Abstract
Background
Borderline personality disorder (BPD) is a severe psychiatric disorder involving a range of symptoms including marked affective instability and disturbances in interpersonal interactions. Neuroimaging studies are beginning to provide evidence of altered processing in fronto-limbic network deficits in the disorder, however, few studies directly examine structural connections within this circuitry together with their relation to proposed causative processes and clinical features.
Methods
In the current study, we investigated whether individuals with BPD (n = 20) have deficits in white matter integrity compared to a matched group of healthy controls (n = 18) using diffusion tensor MRI (DTI). We hypothesized that the BPD group would have decreased fractional anisotropy (FA), a measure of white matter integrity, compared to the controls in white matter tracts connecting frontal and limbic regions, primarily the cingulum, fornix and uncinate fasciculus. We also investigated the extent to which any such deficits related to childhood adversity, as measured by the childhood trauma questionnaire, and symptom severity as measured by the Zanarini rating scale for BPD.
Results
We report decreased white matter integrity in BPD versus controls in the cingulum and fornix. There were no significant relationships between FA and measures of childhood trauma. There were, however, significant associations between FA in the cingulum and clinical symptoms of anger, and in the fornix with affective instability, and measures of avoidance of abandonment from the Zanarini rating scale.
Conclusions
We report deficits within fronto-limbic connections in individuals with BPD. Abnormalities within the fornix and cingulum were related to severity of symptoms and highlight the importance of these tracts in the pathogenesis of the disorder.
Keywords: Borderline personality, Fornix, Cingulum, Diffusion tensor imaging, Anger, Abandonment
Highlights
-
•
Decreased white matter integrity was reported in patients with borderline personality disorder in the cingulum and fornix.
-
•
Decreases in white matter integrity were related to severity of affective and interpersonal symptoms.
-
•
Findings highlight the importance of fronto-limbic tracts in the pathogenesis of the disorder.
1. Introduction
Borderline personality disorder (BPD) is a severe complex psychiatric disorder estimated to affect 0.5–5.9% of the population (Lenzenweger et al., 2007; Grant et al., 2008). BPD is characterized by impulsivity, affective instability, maladaptive cognitive processes, and interpersonal disturbances. Causal mechanisms are at present incompletely understood, however genetic factors, adverse traumatic childhood events, and altered neurobiology are central to its etiology (O'Neill and Frodl, 2012).
Currently, research into BPD lags behind the other major psychiatric disorders such as bipolar disorder and schizophrenia, however there is a growing neuroimaging literature focusing on understanding its underlying neurobiology. Studies of morphology and function have generally indicated disturbances of fronto-limbic networks involved in the regulation and recognition of emotion and response to stress. Structural imaging studies generally report volumetric reductions in limbic structures involved in emotion processing in BPD including the hippocampus and amygdala, a finding which is supported by meta-analysis (Schmahl et al., 2003a; Tebartz van Elst et al., 2003; Brambilla et al., 2004; Zetzsche et al., 2007; O'Neill and Frodl, 2012; Richter et al., 2014). Functional imaging studies have also reported altered activation of amygdala to emotional stimuli (Herpertz et al., 2001; Donegan et al., 2003; Minzenberg et al., 2007; Hazlett et al., 2012), along with abnormal activation of regulatory regions in the prefrontal cortex (Silbersweig et al., 2007; O'Neill and Frodl, 2012; Kamphausen et al., 2013; Koenigsberg et al., 2014).
