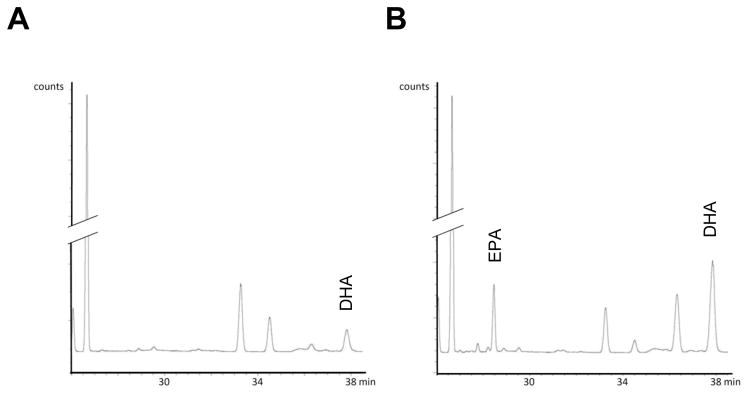
The n-3 PUFA content in lung tissue, as demonstrated by gas chromatography, was lower in WT mice (A) than in Fat-1 mice (B). The retention time for EPA and DHA are indicated. The chromatographs are representative of n = 11 in each cohort.
Table 1.
Content of EPA and DHA is increased in the lung tissue of Fat-1 mice.
| ng/mg | AA | EPA | DHA | AA/(EPA+DHA) |
|---|---|---|---|---|
| mean +/− SEM | ||||
| WT (n = 11) | 1287.4 +/− 191.8 | 5.2 +/− 5.2 | 197.7 +/− 33.2 | 6.3 |
| Fat-1 (n = 11) | 1074.4 +/− 79.3 | 146.7 +/− 29.9 | 520.4 +/− 54.8 | 1.6 |
| T-test | 0.317 | < 0.001 | < 0.0001 | |
3.2. Allergic airway Inflammation is decreased in Fat-1 transgenic mice
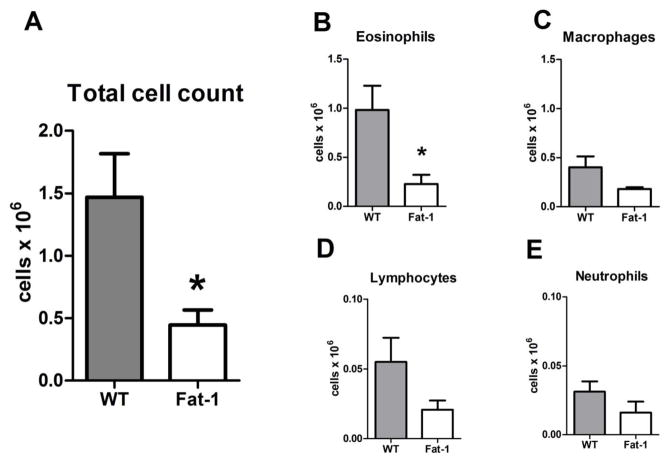
To determine the impact of endogenous n-3 PUFA on the development of allergic airway inflammation, Fat-1 transgenic mice and their littermate WT controls were systemically sensitized to OVA and then aerosol challenged for 25 min with 6% OVA on four consecutive days (see Methods). Twenty-four hours after the last allergen challenge, lungs were prepared for histopathological analyses. Samples from Fat-1 transgenic mice displayed markedly fewer leukocytes and the airway epithelium was less reactive than in WT animals that were also OVA sensitized and challenged (Fig. 2a, b). Periodic Acid-Schiff staining revealed decreased mucus metaplasia of airway epithelium in the Fat-1 compared to littermate WT control mice (Fig. 2c, d). In lieu of lung harvest for histology, some animals underwent bronchoalveolar lavage (BAL) 24h after the last allergen challenge. BAL fluids (BALF) from the Fat-1 mice revealed a marked decrease in total cells as compared to WT mice (n = 6 for Fat-1 as well as WT mice), and leukocyte differential counts in these samples demonstrated significant decrements in BALF eosinophils (9.8 +/− 2.5 × 105 in WT versus 2.3 +/− 0.9 × 105 in Fat-1, p < 0.05), the differences for lymphocytes (5.5 +/− 1.7 × 104 in WT versus 2.1 +/− 0.6 × 104 in Fat-1) and macrophages (4.0 +/− 1.1 × 105 in WT versus 1.8 +/− 0.2 × 105 in Fat-1) did not reach statistical significance at this sample size (Fig. 3). These results indicate that increased lung levels of n-3 PUFA were associated with a dramatic decrease in the development of allergic airway inflammation.
