Abstract
This work was undertaken to obtain information on levels of metabolism in dormant spores of Bacillus species incubated for weeks at physiological temperatures. Spores of Bacillus megaterium and Bacillus subtilis strains were harvested shortly after release from sporangia and incubated under various conditions, and dormant spore metabolism was monitored by 31P nuclear magnetic resonance (NMR) analysis of molecules including 3-phosphoglyceric acid (3PGA) and ribonucleotides. Incubation for up to 30 days at 4, 37, or 50°C in water, at 37 or 50°C in buffer to raise the spore core pH from ∼ 6.3 to 7.8, or at 4°C in spent sporulation medium caused no significant changes in ribonucleotide or 3PGA levels. Stage I germinated spores of Bacillus megaterium that had slightly increased core water content and a core pH of 7.8 also did not degrade 3PGA and accumulated no ribonucleotides, including ATP, during incubation for 8 days at 37°C in buffered saline. In contrast, spores incubated for up to 30 days at 37 or 50°C in spent sporulation medium degraded significant amounts of 3PGA and accumulated ribonucleotides, indicative of RNA degradation, and these processes were increased in B. megaterium spores with a core pH of ∼7.8. However, no ATP was accumulated in these spores. These data indicate that spores of Bacillus species stored in water or buffer at low or high temperatures exhibited minimal, if any, metabolism of endogenous compounds, even when the spore core pH was 7.8 and core water content was increased somewhat. However, there was some metabolism in spores stored in spent sporulation medium.
INTRODUCTION
Spores of various Bacillus species are generally referred to as metabolically dormant as metabolism of these spores of both exogenous and endogenous compounds is extremely low (1, 2). However, there are reports that there is metabolic activity in these supposedly dormant spores, including oxidation of exogenous compounds such as glucose (3) and degradation of endogenous rRNA and even transcription, when B. subtilis spores are incubated for a number of days at physiological temperatures (4). In the latter two processes, this metabolism was suggested to take place shortly after spores were released from sporangia and to be important in adaptation of the spores to the environments in which they were released and incubated.
There have been relatively few detailed studies of metabolism of endogenous compounds in dormant spores although it is known that spores of both Bacillus and Clostridium species have minimal levels, if any, of normal high-energy compounds such as nucleoside triphosphates and reduced pyridine nucleotides (1). However, spores do have significant levels of ribonucleoside monophosphates, with AMP being the most abundant, as well as much smaller amounts of ADP. More importantly, spores have rather significant levels of 3-phosphoglyceric acid (3PGA), a potential rapid source of ATP, and spore 3PGA levels are generally significantly higher than those of AMP. Spores of Bacillus species also have significant levels of the enzymes needed for utilization of 3PGA to convert ADP to ATP, and the 3PGA depot in dormant Bacillus megaterium spores is sufficient to generate much of the ATP needed for even macromolecular synthesis in the first ∼10 min following initiation of spore germination (5). The enzyme that is regulated to allow 3PGA accumulation late in sporulation is phosphoglycerate mutase (PGM), and it appears likely that during sporulation there is a change in the pH of the developing spore core that results in loss of PGM activity and 3PGA accumulation (1, 5–7). However, the extremely low water content in the spore core (35 to 40% of wet weight) in Bacillus subtilis and Bacillus megaterium spores (8) is most likely responsible for maintaining the inactivity of PGM and other spore core enzymes during the potentially long periods of spore dormancy. Indeed, spore core proteins are relatively immobile in dormant spores (9), which would certainly be consistent with inactivity of enzymes in the spore core.
There have been statements in several publications that the 3PGA depot in spores is stable for long periods when spores are stored at 4°C (10–12) although no data have been obtained in which purified spores are incubated for long periods at temperatures such as 37°C that are appropriate for enzymatic activity of mesophiles. As a consequence, it could be argued that tests to date of metabolic activity of endogenous small molecules in spores have not been sufficiently rigorous to allow a definitive statement about the presence or absence of such metabolism in spores. However, prolonged incubation of spores in water or buffer at temperatures of ∼37°C is complicated by the fact that spores often germinate well at such temperatures and, in some cases, spontaneously, and in doing so excrete compounds such as amino acids and the 1:1 chelate of Ca2+ and dipicolinic acid (CaDPA) that can trigger other spores in populations to germinate. Since initiation of metabolism in spores begins soon after initiation of germination (1, 2, 5), having spores germinate can complicate analyses of spore metabolism of endogenous compounds. Although germinated spores can be separated from dormant spores by a variety of relatively simple procedures, it is not uncommon for the germination of a few spores in a moderately concentrated population to trigger the germination of the majority of the population, in particular, at elevated temperatures. However, a potentially simpler way to deal with this problem is to use spores that do not respond to external nutrient germinants because they lack the spore proteins that recognize and trigger germination in response to nutrient germinants (2). These proteins are termed germinant receptors (GRs), and molecular genetic manipulation has generated strains of B. megaterium and B. subtilis that lack all functional GRs (13, 14).
In this work we have primarily used GR-less spores to examine metabolism of endogenous compounds in Bacillus spores during extended incubation at 4, 37, and 50°C in either water or spent sporulation medium and analyzed levels of low-molecular-weight phosphorylated molecules in spores by 31P nuclear magnetic resonance (NMR). Spores of both B. megaterium and B. subtilis were used in this work because GR-less strains are available only for these two species, and data from two species would allow more general conclusions from the results. Throughout this work, metabolism was defined as both energy (i.e., ATP)-generating reactions and degradative reactions, such as nuclease or phosphatase action. We also analyzed low-molecular-weight phosphorylated metabolites in spores lacking the two redundant cortex-lytic enzymes, CwlJ and SleB, that had undergone only stage I of germination in which the spore core's large depot CaDPA has been released and replaced by water (15, 16). The spore core pH also rises from ∼6.3 in dormant spores to ∼7.8 in these stage I germinated spores (11, 16). However, subsequent stage II germination events, most notably the hydrolysis of the spore peptidoglycan cortex, do not take place in cwlJ sleB spores (15, 16). Consequently, while the spore core water content rises to ∼80% of wet weight upon completion of germination in stage II, in stage I the core water content rises from only 35 to 40% of wet weight in dormant B. megaterium spores to ∼55% of wet weight in germinated spores (15–17). However, even with these increases in core pH and water content, ATP levels in these germinated spores blocked prior to stage II are >40-fold lower than in spores immediately after completion of spore germination (15, 16). The effects on spore metabolism of the elevation of the spore core pH alone, achieved by incubating dormant GR-less B. megaterium spores in either water or spent sporulation medium plus a high-pH buffer and ammonium sulfate, conditions that have been shown to elevate spore core pH from ∼ 6.3 to 7.8, have also been examined (11).
MATERIALS AND METHODS
Bacillus strains used.
The B. megaterium strains used were QM B1551 (wild type) (originally obtained from H. S. Levinson) and two isogenic derivatives, GC614 (13), lacking genes encoding all functional GRs, and PS4241 (15), lacking the genes encoding the two redundant cortex-lytic enzymes (CLEs), CwlJ and SleB, that degrade spore cortex peptidoglycan during germination. The B. subtilis strains used were PS533 (wild type) (18), a prototrophic laboratory 168 strain that also carries plasmid pUB110 expressing kanamycin resistance, and an isogenic derivative, FB73 (14), that lacks plasmid pUB110 and genes for all functional GRs.
Spore preparation, purification, and extraction.