There have, however, been fewer neuroimaging studies directly examining the integrity of white matter tracts within fronto-limbic circuits in BPD (Rusch et al., 2010; Carrasco et al., 2012; Maier-Hein et al., 2014). Such structural connectivity is typically measured using diffusion tensor MRI (DTI). DTI measures the random motion of water molecules within white matter fiber tracts, quantified as fractional anisotropy (FA), which is considered to reflect the underlying tissue structure (Schmierer et al., 2007). FA refers to the non-random/constrained water diffusion imposed by axonal fiber structure and myelination, however since DTI measures this diffusion at the macroscopic level, FA can be influenced by a number of other factors including multiple fiber orientations within individual voxels. FA is therefore viewed only as an approximation of white matter integrity. DTI studies in BPD have typically reported reduced white matter integrity within the frontal cortex (Grant et al., 2007; Rusch et al., 2010; Carrasco et al., 2012), in the association tracts connecting with hippocampal and thalamic regions (Maier-Hein et al., 2014), together with evidence for deviation in normal developmental trajectories in individuals with BPD (New et al., 2013). There is still however limited literature linking neurobiological abnormalities with individual trait-features of the disorder. Few studies have, for example, examined these deficits in relation to childhood adversity and symptom severity despite findings in other disorders which suggest associations between white matter integrity and early life adversity and with affective symptomatology (Sprooten et al., 2011; Emsell et al., 2013; Lu et al., 2013).
In the current study we therefore investigated whether there were abnormalities in key fronto-limbic white matter tracts in individuals with BPD compared with healthy age and sex matched controls. In addition we sought to determine whether white matter FA in individuals with BPD was associated with early life adversity or symptom severity. We focused on three fronto-limbic tracts implicated in affective processing; the cingulum, the fornix and the uncinate fasciculus. We hypothesized that there would be reductions in white matter integrity in the BPD group compared with controls, and that FA within these tracts would relate to measures of childhood trauma and BPD-associated symptom severity, particularly in affective and social symptom domains.
2. Methods and materials
2.1. Study population
Twenty individuals meeting DSM-IV criteria for BPD were recruited from local clinical services. A diagnosis of BPD was established through the use of SCID-II interview and screening for comorbidity was conducted using the SCID-I and by case note review. Individuals with BPD were excluded based on a history of bipolar I disorder or schizophrenia, current alcohol or drug dependency, or any form of neurological illness. Healthy controls were recruited from community volunteers. All participants provided written informed consent and were able to withdraw from the study at any time. The study was approved by the local research ethics committee.
All participants IQ levels were assessed by the administration of The National Adult Reading Test (NART) (Nelson, 1982). Participants also completed the Hamilton Rating Scale for Depression (HAM-D) (Hamilton, 1960), Young Mania Rating Scale (YMRS) (Young et al., 1978), Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 1997; Bernstein et al., 2003) and the Zanarini rating scale for borderline personality disorder (ZAN-BPD) (Zanarini et al., 2003). The CTQ was grouped into subscale ratings of emotional abuse, emotional neglect, physical abuse, physical neglect, sexual abuse and total scores.
2.2. Scan acquisition and preprocessing
The MRI data were collected on a 3-T MAGNETOM Verio (Siemens AG, Healthcare Sector, Erlangen, Germany) running the software Syngo MR B17 at the Clinical Research Imaging Centre, University of Edinburgh. Whole brain DTI scans were acquired for each subject with a prototype single-shot pulsed gradient spin-echo echo-planar imaging sequence with diffusion gradients (b = 1000 s/mm2) applied in 56 non-collinear directions and 6 T2-weighted echo-planar imaging baseline scans. Fifty-five 2.5-mm contiguous axial slices were acquired with a field of view of 220 × 220 mm2 which yields an isotropic acquisition voxel of dimensions 2.5 × 2.5 × 2.5 mm3. Standard pre-processing procedures were employed involving conversion to NifTI format, eddy current correction, linear motion correction and brain extraction (http://www.fmrib.ox.ac.uk/fsl). Finally, ‘DTIFit’ (http://www.fmrib.ox.ac.uk/fsl) was used to fit diffusion tensors to the eddy- and EPI-corrected data to generate FA values for each subject using standard formulas in FSL.