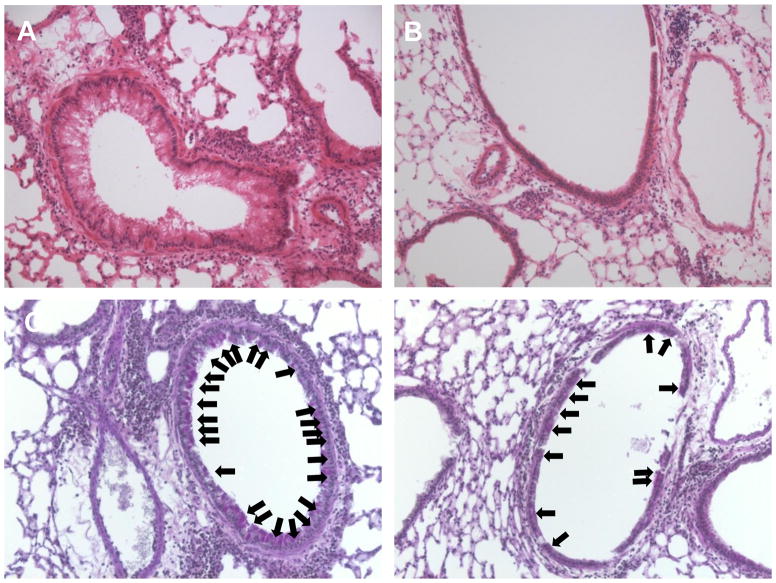
Figure 2. Decreased airway inflammation and mucus metaplasia in Fat-1 transgenic mice.
Lung tissue from WT (left) and Fat-1 (right) mice was obtained and prepared for histopathological analysis (see Methods). (A,B) Lung sections stained with H & E revealed decreased leukocyte infiltration and less reactive airway epithelium in the lungs of Fat-1 mice compared to WT littermate controls. (C,D) Periodic Acid-Schiff staining revealed decreased mucus (arrows) in the airway epithelium in Fat-1 mice as compared to WT controls. Images are representative for n = 3 in each cohort.
Figure 3. Fat-1 mice exhibit decreased airway WBC in allergic inflammation.
Bronchoalveolar lavage was performed on OVA sensitized animals 24 h after the last of 4 daily OVA aerosol challenges (see Methods).. (A) Total BALF cells were enumerated and WBC differential counts were performed manually after Cytospin and Wright-Giemsa stain (B–E). (n=6 for each cohort), * p < 0.05.
3.3. Fat-1 mice are protected from methacholine-induced airway hyperresponsiveness
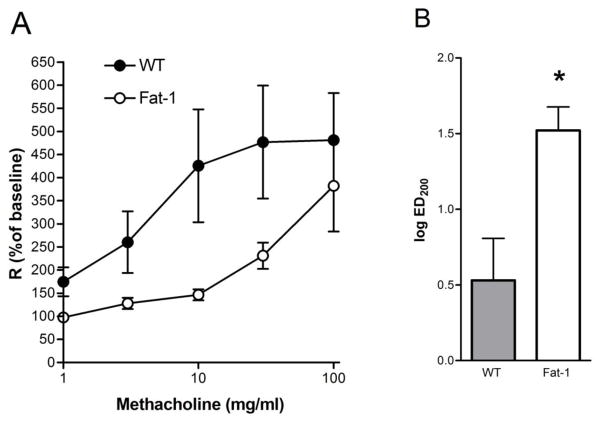
In addition to airway inflammation, airway hyper-responsiveness (AHR) is another diagnostic hallmark of asthma. To determine the impact of n-3 PUFA on AHR, animals were anesthetized, mechanically ventilated and bronchoprovocation challenge was performed (flexiVent, SciREQ) with inhaled methacholine at escalating doses from 1 to 100 mg/ml. Similar to the changes in airway inflammation, Fat-1 mice displayed a significant dampening of methacholine-induced increases in lung mean resistance (R) with a shift in the dose response relationship to the right (Fig. 4a). The effective dose of methacholine leading to a 200% increase in lung mean resistance (ED200) was significantly increased in Fat-1 transgenic mice (Fig. 4b).
Figure 4. Methacholine-induced airway hyperresponsiveness is decreased in fat-1 mice.