In initial experiments to mimic normal spore storage, B. megaterium cells were inoculated in ∼ 3 liters of well-aerated liquid supplemented nutrient broth (SNB) medium (19), and spores were harvested after ∼24 h at 30°C and washed at least twice with 4°C water. The final spores were free (>98%) of growing or sporulating cells and cell debris and contained >95% refractile forms when viewed in a phase-contrast microscope. The purified spores were suspended in 4°C water at an optical density at 600 nm (OD600) of 50, and multiple 12-ml aliquots were prepared. Two tubes were immediately used (labeled the zero time sample) to obtain small molecules using procedures described previously (5, 10, 12, 20–22), as follows. (i) To remove any growing cells or germinated spores, the spores were centrifuged through a high-density solution of 60% Histodenz (Sigma Chemical Co., St. Louis, MO) since under these conditions dormant spores pellet, and germinated spores, growing and sporulating cells, and cell debris float (23). (ii) The resulting dormant spore pellet (>98% dormant spores) was washed with cold water to remove Histodenz and suspended at an OD600 of 125. (iii) Two 1-ml aliquots of the Histodenz-purified spores were added to two loosely capped 20-ml glass tubes with 4 ml of boiling 1-propanol and boiled for 5 min followed by flash evaporation. Cold water (1 ml) was added to each dried residue, followed by vigorous vortexing, incubation on ice for 1 h with intermittent vortexing, and transfer to a microcentrifuge tube. The glass tube was rinsed with 1 ml of cold water, and the rinse was transferred to a microcentrifuge tube. The microcentrifuge tubes were centrifuged for 1 min at 14,000 × g; all supernatant fluids were pooled and applied to a 1.5-ml Chelex (Na form) column to remove divalent cations, especially paramagnetic Mn2+ as described previously (22), and the column was washed with 1 ml of water. This process was carried out in duplicate for samples taken after various times of incubation at 4, 37, or 50°C for 7 or 25 days.
Spores of B. megaterium and B. subtilis strains lacking GRs were also prepared by growth and sporulation in 2 liters of SNB medium at 30°C (B. megaterium) or 2× Schaeffer's glucose (2× SG) medium (19) at 37°C (B. subtilis) in a fermentor (BioFlo 110 modular benchtop fermentor; New Brunswick Scientific Company, Enfield, CT). Approximately 24 h after the fermentor was inoculated, >90% of spores had been formed and released from the sporangia. At this point the culture was divided into seven aliquots each with 285 ml of spent sporulation medium in a 1-liter flask, and two aliquots each were incubated with swirling at 4, 37, and 50°C. The culture in the remaining flask was harvested immediately by centrifugation (10 min at 6,000 × g); the pellet was washed by suspension in ∼100 ml of 4°C water, centrifuged, washed again by suspension with 10 ml of 4°C water, centrifuged, and then suspended in 4 ml of 20% Histodenz. Aliquots of the latter suspension (∼250 μl) were layered on 800 μl of 60% Histodenz (B. megaterium) or 50% Histodenz (B. subtilis) in 1.5-ml microcentrifuge tubes, and the tubes were centrifuged for 20 min at 4°C and 14,000 rpm in a microcentrifuge. Under these conditions, dormant spores pellet, and sporulating and growing cells, germinated spores, and large cell debris float. The supernatant fluid was discarded, and the dormant spore pellet was washed with water to remove Histodenz and suspended in ∼3 ml of water, and the OD600 of the suspension was measured. Flasks incubated for 10 or 30 days at various temperatures were harvested, and the spores were purified similarly. The spores (2 ml at an OD600 of 140; ∼35 mg of dry weight) were then extracted with boiling 1-propanol, and extracts were processed as described above. In these incubations, phase-contrast microscopy of samples incubated for 30 days at the various temperatures revealed minimal, if any, levels of germinated spores. The minimal germination and contamination of these cultures were also indicated by the small amounts of material that floated in the Histodenz solutions following centrifugation (data not shown).
In order to elevate the spore core pH during incubations, newly harvested B. megaterium GC614 spores were incubated in either water or spent sporulation medium as described above. However, the water or spent medium was made to 200 mM Tris-HCl (pH 8.8) and 20 mM (NH4)2SO4 to raise the spore core pH from ∼6.3 to ∼7.8 as described previously (11). The pH of the spent sporulation medium with these additions was measured as 8.6. After incubation at various temperatures for 0, 10, and 30 days, spores were harvested and purified, and small molecules were extracted and purified as described above.
Wild-type and GR-less B. subtilis spores were also prepared on 2× SG medium agar plates (∼60 plates) that were incubated at 37°C for ∼5 days and harvested and purified as described previously (19, 24). The spores were then incubated at 23°C for a few days and further purified, and small molecules were extracted and purified as described above.
Spore germination.
The cwlJ sleB B. megaterium PS4241 spores were harvested ∼24 h after fermenter inoculation as described above. Heat-activated (60°C; 15 min) PS4241 spores were germinated in 1.5 liters at an OD600 of 1 with 10 mM glucose in 25 mM KPO4 buffer (pH 7.4) for 2 h at 30°C. In these germinations, >90% of the spores released their CaDPA, as determined as described previously (25). However, since the redundant CLEs are absent in this strain, the spore cortex cannot be degraded, and these spores do not complete germination. Consequently, the water content of these partially germinated spores is intermediate between dormant and fully germinated spores, and these spores do not pellet in 60% Histodenz but float. Therefore, the spores were isolated as described above but as those spores that floated upon centrifugation in 50% Histodenz, thus removing any ungerminated spores. These purified germinated spores were incubated in 25 mM KPO4 buffer (pH 7.4)–0.15 M NaCl for 3 and 8 days at 4 and 37°C and then extracted, and extracts were processed as described above.
Quantitation of levels of small molecules.
The final dried samples equivalent to 1 ml of spores at an OD600 of 250 to 280 (31 or 35 mg of dry weight) were dissolved in 700 μl of NMR buffer containing 50 mM K-HEPES buffer (pH 7.4), 25 mM EDTA, 10% D2O, and 0.5 mM trimethyl phosphate or 0.25 mM dTMP (TMP) (Sigma); the latter two compounds served as internal standards. Preliminary experiments were done with trimethyl phosphate that had to be added after lyophilization of the Chelex flowthrough since trimethyl phosphate evaporates upon lyophilization. Subsequently, it was decided that it would be more appropriate to use an internal standard that could be added before running the Chelex column so as to better represent the conditions used for extraction and processing of endogenous small molecules; consequently, TMP was used as the internal standard in most experiments. As expected, 31P NMR peak intensities of various concentrations (0.1 to 5 mM) of trimethyl phosphate or TMP showed a linear response. 31P one-dimensional (1D) inverse-gated decoupling NMR experiments were collected on a Varian Inova 400 MHz NMR equipped with a 5-mm broadband probe with a 45° excitation pulse of 3.5 μs, a spectral width of 17.5 kHz, WALTZ (wideband alternating-phase low-power technique for zero-residual splitting) 1H decoupling with an effective radio frequency (RF) field strength of 5.58 kHz, a recycle delay of 5 s, and an acquisition time of 3.74 s, for a total relaxation delay between pulses of 8.74 s. Spectra were processed with 1 Hz line broadening, and the volumes of 31P NMR peaks were quantitated by integration using MestReNova software. Peak identities were verified by doping samples with 0.17 μmol of pure compounds. The 31P NMR peak given by GMP was extremely close to the invariably much larger AMP peak. Consequently, the GMP peak, while sometimes evident as a shoulder in the AMP peak, could not be quantitated accurately although its presence was clear in some samples (see Results). All 31P NMR analyses were carried out on duplicate aliquots for each time point analyzed, and all experiments were carried out in duplicate with similar results.