2.3. Tract based spatial statistics
Tract Based Spatial Statistics (TBSS) (Behrens et al., 2003; Smith et al., 2006) was performed using the standard FSL procedures (Sprooten et al., 2011). First, each subject's FA volume was linearly and non-linearly registered to the ‘most representative’ participant, i.e. that which minimized the amount of warping required for all other subjects to be aligned to it. All subjects were then subsequently aligned to MNI152 space. The resulting FA volumes were then averaged and a mean FA skeleton mask generated by searching along all tracts in a perpendicular direction; the voxel with the highest FA was then considered to be the center of the tract. A threshold of FA > 0.2 was applied to the skeleton with the aim of removing voxels which consisted primarily of gray matter or CSF. Each subject's aligned FA images were then projected onto the fiber skeleton template resulting in one FA skeleton map per subject assumed to contain anatomically corresponding centers of white matter structure.
Having obtained the skeletons for each subject from TBSS, a two-sample t-test was conducted to assess between group differences in FA using ‘randomize’ functions within FSL. Three separate masks were used at the randomize command in FSL for the region of interest (ROI) analyses (the cingulum, fornix, and uncinate fasciculus), as well as the mean FA skeleton mask. White matter masks were derived from the Johns Hopkins University DTI-based white matter atlas and the Johns Hopkins University white matter tractography atlas digitally available in FSL. Threshold-Free Cluster Enhancement (TFCE) was used to obtain cluster-based statistics corrected for multiple comparisons, a method of enhancing cluster-like structures in the voxel-based data. The current analyses present p-values corrected for ROI with family-wise error (pFWE) via permutation testing with 5000 permutations. The threshold-free cluster enhancement corrected p-maps were thresholded at pFWE < 05. Motion did not differ between the patients and controls (patients: mean = 0.68 mm, std dev = 0.15, controls: mean = 0.60 mm, std dev = 0.17, p = 0.94).
2.4. Statistical analysis
Statistical analysis of demographic and clinical data were conducted using two sample t-tests (for age, IQ), Kruskal–Wallis (for CTQ and Zanarini scores) or chi-squared tests (for sex, handedness) where appropriate. All analyses were conducted in ‘R’ (http://www.R-project.org). Regression analysis of the CTQ (for subscale ratings of emotional abuse, emotional neglect, physical abuse, physical neglect, sexual abuse and total scores) and Zanarini scores with measures of FA were conducted for regions demonstrating significant group differences. The CTQ and Zanarini scales were examined separately. These analyses were also performed in R and were restricted to the BPD group only.
3. Results
3.1. Demographic and clinical measures
Demographic details are presented in Table 1. The BPD group consisted of 17 females and 3 males with a mean age of 35.8 years (SD 8.61) and a mean IQ of 114.8 (SD 7.89). Of the 20 individuals with BPD, 12 were being treated with antipsychotic medication and 15 were being treated with antidepressant medication. The healthy controls comprised 14 females and 4 males with mean age of 34.9 years (SD 9.85) and a mean IQ of 113.2 (SD 5.59). No significant differences were found in age, IQ, handedness or gender between the groups. Clinical measures for the BPD group are described in Table 1.
Table 1.
Demographics and clinical measures.