(A) Airway hyper-responsiveness to methacholine was determined using amouse ventilator with in-line nebulizer (see Methods). (A) Mean lung resistance (R) was measured at baseline and after each dose of methacholine. R was significantly decreased in Fat-1 relative to WT mice. (B) The effective dose of methacholine needed to increase mean lung resistance by 200% from baseline (ED200) was significantly higher for Fat-1 compared to WT mice. Values represent the mean +/− SEM for n = 6 WT and n = 5 Fat-1 mice. * p < 0.05.
3.4. BALF cytokine and chemokine levels are decreased in Fat-1 mice
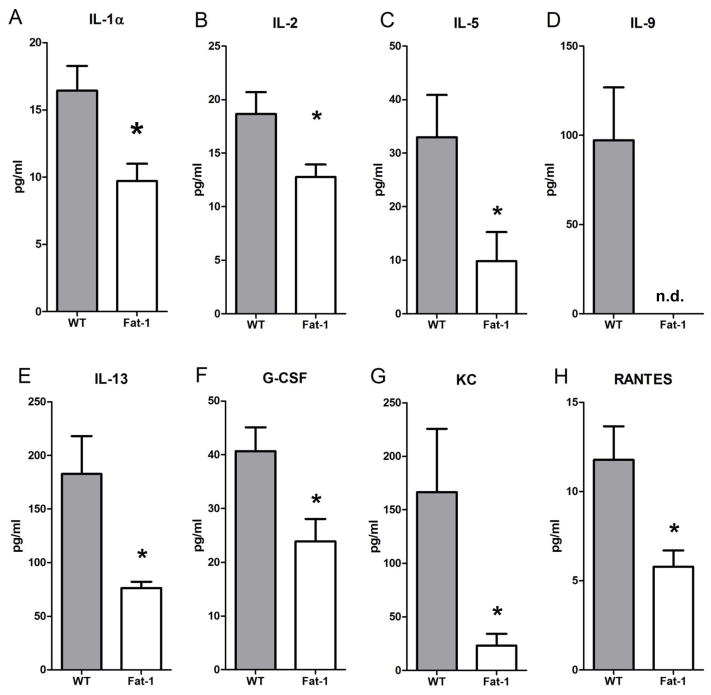
To determine mechanisms for the observed effect on allergic airway responses in Fat-1 mice, BALF aliquots were analyzed using a protein bead array for cytokines and chemokines. There were significant decrements in the levels of several pro-inflammatory mediators in Fat-1 mice, including IL-1α, IL-2, IL-5, IL-13, G-CSF, KC (CXCL1) and RANTES (CCL5) (Fig. 5). In addition, there were concomitant decreases in IL-1β, IL-3, IL-10, IL-12 (p40), IL-17, INF-γ, MCP-1, MIP-1α, MIP-1β and TNF-α in the Fat-1 mice, but these trends did not reach statistical significance at this sample size (data not shown). Of interest, IL-9 was detectable only in BALF from WT animals but not in samples from Fat-1 mice (Fig. 5). None of the peptide mediators that were assayed were increased in the Fat-1 mice, but levels of IL-12(p70), IL-17 and GM-CSF were essentially unaltered.
Figure 5. Select cytokine and chemokine levels were lower in BALF from Fat-1 mice.
BALF was analyzed for cytokines and chemokines using a Multiplex Suspension Array System (Bio-Rad). Values represent the mean +/− SEM for n=5 WT and n=6 Fat-1 mice. * p < 0.05 n.d., not detected.
3.5. Lipid Mediator Profile
To determine the impact of the increased n-3 PUFA on RvE1 and PD1, lung tissue homogenates were subjected to solid phase extraction and then materials were analyzed by LC-MS/MS. Because of limited availability of fat-1 mice, murine lungs were harvested after bronchoprovocative testing with methacholine. There was a marked difference in lung tissue levels of both RvE1 and PD1 between Fat-1 and WT mice. Both RvE1 and PD1 were detectable in WT lung after methacholine challenge (Fig. 6).
Figure 6. Formation of n-3 PUFA derived lipid mediators in Fat-1 mice was increased.
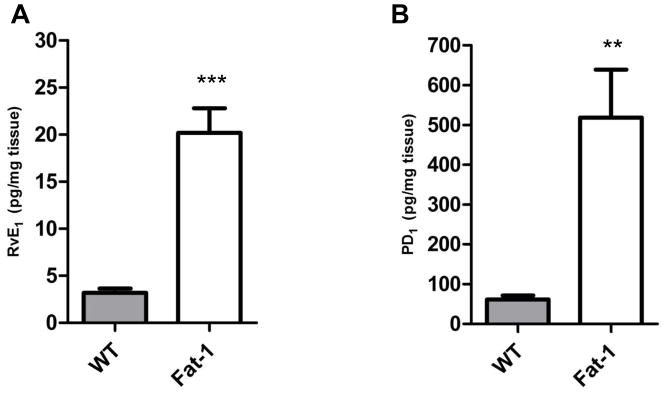
There were significant increases in the formation of resolvin E1 (RvE1, A) and protectin D1 (PD1, B) in lung tissue from methacholine treated animals (*** p < 0.001, ** p < 0.01).