Dipicolinic acid (DPA) was extracted from Histodenz-purified spores by boiling in water and was assayed in extracts by its fluorescence with Tb3+ as described previously (25).
RESULTS
Levels of low-molecular-weight phosphorylated molecules in spores.
The major small molecules observed by 31P NMR of extracts of wild-type B. megaterium and B. subtilis spores were AMP, inorganic phosphate (Pi), and 3PGA, and levels of these molecules were similar to those found previously using other analytical techniques (Fig. 1; Table 1) (1). Similar levels of these compounds were also found in spores lacking GRs or CLEs, and 3PGA levels were significantly higher in B. megaterium spores than in spores of B. subtilis (Table 1), as found previously (10). Incubation of these spores in water for 7 days even at 4°C led to release of a significant amount of Pi which was found in the supernatant fluid, but no 3PGA or AMP was released (Table 1; also data not shown). Lack of release of 3PGA upon extended incubation of B. megaterium spores at 4°C has been reported previously (10, 11). The loss of some Pi from the spores of the two species suggests that this Pi was loosely bound in spores' outer layers, while 3PGA and AMP are all in the spore core. The location of AMP and 3PGA in the spore core has also been indicated by previous work (5, 10).
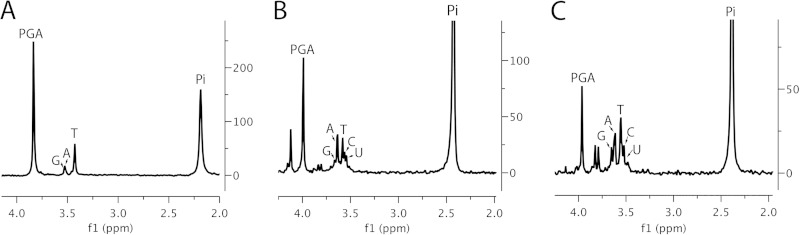
FIG 1.
31P NMR spectra of small molecules extracted from dormant wild-type spores of B. megaterium and B. subtilis. Spores of B. megaterium QM B1551 (wild type) (A) and B. subtilis PS533 (wild type) (B) were prepared in liquid medium (B. megaterium) or on solid medium (B. subtilis); spores were purified and extracted, extracts were processed, and small molecules were analyzed by 31P NMR as described in Materials and Methods. Peaks labeled 1, 2, 3, and 4 are 3PGA, AMP, TMP, and Pi, respectively.
TABLE 1.
Levels of low-molecular-weight phosphate-containing molecules in dormant spores of B. megaterium and B. subtilis strainsa
| Strain | Amt of molecule (μmol/g dry spores) |
||
|---|---|---|---|
| AMP | 3PGA | Pib | |
| B. megaterium strains | |||
| QM B1551 (wild type) | 2.1c | 24 | 4.5 (3.9) |
| GC614 (GR-less) | 2.3c | 25 | 23 (5.4) |
| PS4241 (cwlJ sleB) | 2.1c | 24 | 27 |
| B. subtilis strains | |||
| PS533 (wild type) | 0.7 | 2.8 | 2.3 (1.9) |
| FB73 (GR-less) | 0.8 | 2.9 | 2.4 (1.7) |
Values are for spores immediately after their isolation from (i) a fermentor (GR-less and cwlJ sleB strains), (ii) SNB liquid medium in shake flasks (wild-type B. megaterium), or (iii) 2× SG medium plates (wild-type or GR-less B. subtilis). Variations in values for 3PGA and AMP levels did not deviate by more than 13 and 18%, respectively, in B. megaterium spores and 17 and 31%, respectively, in B. subtilis spores.
Values for Pi levels in parentheses are after incubation for 7 days at 4°C. Levels of AMP and 3PGA changed minimally, if at all, after extended incubation at any temperature.
The 31P NMR peak for GMP is very close to that for AMP, making GMP levels difficult to quantitate. However, previous work (10) has shown that the GMP level in dormant B. megaterium spores is only 15% of the AMP level, so only 0.3 to 0.4 μmol of the AMP level is due to GMP. The level of CMP plus UMP in dormant B. megaterium spores is only ∼40% of the AMP level (10).
With wild-type B. subtilis spores, incubation in water for 2 days at temperatures from 4 to 50°C led to some (10 to 15%) spore germination (data not shown), but no notable changes in levels of 3PGA, AMP, or other ribonucleotides (Table 2; also data not shown). However, longer incubation of wild-type B. megaterium or B. subtilis spores in water at 37°C or 50°C resulted in significant germination; as with B. megaterium spores, >70% of the spores had germinated in a week, with only slightly less germination with wild-type B. subtilis spores (data not shown).
TABLE 2.
AMP and 3PGA levels in wild-type B. subtilis spores incubated for 2 days at various temperaturesa
| Incubation temp (°C) | Amt of molecule (μmol/g dry spores) |
|
|---|---|---|
| AMP | 3PGA | |
| 4 | 0.7 | 2.7 |
| 37 | 0.7 | 2.6 |
| 50 | 0.8 | 2.7 |
Spores of B. subtilis strain PS533 (wild type) were prepared on 2× SG plates at 37°C and incubated in water at various temperatures for 2 days. Spores were harvested and purified, and AMP and 3PGA were extracted, purified, and quantitated as described in Materials and Methods. Variations in values for AMP and 3PGA did not deviate by more than 25 and 15%, respectively.
Use of spores of various strains for extended incubation at 37°C and 50°C.
The results given above indicated that it would be difficult to do extended incubation of wild-type spores at 37 or 50°C in water without significant spore germination. While it was possible that incubating spores at lower concentrations reduced the amounts of germination by lowering the concentrations of germinants released by germinating spores, we decided to prevent nutrient germination by using spores of strains that lack genes for all functional GRs. Indeed, previous work has shown that GR-less spores of B. megaterium and B. subtilis exhibit extremely low rates of germination even with mixtures of normally good nutrient germinants (13, 14). We anticipated that using GR-less spores would greatly decrease spore germination during extended incubation of spores at elevated temperatures, and the absence of GRs would not affect the spore response to CaDPA. This molecule triggers spore germination by activating the CLE CwlJ (2, 26). While CaDPA released from a few spores that spontaneously germinate could cause germination of GR-less spores, CaDPA is effective only at rather high concentrations (14, 26, 27). Thus, we felt that using GR-less spores alone would reduce the germination of spores incubated at 37 and 50°C sufficiently such that more than enough dormant spores would remain after extended incubation to allow for analysis of small molecules in these spores. This was indeed the case as GR-less B. megaterium and B. subtilis spores exhibited ≤5% germination upon incubation for >3 weeks in water at 37 or 50°C (data not shown).
Phosphorylated low-molecular-weight molecules in GR-less spores incubated in water or buffer at various temperatures.
As expected, there were no significant changes in the levels of AMP and 3PGA in GR-less spores of B. megaterium and B. subtilis when these spores were incubated in water at 4°C for up to 25 days (Fig. 2A and B). However, there was loss of some Pi in the first week of incubation, in particular, from the GR-less B. megaterium spores as also found with wild-type spores (Table 1; Fig. 2A and C); this Pi was found in the incubation fluid (data not shown).