| Healthy control participants (n = 18) |
Participants with a diagnosis of BPD (n = 20) |
Significance Z score/chi sq (p-value) | |
|---|---|---|---|
| Demographics mean (std dev) | |||
| Age (years) | 34.9 (9.85) | 35.8 (8.61) | 0.28 (0.77) |
| Sex F:M | 14:4 | 17:3 | 1.58 (0.46) |
| IQ (NART) | 113.2 (5.59) | 114.8 (7.89) | 0.62 (0.54) |
| Handedness R:L plus mixed | 17:1 | 18:2 | 0.26 (0.61) |
| Clinical measuresa | |||
| Hamilton Rating Scale for Depression | NA | 16.5 (15) | NA |
| Young Mania Rating Scale | NA | 0.0 (2.75) | NA |
| Zanarini rating scale | |||
| Z1 (anger) | NA | 1.0 (1.75) | NA |
| Z2 (affective instabil) | NA | 2.5 (2.00) | NA |
| Z3 (emptiness) | NA | 2.0 (2.75) | NA |
| Z4 (identity disturb) | NA | 1.5 (1.00) | NA |
| Z5 (dissociative sympt) | NA | 2.0 (2.00) | NA |
| Z6 (abandonment) | NA | 1.0 (1.00) | NA |
| Z7 (self-harm) | NA | 1.5 (1.75) | NA |
| Z8 (impulsivity) | NA | 1.0 (2.00) | NA |
| Z9 (interpersonal rel) | NA | 1.0 (2.00) | NA |
| Childhood trauma questionnaire (CTQ) | |||
| Emotional abuse | NA | 3.8 (1.11) | NA |
| Emotional neglect | NA | 3.68 (1.26) | NA |
| Physical abuse | NA | 2.5 (1.49) | NA |
| Physical neglect | NA | 1.95 (1.25) | NA |
| Sexual abuse | NA | 3.14 (1.74) | NA |
| CTQ total | NA | 15.1 (5.10) | NA |
| Antipsychotic medication (Y:N) | 0 | 12:8 | NA |
| Antidepressant medication (Y:N) | 0 | 15:5 | NA |
| Comorbid diagnoses, current and past (number) | NA | BPADii (1), OCD (1), PTSD (3), eating disorder (4), depression (11); anxiety (8) | NA |
Median (interquartile range).
3.2. Between group differences in fractional anisotropy
Decreases in FA were found between individuals with BPD and controls in the anterior portion of the cingulum bundle (125 voxels at pFWE = 0.005, MNI co-ordinates: x = 107, y = 160, z = 87, controls: mean FA = 0.4582, sd = 0.05; BPD: mean FA = 0.3858, sd = 0.07) and in the fornix (139 voxels at pFWE = 0.015, MNI co-ordinates: x = 90, y = 117, z = 88, controls: mean FA = 0.5324, sd = 0.09; BPD: mean FA = 0.4292, sd = 0.14) using ROIs as described above (Figs. 1 and 2). No differences were found in the uncinate fasciculus between groups, and no significant differences were found between groups using the mean FA skeleton mask.
Fig. 1.
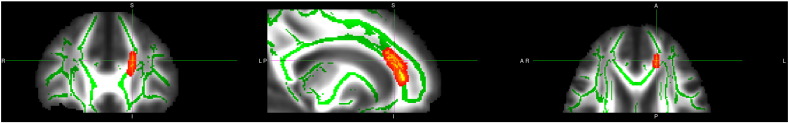
Decreased FA in BPD group in cingulum. Deceased white matter integrity in patients with BPD versus controls in the cingulum. For further details see text. To aid visualization the results (pFWE < 0.05) are thickened using the “tbss-fill” command.
Fig. 2.
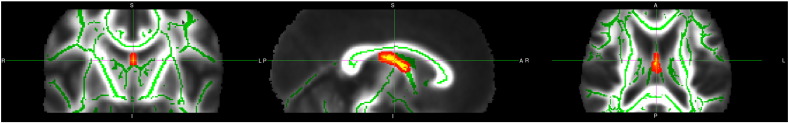
Decreased FA in BPD group in fornix. Deceased white matter integrity in patients with BPD versus controls in the fornix. For further details see text. To aid visualization the results (pFWE < 0.05) are thickened using the “tbss-fill” command.