4. Discussion
In the present study, we used the Fat-1 mouse model to investigate the effect of increased tissue status of n-3 PUFA on allergic airway responses. Our results demonstrate that endogenously increased lung n-3 PUFA decreases allergic airway inflammation with reduced leukocyte infiltration and inflammatory cytokine and chemokine levels, and also suppresses airway hyper-responsiveness to methacholine, which was associated with an increase in formation of the n-3 PUFA derived pro-resolving mediators RvE1 and PD1.
One key objective of this study was to address the potential impact of tissue status of n-6 and n-3 PUFA on allergic airway inflammation. We utilized a genetic approach instead of traditional dietary supplementation to modify the n-6 and n-3 PUFA content in lung tissue by expression of the Fat-1 gene, which led to endogenous conversion of n-6 to n-3 fatty acids. Use of this approach can not only significantly increase tissue n-3 content, but can also decrease n-6 content, resulting in a large difference in lung tissue n-6/n-3 fatty acid ratio between Fat-1 and WT mice. The tissue content of n-3 PUFA may be critical in airway inflammation as previous studies observed the presence of DHA in human airway mucosa and decreases in the setting of airway inflammation in asthma and cystic fibrosis [20].
n-3 PUFAs are important precursors for anti-inflammatory and pro-resolving mediators [4], so this study focused on RvE1 and PD1. These representative members of the n-3 PUFA-derived resolvins and protectins have potent anti-inflammatory and pro-resolution effects [7], in particular in the lung [8, 9]. Levels of RvE1 and PD1 were 6–8 times higher in the lungs of Fat-1 transgenic mice than those of WT littermates and in the pg to ng range. Systemic administration of 2 to 200 ng of PD1 or RvE1 is pharmacologically active in this model [8, 9], consistent with a potent regulatory activity for these amounts of RvE1 and PD1 in this model. PD1 and its biosynthetic precursor, 17-hydroxy-DHA, are present in murine lung and human exhaled breath condensates [8]. In inflamed murine lung, levels of RvE1 are below the limits of detection, but here RvE1 could be detected after methacholine challenge. These findings point to the rapid formation and metabolism of RvE1, a potent locally acting autacoid.
Given the importance of cytokines and chemokines in airway inflammation in asthma, their content and composition in the BAL fluids were analyzed. The results suggest that the increased n-3 PUFA preferentially modulate the Th2-cell response, as in Fat-1 mice there was a marked down-regulation of IL-5, IL-9, IL-13 and RANTES, which are chemokines and cytokines that are integral to Th2 driven immune responses [21–25]. IL-5 is an eosinophil-specific colony-stimulating factor [26] and decrements in this Th2 cytokine correlated well with our observations of lower eosinophil counts in Fat-1 mice compared to WT animals.
Our results provide evidence that airway inflammation and hyper-responsiveness can be reduced by changing tissue n-6 PUFA to n-3 PUFA, highlighting the importance of the n- 6/n-3 PUFA ratio in asthma. The increased incidence of asthma in Western societies can be attributed in part to the consumption of an n-6-rich pro-inflammatory diet. In a typical Western diet, the ratio of n-6 to n-3 PUFA is 20:1 or even higher [27]. On this basis, decreasing the tissue n-6/n-3 PUFA ratio by dietary means to both lower n-6 and increase n-3 PUFA would be expected to lessen the incidence and severity of asthma. The decreased inflammation observed with a lower n-6/n-3 PUFA ratio could be partially due to reduced n-6 PUFA-derived lipid mediator formation as a result of competitive inhibition or down-regulation of cyclooxogenase/lipoxygenase enzymes. However, the data presented here point to another important effect, the formation of n-3 PUFA derived anti-inflammatory and pro-resolving mediators.
Increased formation of n-3 PUFA derived anti-inflammatory and pro-resolving mediators by fat-1 mice also suggest the direct administration of these natural compounds, namely RvE1 and PD1, as a potential therapeutic intervention for asthma, given their potent activity in regulating allergic airway responses in model systems. Of interest, increased PUFA availability does not always translate into increased formation of protective mediators, as asthma exacerbations and severe asthma are associated with defects in PD1 and lipoxin A4 generation [8, 28].