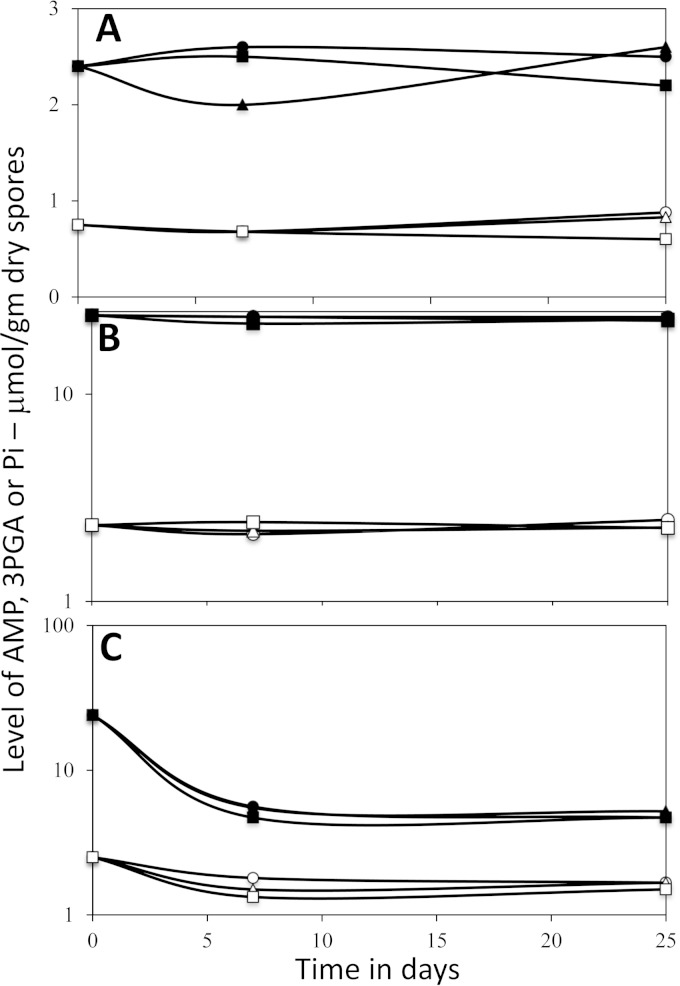
FIG 2.
Levels of AMP, 3PGA, and Pi in dormant spores of B. megaterium and B. subtilis incubated in water for different times and at different temperatures. Spores of the GR-less strains B. megaterium GC614 (filled symbols) or B. subtilis FB73 (open symbols) were prepared (on plates for B. subtilis spores), incubated in water at 4°C (circles), 37°C (triangles), or 50°C (squares); small molecules were extracted, extracts were processed, and levels of AMP (A), 3PGA (B), and Pi (C) were determined by 31P NMR as described in Materials and Methods. Values shown are averages from duplicate determinations in two separate experiments, and individual values for 3PGA and Pi did not deviate by more than 9% from average values; individual values for AMP in B. megaterium and B. subtilis spores did not deviate by more than 15 and 27%, respectively, from average. Note that values on the vertical axes in B and C are a log scale. There were no detectable NMR peaks from ADP or ATP in samples from B. subtilis and B. megaterium spores, and this allowed estimates that levels of ADP and ATP were ≤20% and 9% of AMP levels in all B. subtilis and B. megaterium spore samples, respectively.
As noted above, incubation of B. subtilis wild-type spores in water for 2 days at 37°C or 50°C resulted in no major changes in levels of 3PGA or ribonucleotides (Table 2), but these incubations could not be extended because of the high levels of spore germination that took place, especially for B. megaterium spores. However, this was not a problem with the GR-less spores, as noted above. Strikingly, analysis of low-molecular-weight phosphate compounds in either B. megaterium or B. subtilis GR-less spores incubated in water for up to 25 days at 37 or 50°C revealed no notable changes in levels of 3PGA and only small increases in levels of AMP while levels of no other low-molecular-weight compounds increased significantly (Fig. 2A and B; also data not shown). There was also no release of low-molecular-weight phosphate-containing molecules from these spores other than the Pi noted above (data not shown).
Two of the major factors that cause the relative lack of enzyme activity in dormant spores are the low water content in the spore core and the low core pH, which is ∼1.5 units below that in growing cells or germinated spores (1, 9, 11, 28, 29). To determine if elevation of spore core pH could stimulate dormant spore metabolism, newly harvested GR-less B. megaterium spores were incubated in a high-pH buffer plus (NH4)2SO4 to raise the spore core pH from ∼6.3 to ∼7.8 but with no effects on the spore core water content (11). These conditions were chosen because Bacillus PGM, the enzyme that is regulated to allow 3PGA accumulation in the developing spore, is extremely pH sensitive such that its activity is extremely low at the rather low pH of ∼6.3 in the developing and dormant spore core but very much higher at pH ∼7.8 (28, 29). This elevation of core pH had no significant effects on 3PGA catabolism during incubation of spores at 37°C, but there was an ∼40% decrease in 3PGA content after 30 days at 50°C (Fig. 3A). In addition, there were increases in levels of AMP and CMP plus UMP and probably GMP, even after 10 days of incubation at 37 or 50°C under conditions giving a high core pH although these levels either increased only minimally or even decreased by 30 days (Fig. 3B; also data not shown). Pi levels in samples from spores incubated at 37 or 50°C for 10 or 30 days in buffer at high pH were all 12.1 ± 1.2 μmol/g dry spores (data not shown), which is significantly higher than in spores incubated at these temperatures in water alone (∼5 μmol/g dry spores) (Fig. 2C; Table 1). However, 31P NMR peaks from ADP and ATP were not detected in all samples, indicating that ATP levels in spores incubated at elevated temperatures were below detection limits, if present at all.
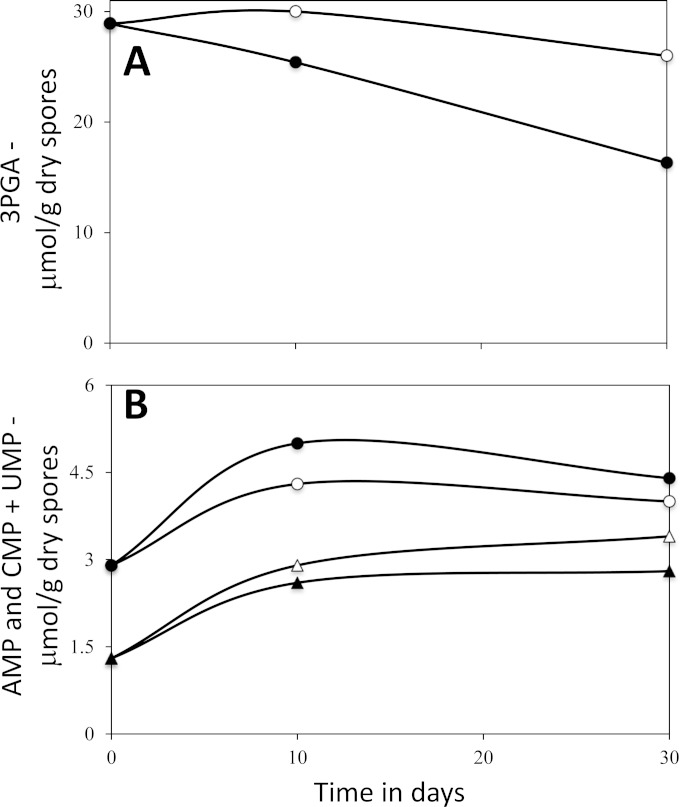
FIG 3.