3.3. Regression analysis
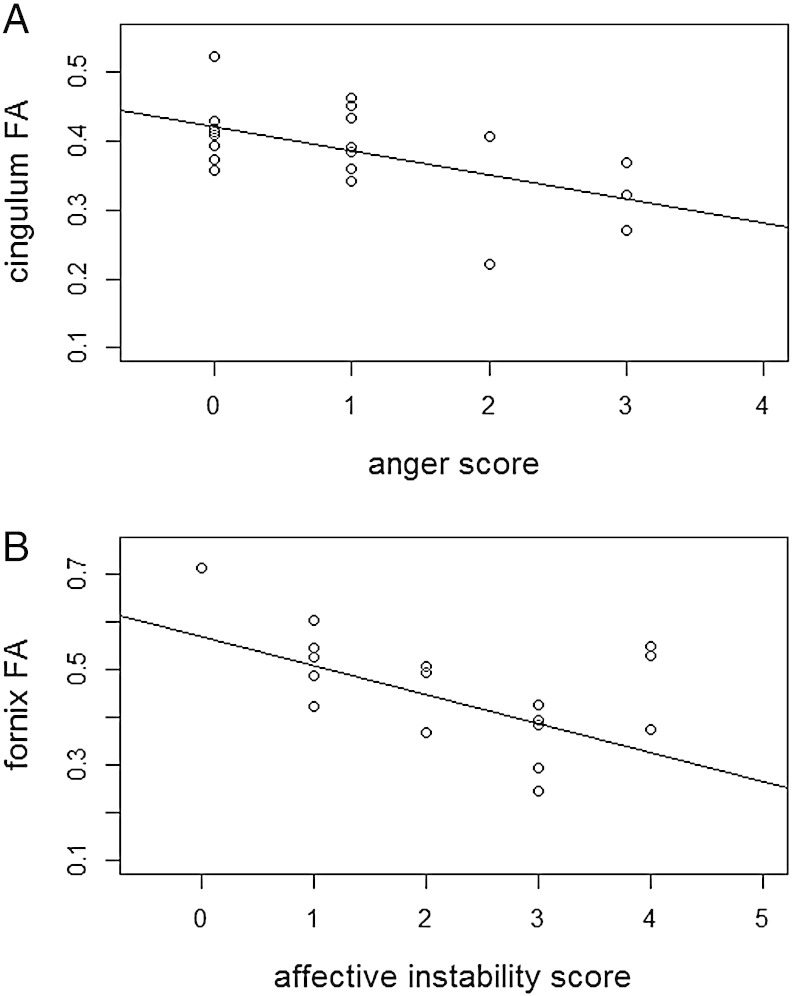
Regression analyses were performed using FA values from the clusters of group difference in the cingulum and fornix within the BPD group. No significant relationships were reported for any of the measures from the CTQ. However there were significant associations between FA values in the cingulum and measures of anger from the Zanarini scale (r = −0.56, p = 0.009), and between fornix FA values and measures of affective instability (r = −0.49, p = 0.027) and symptoms of avoidance of abandonment (r = 0.47, p = 0.036) also from the Zanarini scale (Fig. 3). Finally there was a trend level association between fornix FA values and measures of impulsivity (r = 0.438, p = 0.053).
Fig. 3.
Association between symptom severity measures from the Zanarini scale and FA values. (A) Between cingulum and anger, (B) between fornix and affective instability
3.4. Analysis of potential confounders
Within the BPD group, measures of FA from the cingulum and fornix were not found to correlate significantly with the severity of depressive symptoms from the HAM-D, nor with age or IQ. To explore potential confounding effects of medication, FA values for these structures in those individuals on antipsychotic medication were compared to those not taking it, similarly for antidepressant medication. There were no significant differences for FA measures from the cingulum (t = 0.189, p = 0.852; t = 0.205, p = 0.840) or fornix (t = 0.51, p = 0.613; t = 1.14, p = 0.268) for anti-psychotic or antidepressant medication respectively.
4. Discussion
In this study we report reductions in white matter integrity as measured using FA in individuals with BPD versus a matched group of healthy controls in white matter tracts connecting frontal and limbic regions, specifically the cingulum and fornix. These findings represent deficits of structural connectivity in tracts connecting brain regions involved in affective and social processing previously implicated in BPD using structural and functional neuroimaging approaches. In addition, we also report associations between FA measures in the cingulum and fornix with symptom severity measures within the BPD group, particularly for affective and social symptom domains. Notably, however, we did not find evidence of an association of early trauma, as assessed by the CTQ, with white matter integrity in BPD.