In conclusion, increasing lung tissue levels of n-3 PUFA may provide a protective effect against asthma. One of the underlying mechanisms for this protection was an increase in enzymatic conversion of EPA and DHA to pro-resolving mediators, such as protectins and resolvins. These findings address some of the observed health benefits for diets rich in marine oils, and also point to potentially important roles for tissue n-3 PUFA and the n-6/n-3 PUFA ratio in asthma prevention and treatment.
Fat-1 mice had increased endogenous lung omega-3 polyunsaturated fatty acids
These mice showed decreased allergen-induced airway inflammation
This was associated with alleviated methacholine-induced bronchoconstriction
Lavage fluid concentrations of IL-1α, IL-2, IL-5, IL-9, IL- 13, G-CSF, KC and RANTES were decreased
Anti-inflammatory protectin D1 and resolvin E1 were increased in fat-1 lungs
Acknowledgments
This work was supported by the NIH (NIH R01 113605 to J.X.K. and AI068084 to B.D.L.), and a Boehringer Ingelheim Fonds Travel Grant to S.B..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402:B2–4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2007 doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 7.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208:401–406. [PubMed] [Google Scholar]
- 11.Schwartz J, Weiss ST. The relationship of dietary fish intake to level of pulmonary function in the first National Health and Nutrition Survey (NHANES I) Eur Respir J. 1994;7:1821–1824. doi: 10.1183/09031936.94.07101821. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama A, Hamazaki T, Ohshita A, Kohno N, Sakai K, Zhao GD, Katayama H, Hiwada K. Effect of aerosolized docosahexaenoic acid in a mouse model of atopic asthma. Int Arch Allergy Immunol. 2000;123:327–332. doi: 10.1159/000053645. [DOI] [PubMed] [Google Scholar]
- 13.Reisman J, Schachter HM, Dales RE, Tran K, Kourad K, Barnes D, Sampson M, Morrison A, Gaboury I, Blackman J. Treating asthma with omega-3 fatty acids: where is the evidence? A systematic review. BMC Complement Altern Med. 2006;6:26. doi: 10.1186/1472-6882-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 15.Mayer K, Kiessling A, Ott J, Schaefer MB, Hecker M, Henneke I, Schulz R, Gunther A, Wang J, Wu L, Roth J, Seeger W, Kang JX. Acute lung injury is reduced in fat-1 mice endogenously synthesizing n-3 fatty acids. Am J Respir Crit Care Med. 2009;179:474–483. doi: 10.1164/rccm.200807-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmocker C, Weylandt KH, Kahlke L, Wang J, Lobeck H, Tiegs G, Berg T, Kang JX. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology. 2007;45:864–869. doi: 10.1002/hep.21626. [DOI] [PubMed] [Google Scholar]
- 18.Weylandt KH, Nadolny A, Kahlke L, Kohnke T, Schmocker C, Wang J, Lauwers GY, Glickman JN, Kang JX. Reduction of inflammation and chronic tissue damage by omega-3 fatty acids in fat-1 transgenic mice with pancreatitis. Biochim Biophys Acta. 2008;1782:634–641. doi: 10.1016/j.bbadis.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem. 2005;6:5. doi: 10.1186/1471-2091-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O’Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 21.Izuhara K, Arima K, Kanaji S, Ohta S, Kanaji T. IL-13: a promising therapeutic target for bronchial asthma. Curr Med Chem. 2006;13:2291–2298. doi: 10.2174/092986706777935140. [DOI] [PubMed] [Google Scholar]
- 22.Lopez AF, Begley CG, Williamson DJ, Warren DJ, Vadas MA, Sanderson CJ. Murine eosinophil differentiation factor. An eosinophil-specific colony-stimulating factor with activity for human cells. J Exp Med. 1986;163:1085–1099. doi: 10.1084/jem.163.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori A, Ogawa K, Kajiyama Y, Suko M, Kaminuma O. Th2-cell-mediated chemokine synthesis is involved in allergic airway inflammation in mice. Int Arch Allergy Immunol. 2006;140(Suppl 1):55–58. doi: 10.1159/000092712. [DOI] [PubMed] [Google Scholar]
- 24.Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, Zhou T, Kleeberger SR, Buetow KH, Levitt RC. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci U S A. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechelle TL, O’Neill KR, Ansay TL, Colbert DC, Cormier SA, Justice JP, Lee NA, Lee JJ. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 26.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 27.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 28.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]