Changes in levels of 3PGA, AMP, and CMP plus UMP in dormant GR-less B. megaterium spores incubated in buffer giving a high spore core pH. Dormant B. megaterium GC614 spores were sporulated in a fermentor; spores were harvested and purified and incubated at 37°C (open symbols) or 50°C (filled symbols) in the spent sporulation medium with 200 mM Tris-HCl buffer (pH 8.8) and 20 mM (NH4)2SO4, as described in Materials and Methods. The pH of the incubations was 8.6. At various times, samples were isolated, dormant spores were purified, small molecules were extracted, extracts were processed, and levels of 3PGA (A), AMP (B, circles), and CMP plus UMP (B, triangles) were determined by 31P NMR, as described in Materials and Methods. All values shown are average values from duplicate determinations in one experiment, and individual values for 3PGA, AMP and CMP plus UMP did not deviate from average values by more than 14, 22, and 26%. NMR peaks corresponding to ADP or ATP were not detected in these samples, indicating that levels of these two nucleotides were not more than ∼5% of AMP levels in spores incubated for 10 or 30 days.
Phosphorylated low-molecular-weight molecules in GR-less spores incubated in spent sporulation medium at various temperatures.
To further extend the analysis of possible dormant spore metabolism, newly harvested GR-less B. megaterium and B. subtilis spores were incubated at various temperatures in spent sporulation medium, and levels of 3PGA and ribonucleotides were determined (Fig. 4). As found with GR-less spores incubated in water, spores incubated in spent sporulation medium at 4°C exhibited no notable changes in AMP or 3PGA levels over 30 days. However, extended incubation in spent sporulation medium at 37 or 50°C led to significant loss of 3PGA, especially at 50°C, with B. subtilis spores losing 33% in 30 days and B. megaterium spores losing ∼60%. There were also increases in the B. megaterium spore level of AMP, as well as GMP, CMP, and UMP during incubation at elevated temperatures, in particular, at 50°C. Despite the changes in 3PGA and ribonucleotide levels after 30 days, there were no significant changes in the spore DPA levels (≤10%) after 30 days at 37 or 50°C under these conditions, and a 31P NMR peak due to ATP was not observed (data not shown).
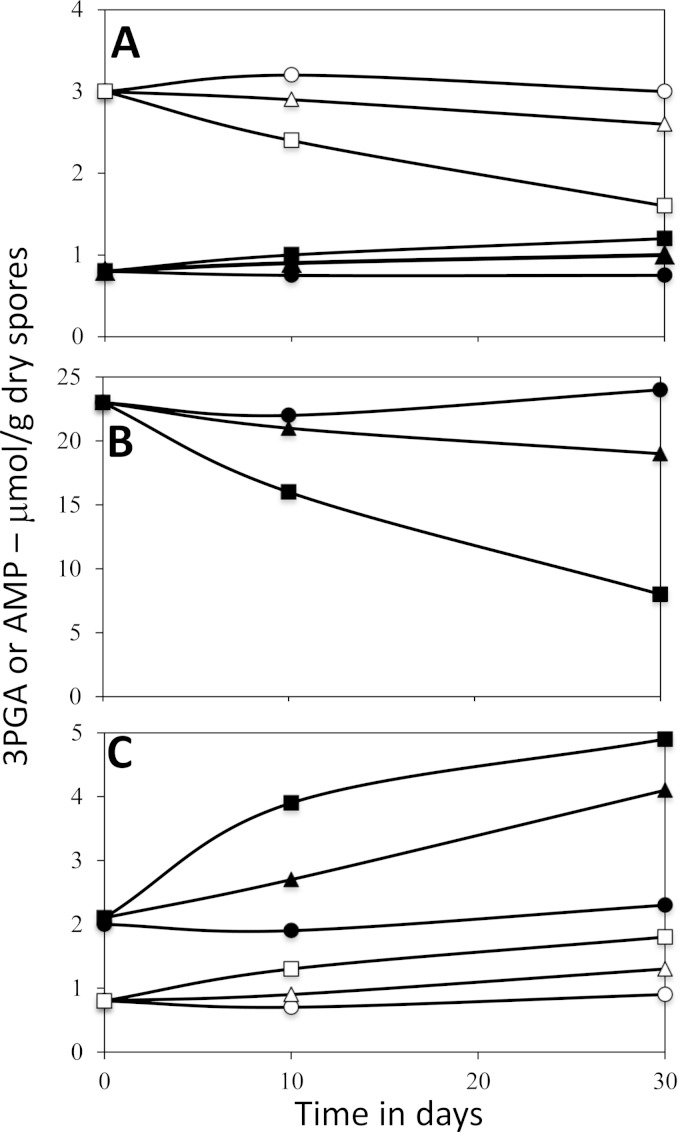
FIG 4.
Levels of 3PGA, AMP, and CMP plus UMP in B. subtilis and B. megaterium dormant spores incubated in spent sporulation medium for different times and at different temperatures. Spores of the GR-less strains B. subtilis FB73 (A) or B. megaterium GC614 (B and C) were prepared in a fermentor, isolated, and incubated in the spent sporulation medium at 4°C (circles), 37°C (triangles), or 50°C (squares); small molecules were extracted, extracts were processed, and levels of 3PGA (A, open symbols) and AMP (A, filled symbols), 3PGA (B), and AMP (C, filled symbols) or CMP plus UMP (C, open symbols) were determined by 31P NMR, as described in Materials and Methods. All values shown are averages from duplicate determinations in two independent experiments, and individual values for 3PGA in B. megaterium and B. subtilis spores did not deviate by more than 14 and 22%, respectively; individual values for AMP in B. subtilis spores differed by ≤32% from average values, and individual values for AMP and CMP plus UMP in B. megaterium spores did not deviate by more than 18 and 31%, respectively, from average values. NMR peaks corresponding to ADP or ATP were not detected in these samples, indicating that levels of these two nucleotides were not more than ∼5% and ∼15% of AMP levels in B. megaterium and B. subtilis spores, respectively, incubated for 10 or 30 days.
In a further attempt to stimulate metabolism of endogenous compounds in dormant spores, GR-less B. megaterium spores were incubated at 37 or 50°C in spent sporulation medium containing a high-pH buffer plus (NH4)2SO4 to raise the spore core pH from ∼6.3 to 7.8 as noted above (11). However, even when the core pH was elevated in spent sporulation medium, 3PGA was not lost rapidly although 75% was lost in 30 days at 50°C (Fig. 5A and 6), but NMR peaks from ATP were not observed (data not shown). There were also marked increases in levels of AMP and CMP plus UMP after 10 days of incubation at 37 or 50°C although these levels decreased by 30 days (Fig. 5B and 6). As expected from the latter results, the 31P NMR spectra of small molecules from the spores with a high core pH incubated for 10 or 30 days contained a number of additional peaks, three of which were at positions of GMP, CMP, and UMP (Fig. 1B and 6). The heights of the latter peaks were consistent with levels of these mononucleotides in the 10-day samples that were 10 to 25% of AMP levels (Fig. 6B; also data not shown) even though accurate GMP quantitation was not possible, and some of the peak quantitated as AMP may include a contribution from GMP.
FIG 5.
Levels of 3PGA and AMP or CMP plus UMP in dormant B. megaterium spores incubated in spent sporulation medium and with spore core pH adjusted to ∼7.8. Spores of the B. megaterium GR-less strain GC614 were prepared in a fermentor, isolated, and incubated in the spent sporulation medium plus 200 mM Tris-HCl buffer (pH 8.8) and 20 mM (NH4)2SO4 at either 37°C (circles) or 50°C (triangles), giving a pH of 8.6 in the spent medium plus spores, in contrast to a pH of ∼7 in spent sporulation medium by itself. At various times spores were harvested, small molecules were extracted, and levels of 3PGA (A) or AMP (B, filled symbols) or CMP plus UMP (B, open symbols) were determined by 31P NMR, as described in Materials and Methods. All values are averages based on duplicate determinations in one experiment, and individual values for 3PGA, AMP, and CMP plus UMP did not deviate by more than 11, 14, and 23%, respectively, from average values. NMR peaks corresponding to ADP or ATP were not detected in these samples, indicating that levels of these two nucleotides were not more than ∼5% of AMP levels in spores incubated for 10 or 30 days.