The current study indicated white matter deficits in individuals with BPD within the anterior section of the cingulum bundle. The cingulum bundle forms the white matter structure of the cingulate gyrus. On sagittal section it is C-shaped bundle of fibers running from the rostral subcallosal region, along the corpus callosum, through to the parahippocampal gyrus. It is the main tract connecting the cingulate cortex with other limbic areas of the brain, specifically the medial temporal lobe, and wider networks including prefrontal, parietal, temporal areas and the thalamus (Pandya et al., 1981). Hence, it is one of the most important tracts of the limbic system and plays a central role in co-ordinating emotional processing and affect regulation, and impulse control which relate to core areas of difficulty in BPD. Abnormalities within this tract may therefore underlie disrupted regulatory inhibitory influence of higher order cortical regions, such as the anterior cingulate and prefrontal cortex, over limbic regions such as the amygdala and insula, resulting in the typical limbic hyper-responsiveness commonly reported in the disorder (Herpertz et al., 2001; Donegan et al., 2003; Minzenberg et al., 2007; Hazlett et al., 2012). This finding is indeed consistent with a previous DTI study examining adults with BPD (Rusch et al., 2010), and with numerous studies reporting volumetric abnormalities within the anterior segments of the cingulate cortex itself (Tebartz van Elst et al., 2003; Hazlett et al., 2005; Minzenberg et al., 2008; Soloff et al., 2008; Whittle et al., 2009).
The current analysis focused on tracts connecting fronto-limbic regions of the brain. It is noteworthy however that in the case of both the cingulum and fornix that these are tracts adjacent to the corpus callosum which itself has been previously implicated in BDP (Rusch et al., 2010; Carrasco et al., 2012). In the present study we also examined the relationship between FA values within fronto-limbic tracts and measures of childhood adversity and symptom severity. There is currently limited literature linking deficits in specific tracts with individual symptoms in BPD, we therefore attempt within the following discussion to draw on other imaging modalities in other patient populations and in healthy controls with respect to anger processing and affective symptomatology in order to interpret these findings. Although we did not find associations with childhood adversity, FA values within the cingulum were related to measures of inappropriate anger from the Zanarini rating scale. This is consistent with previous studies implicating the importance of regions connected by the cingulum, specifically the cingulate cortex, in functions such as trait aggression and self-reported feelings of anger (Denson et al., 2009; Pawliczek et al., 2013), and with previous functional imaging studies of BPD reporting decreased activation of the subgenual anterior cingulate during anger induction (Jacob et al., 2013). A recent study of adolescents with BPD, however, did not report deficits in this tract (Maier-Hein et al., 2014). Although there maybe methodological explanations for the differences in findings, this may reflect the fact that the maturation of the cingulum bundle as well as other fronto-limbic tracts is reported to continue through childhood and into adolescence (Lebel et al., 2008). The difference between our study and previous work may therefore reflect the development stage at which participants were assessed. In terms of white matter development, FA proceeds along an inverted U-shape curve, increasing through adolescence, and decreasing into adulthood, the latter described as ‘pruning’ of neural connections (New et al., 2013). Studies of normal development have indeed suggested there are significant relationships between FA values and inhibitory performance on cognitive tasks, as well as measures of impulsiveness (Silveri et al., 2006; Treit et al., 2014). Further longitudinal studies would therefore be required to fully explore timings of this deficit in the course of illness development.