FIG 6.
31P NMR spectra of small molecules from dormant GR-less B. megaterium spores either as just harvested or incubated in spent sporulation medium under various conditions. GR-less spores of B. megaterium GC614 were prepared in a fermentor, harvested, and purified as described in Materials and Methods. Samples of spores were taken and processed, and small molecules were extracted and purified, as described in Materials and Methods either without further incubation (A) or after 10 (B) or 30 (C) days of incubation at 50°C in spent sporulation medium containing 200 mM Tris-HCl buffer (pH 8.8) and 20 mM (NH4)2SO4. 31P NMR spectra of the purified extracts were then acquired as described in Materials and Methods. Peaks are labeled as determined by positions of known standards added to spore extracts. Note that the appearance of the NMR peak just upfield of PGA in panel B was quite variable, and its identity is not clear.
Levels of low-molecular-weight phosphorylated molecules in dormant and germinated B. megaterium cwlJ sleB spores.
The results noted above indicated that 3PGA levels are relatively constant in dormant spores incubated in water, presumably in large part due to the spore core's low pH and water content although elevation of core pH alone did lead to significant 3PGA loss upon incubation at elevated temperatures. Upon completion of spore germination, the spore core pH rises to ∼7.8, and the spore core water content rises to ∼80%, both values similar to those in growing cells (1, 2, 8, 11). Indeed, when B. megaterium spores germinate completely, (i) 3PGA is converted to acetate in ∼10 min to generate ATP via enzymes of glycolysis as well as pyruvate dehydrogenase (5, 30) and (ii) ∼10% of dormant spore RNA is degraded to mononucleotides in 50 min although the RNA molecules degraded are not known (10). It is also possible to block B. megaterium spore germination at stage I in which spores release their large CaDPA depot (∼20% of core dry weight) by abolishing hydrolysis of the spore peptidoglycan cortex (2, 15, 16). The resulting stage I germinated B. megaterium spores have a moderately increased core water content of ∼55% of wet weight compared to 35 to 40% in fully dormant spores. The spore core pH also rises to ∼7.8 in stage I germinated spores (11). An obvious question is whether these changes in stage I germinated spores are sufficient to lead to 3PGA utilization and RNA degradation. To answer this question, we used B. megaterium spores lacking the redundant CLEs CwlJ and SleB, in which spore germination can proceed only through stage I (15), and measured levels of 3PGA and ribonucleotides in the dormant and germinated spores as well as in germinated spores incubated at 4 and 37°C (Fig. 7). In contrast to complete spore germination in which 3PGA disappears within 15 min, much ATP is generated, and much RNA is degraded to mononucleotides (5, 10), there were only small decreases in levels of AMP, 3PGA, and CMP plus UMP in the stage I germinated spores incubated for 8 days at 37°C (Fig. 7). Notably, the same small decreases in 3PGA and nucleotide levels were seen in the stage I germinated spores incubated at 4°C, suggesting that these partially germinated spores may simply be undergoing some general release of small molecules during extended incubation in buffer. 31P NMR peaks for ADP and ATP in spectra of small molecules from stage I germinated spores were not detected (data not shown), consistent with previous findings using direct assays for ATP in stage I germinated cwlJ sleB B. subtilis and B. megaterium spores (15, 16).
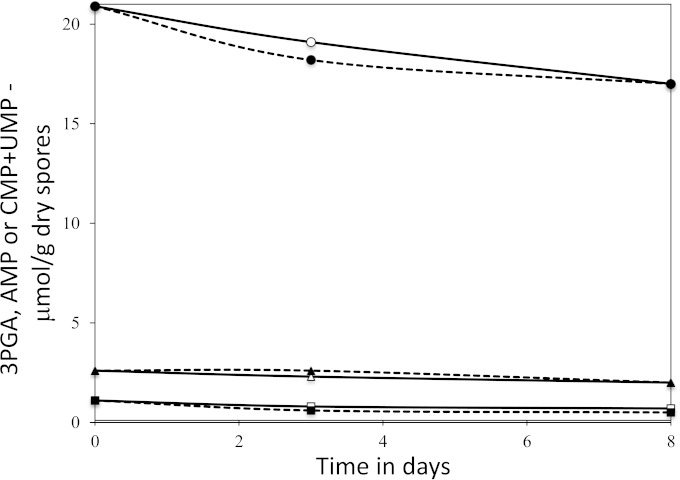
FIG 7.
Levels of 3PGA, AMP, and CMP plus UMP in germinated cwlJ sleB B. megaterium spores incubated at 4 or 37°C. Spores of B. megaterium strain PS4241 (cwlJ sleB) were germinated with glucose, harvested, washed, and incubated at 4 or 37°C, and 3PGA, AMP, and CMP plus UMP were extracted and determined as described in Materials and Methods. At most, 8% of the initial dormant spore 3PGA and AMP was lost in germination. Values shown are averages from duplicate determinations in one experiment, and 3PGA, AMP, and CMP plus UMP values did not deviate by more than 12, 18, and 30%, respectively, from average values. The symbols used are as follows: circles, 3PGA; triangles, AMP; squares, CMP plus UMP; solid lines, incubation at 4°C; dashed lines, incubation at 37°C. NMR peaks corresponding to ADP or ATP were not detected in these samples, indicating that levels of these two nucleotides were not more than ∼5% of AMP levels in spores incubated for 3 to 8 days.
DISCUSSION
It has long been accepted that there is minimal metabolism of exogenous or endogenous compounds in the core of dormant spores of Bacillus species. However, there have been few direct measurements of spore metabolism at physiological temperatures. One exception is evidence for significant rRNA degradation in newly harvested B. subtilis spores held at physiological temperatures, as well as suggestions that there is some gene expression in these spores (4). Some changes do take place in the spore outer layers within a few days after spore release from sporangia (23, 31, 32). However, the core water content of B. subtilis spores immediately after their release from sporangia is identical to that of spores purified with multiple water washes over ∼7 days at 4°C (24), so changes in this parameter presumably cannot affect spore metabolism.
That there is metabolism in dormant spores, in particular, transcription and translation, is counter to long-held beliefs, especially given the immobility of proteins in the dormant spore core (9). The work outlined in this communication was designed to directly measure metabolism in dormant B. megaterium and B. subtilis spores by examining changes in the levels of low-molecular-weight phosphorylated metabolites in newly harvested spores incubated at low and high temperatures and with and without elevation of the core pH to the value in germinated spores and with some elevation in the core water content. One notable finding in this work was that storage of newly harvested spores at 4° in either water or spent sporulation medium resulted in no notable changes in 3PGA or ribonucleotide levels and no generation of detectable ATP although incubation for extremely long times could have led to changes reflecting extremely slow metabolism. We also note that spores contain unphosphorylated low-molecular-weight compounds that disappear in spore germination (33, 34), and perhaps these compounds are metabolized more rapidly in dormant spores. It is also possible that there was some rRNA degradation during spore storage at 4°C in water or spent medium but that the rRNA degradation products were not detected, either because they were large oligonucleotides or because nucleoside monophosphates were rapidly dephosphorylated. However, the latter seems unlikely since AMP levels did not change in spores incubated at 4°C, spore Pi levels did not rise after the release of Pi from outer spore layers, and no other phosphate-containing small molecules were released from spores incubated for up to 25 days in water at elevated temperatures.
A second notable finding was that no significant changes in 3PGA or mononucleotide levels or accumulation of significant levels of other low-molecular-weight phosphorylated compounds, including ATP, were detected in dormant spores incubated in water at elevated temperatures. It is notable that spores of B. megaterium and B. subtilis contain ∼180 and 110 μmol/g dry weight of RNA nucleotides, respectively (10). Since ∼85% of spore RNA is rRNA, degradation of even 5% of the spore rRNA to mononucleotides would increase spore AMP levels more than 2-fold, and this was not observed even after 25 days; levels of Pi did not change after 7 days at 37 or 50°C. Consequently, it appears most likely that spores incubated in water even at physiological or higher temperatures exhibit minimal, if any, metabolism, although we cannot rule out that there is significant RNA degradation, but not to mononucleotides.
The lack of significant metabolism in spores incubated in water at physiological temperatures is also consistent with the lack of significant changes in 3PGA or mononucleotide levels observed in stage I germinated spores incubated in buffer at 37°C for up to 8 days. This was not surprising since the spore core proteins remain immobile in stage I germinated spores even though the core pH rises to ∼7.8 and core water content rises appreciably but is still well below that in fully germinated spores (9, 11, 15, 16). In addition, NADH and FADH2 were previously shown to be absent in stage I germinated spores, and ATP levels were at least 40- to 100-fold higher in spores that have completed germination (15, 16). There were small decreases in mononucleotide and 3PGA levels in stage I germinated spores incubated for up to 8 days at 37°C, but essentially the same decreases were seen during incubation at 4°C. This suggests that these stage I spores may slowly release small molecules although this was not examined further.
In contrast to the results described above, spores incubated at elevated temperatures in buffer to raise the core pH to 7.8, in spent sporulation medium, or in buffered spent medium to elevate the core pH exhibited significant core metabolism. This metabolism included decreases in 3PGA levels of up to 75%, as well as increases in AMP, CMP, GMP, and UMP although GMP levels were hard to quantitate. The increases in mononucleotide levels are consistent with RNA degradation in these dormant spores although the maximal increase represented <5% of total spore RNA. However, RNA degradation in dormant spores may not be complete, and nucleotidase or phosphatase action could lower levels of ribonucleotides in spore extracts. Notably, spores incubated at elevated temperatures in buffer giving a high core pH accumulated Pi levels well above values in dormant spores when the readily lost Pi that was likely in the spore outer layers is accounted for. However, the source of this accumulated Pi is not clear.
The results in the spent medium are then in contrast to the results with dormant spores incubated in water or with stage I germinated spores incubated in buffer at elevated temperatures. We do not know why there might be more metabolism in the dormant spores incubated in spent sporulation medium than in water. One possibility is that one or more molecules in the spent sporulation medium is able to trigger events in the spore core that stimulate spore core metabolism, perhaps triggering germination-like events by interacting with molecules in the spore outer layers. However, the GR-less spores incubated at elevated temperatures exhibited no evidence of normal early germination events as DPA levels in spores incubated in spent sporulation medium at elevated temperatures did not decrease. Another possibility is that some of the many compounds in the spent sporulation medium permeate into the dormant spore core and alter spore metabolism, and both anions and cations can slowly enter the spore core (35, 36). However, permeation of small molecules into spores over periods of weeks at elevated temperatures has not been studied, nor has how such permeation could alter spore metabolism.
A major conclusion from this work is that dormant and stage I germinated spores of Bacillus species exhibit no detectable metabolism of low-molecular-weight phosphorylated molecules during incubation at 4°C in various solutions or in water at elevated temperatures with or without core pH elevation. This conclusion is gratifying in that dormant spores can thus still be considered dormant—at least under these conditions. However, a second major conclusion is that dormant spores do exhibit metabolism of phosphorylated molecules when incubated at elevated temperatures in spent medium with or without additions that increase spore core pH. Thus, dormant spores can exhibit metabolic reactions, or at least enzymatic reactions, in the spore core under some conditions.
In addition to the major conclusions noted above, other points and questions are raised by the work in this communication, as follows. (i) As suggested previously (1, 28, 29), it seems likely that the low pH of the dormant spore core is one factor in the low metabolism of the spore since elevation of the core pH alone increased rates of 3PGA decrease and mononucleotide increase in spores incubated at elevated temperatures. However, in considering the decreases in 3PGA levels, we do not know how this takes place, as noted below. (ii) Despite the increases in metabolism in spores with a high core pH incubated at elevated temperatures, elevation of the core pH and partial elevation of the core water content as seen with stage I germinated spores did not result in increases in spore metabolism. However, it is possible that these stage I germinated spores begin to leak small molecules nonselectively during extended incubation. (iii) Given the small increases in spore metabolism caused by elevation of spore core pH alone, it is clear that the low water content of the spore core is a major factor in the minimal metabolism in dormant spores and also in stage I germinated spores. Perhaps it will be possible to generate dormant spores with even higher core water contents by making use of spores with defects in spore cortex structure (37) in order to determine precisely how much core water is needed for enzymatic action in the dormant spore core. (iv) What is the mechanism of the loss in 3PGA in dormant spores incubated in spent medium? The 3PGA could go through the later reactions of glycolysis giving pyruvate and ATP and then to acetate via pyruvate dehydrogenase as the appropriate enzymes are in the spore core (5, 20). However, we saw no ATP accumulation during the long incubation of spores, and thus it is possible that 3PGA is catabolized by a phosphatase generating glyceric acid, which might not be utilized further by spores. (v) Are any nucleoside triphosphates generated in dormant spores during long incubations in spent medium? There is a previous report of new RNA synthesis in dormant spores (4), and clearly this will require ribonucleotide triphosphates. However, these compounds were not seen in 31P NMR spectra of small molecules. Is this because they are not there or because their steady-state level is simply too low to allow detection even though they are still generated in sufficient amounts such that some RNA could be made? For example, if 50% of the spore 3PGA depot was catabolized to acetate, this would generate ∼40 μmol of ATP/g dry spores, sufficient to make ∼40 μmol of RNA nucleotides. Indeed, 3PGA catabolism supports most RNA synthesis in the first ∼10 min of B. megaterium spore germination in the absence of exogenous nutrients (5, 38). However, will RNA polymerase work with minimal ribonucleoside triphosphate levels in the low-water environment of the spore core in which spore DNA is saturated with DNA-binding proteins that inhibit RNA polymerase action, at least in vitro (39)? (vi) What is the signal(s) supplied by spent sporulation medium that stimulates dormant spore metabolism at elevated temperatures? At present we have no good answers to this question. (vii) While there is almost certainly RNA degradation in dormant spores incubated under some conditions, how much spore RNA is degraded, and what is the fate of the degradation products? It would be informative to determine the fate of these RNA degradation products, in particular, in spore germination when any rRNA lost through degradation would need to be resynthesized and initially from preexisting nucleotides generated from spore RNA breakdown (10, 38). Overall, it appears that there are still aspects of supposedly dormant spores that are worthy of investigation.
ACKNOWLEDGMENTS
This communication is based upon work supported by a STIR award from the U.S. Army Research Office to P.S. and a Department of Defense Multi-Disciplinary Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911NF-09-1-0286 and by support from the Carole and Ray Neag Comprehensive Cancer Center at the University of Connecticut Health Center.