We also report decreased structural integrity of the fornix in the BPD group. The fornix is another major tract of the limbic system and forms an arching bundle of fibers situated below the corpus callosum. It connects the hippocampus, entorhinal and perirhinal areas with the mamillary bodies, the hypothalamus, thalamus, cingulate cortex and nucleus accumbens (Saunders and Aggleton, 2007). As such it is also involved in the regulation of emotional brain regions by higher order cortical regions (Dalgleish, 2004), in reward processing (Salinas and White, 1998), and in the response to stress (Kim and Diamond, 2002), all important in the clinical and behavioral manifestations of BPD. The current finding of decreased integrity of the fornix in BPD is consistent with a recent study reporting deficits in structural integrity of the fornix versus both healthy controls and a ‘clinical control’ sample consisting of subjects with mixed psychiatric diagnoses who did not fulfill more than one of the nine DSM-IV diagnostic criteria of BPD (Maier-Hein et al., 2014). Furthermore, morphometric studies in BPD also indicate volumetric abnormalities in regions connected by the fornix, including the hippocampus, hypothalamus and cingulate cortex (Goodman et al., 2011; O'Neill and Frodl, 2012; Kuhlmann et al., 2013; Richter et al., 2014).
FA measures within the fornix were also related to several clinical features of the disorder. Specifically, there was a negative relationship with affective instability which is consistent with numerous prior studies reporting decreased fornix FA in other disorders with a major affective component, including bipolar disorder and major depressive disorder (Barnea-Goraly et al., 2009; Barysheva et al., 2013; Emsell et al., 2013), and since the fornix connects regions critically involved in emotional regulation. There was also a trend for a significant negative association between FA values in the fornix and the measure of impulsivity from the Zanarini scale. Although definitions of impulsivity differ, it is generally regarded as poorly conceived, risky, or inappropriate behavior, and the inability to appreciate the negative consequences of one's own actions. It therefore broadly involves deficits in the cognitive domains of executive control, reward processing, hence linking to regions such as those connected by the fornix, including the cingulate, thalamus and nucleus accumbens (Miyake et al., 2000; Moeller et al., 2001). Finally there was also a positive association between FA in the fornix and the item on the Zanarini rating abandonment. Other neuroimaging studies have also reported similar relationships between anterior cingulate volumes and activation with abandonment and attachment trauma scores (Schmahl et al., 2003b; Whittle et al., 2009). The current study therefore indicates the involvement of white matter tracts connecting these regions underlying such symptoms in the disorder.
Epidemiological research strongly indicates that early life adversity is highly correlated with the occurrence of the disorder (Zanarini et al., 1997; Zanarini et al., 2002). Previous imaging studies have showed associations between early trauma and altered white matter development (Lu et al., 2013). We did not, however, see this association in present study of BPD, suggesting that white matter deficits in BPD may not be strongly related to developmental experience, but may instead in part reflect genetic vulnerability, perhaps reflecting similar white matter changes in other affective disorders (Emsell et al., 2013).
We acknowledge that under strict statistical conditions it would be desirable to conduct Bonferroni multiple comparison corrections for the 3 regions of interest used in the current study. Under such conditions we note that only the correlation between FA measures from the cingulum and symptoms of inappropriate anger would remain significant. However, we considered the overall exploratory analysis approach justified given the preliminary nature of the study on such a modest sample size. Further replication in larger samples would be necessary to determine the robustness of the findings. The modest sample size and consequent lack of statistical power may have also contributed to the inability to detect similar associations with measures of childhood trauma.
Another limitation is that individuals with BPD often have a variety of co-morbidities and were on a range of treatments as described within Table 1, and this may contribute to sample heterogeneity. This reflects the nature of typical BPD populations and as such increases the generalizability of the current findings. Furthermore, there was no evidence that the observed deficits in whiter matter integrity could be explained by either medication class or measures of depression severity, which represented the most common comorbidity, although, due to modest group sizes, lack of such effects should be interpreted with caution.
It should also be noted that deficits in FA could be due to a number of factors, including differences in axonal density, myelination, diameter, membrane permeability, or in the orientational coherence of axons within voxels (Jones et al., 2013). We are unable therefore to interpret findings as providing evidence of a particular cellular pathology (Jones et al., 2013). Also, FA cannot accurately describe multifiber architecture and might be influenced by motion, eddy currents, misregistration, and partial volume effects (Zhan et al., 2009). Further study with techniques complementary to DTI would be necessary to provide additional information with regard to the true nature of these deficits.