REFERENCES
- 1.Setlow P, Johnson EA. 2012. Spores and their significance, p 45–79. In Doyle MP, Buchanan R (ed), Food microbiology: fundamentals and frontiers, 4th ed. ASM Press, Washington, DC. [Google Scholar]
- 2.Setlow P. 2013. When the sleepers wake: the germination of spores of Bacillus species. J Appl Microbiol 115:1251–1268. doi: 10.1111/jam.12343. [DOI] [PubMed] [Google Scholar]
- 3.Church BD, Halvorson H. 1957. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var. Terminalis. J Bacteriol 73:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segev F, Smith Y, Ben-Yehuda S. 2012. RNA dynamics in aging bacterial spores. Cell 148:139–149. doi: 10.1016/j.cell.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Setlow P, Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem 245:3637–3644. [PubMed] [Google Scholar]
- 6.Jedrzejas MJ, Setlow P. 2001. Comparison of the binuclear metalloenzymes diphosphoglycerate-independent phosphoglycerate mutase and alkaline phosphatase: their mechanism of catalysis by a phosphoserine intermediate. Chem Rev 101:607–618. doi: 10.1021/cr000253a. [DOI] [PubMed] [Google Scholar]
- 7.Chander M, Setlow B, Setlow P. 1998. The enzymatic activity of phosphoglycerate mutase from gram positive endospore forming bacteria requires Mn2+ and is pH sensitive. Can J Microbiol 44:759–7677. doi: 10.1139/w98-060. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt P, Marquis RE. 1989. Spore thermoresistance mechanisms, p 43–63. In Smith I, Slepecky RA, Setlow P (ed), Regulation of prokaryotic development. American Society for Microbiology, Washington, DC. [Google Scholar]
- 9.Cowan AE, Koppel DE, Setlow B, Setlow P. 2003. A soluble protein is immobile in dormant spores of Bacillus subtilis but is mobile in germinated spores: implications for spore dormancy. Proc Natl Acad Sci U S A 100:4209–4214. doi: 10.1073/pnas.0636762100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson DL, Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J Biol Chem 245:1137–1145. [PubMed] [Google Scholar]
- 11.Swerdlow BM, Setlow B, Setlow P. 1981. Levels of H+ and other monovalent cations in dormant and germinated spores of Bacillus megaterium. J Bacteriol 148:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh RP, Setlow B, Setlow P. 1977. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol 130:1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Üstok FI, Johnson CL, Bailey DMD, Lowe CR, Christie G. 2013. Investigating the functional hierarchy of Bacillus megaterium PV361 spore germinant receptors. J Bacteriol 195:3045–3053. doi: 10.1128/JB.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paidhungat M, Setlow P. 2000. Role of Ger proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J Bacteriol 182:2513–2519. doi: 10.1128/JB.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setlow B, Peng L, Loshon CA, Li YQ, Christie G, Setlow P. 2009. Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex. J Appl Microbiol 107:318–328. doi: 10.1111/j.1365-2672.2009.04210.x. [DOI] [PubMed] [Google Scholar]
- 16.Setlow B, Melly E, Setlow P. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J Bacteriol 183:4894–4899. doi: 10.1128/JB.183.16.4894-4899.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popham DL, Helin J, Costello CE, Setlow P. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci U S A 93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood CR, Cutting SM (ed), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom. [Google Scholar]
- 20.Vepachedu VR, Hirneisen K, Hoover DG, Setlow P. 2007. Studies of the release of small molecules during pressure germination of spores of Bacillus subtilis. Lett Appl Microbiol 45:342–348. doi: 10.1111/j.1472-765X.2007.02204.x. [DOI] [PubMed] [Google Scholar]
- 21.Setlow B, Wahome PG, Setlow P. 2008. Release of small molecules during germination of spores of Bacillus species. J Bacteriol 190:4759–4763. doi: 10.1128/JB.00399-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loshon CA, Wahome PG, Maciejewski MW, Setlow P. 2006. Levels of glycine betaine in growing cells and spores of Bacillus species and lack of effect of glycine betaine on spore properties. J Bacteriol 188:3153–315822. doi: 10.1128/JB.188.8.3153-3158.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Salas JL, Setlow B, Zhang P, Li YQ, Setlow P. 2011. Maturation of released spores is necessary for acquisition of full spore heat resistance during Bacillus subtilis sporulation. Appl Environ Microbiol 77:6746–6754. doi: 10.1128/AEM.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512. doi: 10.1128/JB.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192:3424–3433. doi: 10.1128/JB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat M, Ragkousi K, Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol 183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Garner W, Setlow P, Yu J. 2011. Quantitative analysis of spatial-temporal correlations during germination of spores of Bacillus species. J Bacteriol 193:3765–3772. doi: 10.1128/JB.05154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn NJ, Setlow B, Setlow P. 1993. Manganese (II) activation of 3-phosphoglycerate mutase of Bacillus megaterium: pH-sensitive interconversion of active and inactive forms. Arch Biochem Biophys 306:342–349. doi: 10.1006/abbi.1993.1521. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn NJ, Setlow B, Cammack R, Setlow P. 1995. Cooperative manganese(II) activation of 3-phosphoglycerate mutase of Bacillus megaterium: a biological pH-sensing mechanism in bacterial spore formation and germination. Arch Biochem Biophys 320:35–42. doi: 10.1006/abbi.1995.1339. [DOI] [PubMed] [Google Scholar]
- 30.Setlow B, Shay LR, Vary JC, Setlow P. 1977. Production of large amounts of acetate during germination of Bacillus megaterium spores in the absence of exogenous carbon sources. J Bacteriol 132:744–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragkousi K, Setlow P. 2004. Transglutaminase-mediated cross-linking of GerQ in the coats of Bacillus subtilis spores. J Bacteriol 186:5567–5575. doi: 10.1128/JB.186.17.5567-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriques AO, Moran CP Jr. 2007. Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol 61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- 33.Magge A, Granger AC, Wahome PG, Setlow B, Vepachedu VR, Loshon CA, Peng L, Chen D, Li YQ, Setlow P. 2008. Role of dipicolinic acid in the germination, stability, and viability of spores of Bacillus subtilis. J Bacteriol 190:4798–4807. doi: 10.1128/JB.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loshon CA, Wahome PG, Maciejewski MW, Setlow P. 2006. Levels of glycine betaine in growing cells and spores of Bacillus species and lack of effect of glycine betaine on dormant spore resistance. J Bacteriol 188:3153–3158. doi: 10.1128/JB.188.8.3153-3158.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosal S, Leighton TJ, Wheeler KE, Hutcheon ID, Weber PK. 2010. Spatially resolved characterization of water and ion incorporation in Bacillus spores. Appl Environ Microbiol 76:3275–3282. doi: 10.1128/AEM.02485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosal S, Fallon SJ, Leighton TJ, Wheeler KE, Kristo MJ, Hutcheon ID, Weber PK. 2008. Imaging and 3D elemental characterization of intact bacterial spores by high-resolution secondary ion mass spectrometry. Anal Chem 80:5986–5992. doi: 10.1021/ac8006279. [DOI] [PubMed] [Google Scholar]
- 37.Zhang P, Thomas S, Li YQ, Setlow P. 2012. Effects of cortex peptidoglycan structure and cortex hydrolysis on the kinetics of Ca2+-dipicolinic acid release during Bacillus subtilis spore germination. J Bacteriol 194:646–652. doi: 10.1128/JB.06452-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setlow P, Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XXII. Nucleotide metabolism during spore germination. J Biol Chem 245:3645–3652. [PubMed] [Google Scholar]
- 39.Setlow B, Sun D, Setlow P. 1992. Interaction between DNA and α/β-type small, acid-soluble spore proteins: a new class of DNA-binding protein. J Bacteriol 174:2312–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]