In conclusion we report altered structural connectivity of two major fronto-limbic tracts in individuals with BPD indicating that these tracts play a key role in the pathogenesis of the disorder. These findings suggest that there are deficits in structural connectivity in tracts which connect regulatory regions such as the cingulate and prefrontal cortex with subcortical limbic regions, thereby resulting in abnormal emotional processing, emotional dysregulation, and poor impulse control characteristic of the disorder. Associations with symptom severity further indicate that disruption of cingulum and fornix white matter integrity contribute to mechanisms underlying symptomatology in BPD.
Acknowledgements
This work was supported by a Scottish Funding Council Senior Clinical Fellowship awarded to JH. The author HCW is supported by a Dorothy Hodgkin Fellowship from the Royal Society (DH080018) and by a JMAS SIM fellowship from the Royal College of Physicians of Edinburgh. AMM was supported by the Health Foundation through a Clinician Scientist Fellowship (Ref: 2268/4295), by the Brain and Behaviour Research Foundation through a NARSAD Independent Investigator Award and by a Scottish Funding Council Senior Clinical Fellowship. The investigators also acknowledge the financial support of National Health Service (NHS) Research, Scotland, through the Scottish Mental Health Research Network (http://www.smhrn.org.uk) who provided assistance with subject recruitment and cognitive assessments. All imaging aspects also received financial support from the Dr. Mortimer and Theresa Sackler Foundation. We would like to thank all of the participants who took part in the study and the radiographers who acquired the MRI scans. The authors would also like to thank Dr. Thorsten Feiweier from Siemens Healthcare for providing the prototype diffusion sequence used in this study.
References
- Barnea-Goraly N., Chang K.D., Karchemskiy A., Howe M.E., Reiss A.L. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol. Psychiatry. 2009;66(3):238–244. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Barysheva M., Jahanshad N., Foland-Ross L., Altshuler L.L., Thompson P.M. White matter microstructural abnormalities in bipolar disorder: a whole brain diffusion tensor imaging study. Neuroimage Clin. 2013;2:558–568. doi: 10.1016/j.nicl.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E., Woolrich M.W., Jenkinson M., Johansen-Berg H., Nunes R.G. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Ahluvalia T., Pogge D., Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Brambilla P., Soloff P.H., Sala M., Nicoletti M.A., Keshavan M.S. Anatomical MRI study of borderline personality disorder patients. Psychiatry Res. 2004;131(2):125–133. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Carrasco J.L., Tajima-Pozo K., Díaz-Marsá M., Casado A., López-Ibor J.J. Microstructural white matter damage at orbitofrontal areas in borderline personality disorder. J. Affect. Disord. 2012;139(2):149–153. doi: 10.1016/j.jad.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. The emotional brain. Nat. Rev. Neurosci. 2004;5(7):583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- Denson T.F., Pedersen W.C., Ronquillo J., Nandy A.S. The angry brain: neural correlates of anger, angry rumination, and aggressive personality. J. Cogn. Neurosci. 2009;21(4):734–744. doi: 10.1162/jocn.2009.21051. [DOI] [PubMed] [Google Scholar]
- Donegan N.H., Sanislow C.A., Blumberg H.P., Fulbright R.K., Lacadie C. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol. Psychiatry. 2003;54(11):1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Emsell L., Leemans A., Langan C., Van Hecke W., Barker G.J. Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biol. Psychiatry. 2013;73(2):194–201. doi: 10.1016/j.biopsych.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Goodman M., Hazlett E.A., Avedon J.B., Siever D.R., Chu K.W. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. J. Psychiatr. Res. 2011;45(6):803–807. doi: 10.1016/j.jpsychires.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Grant B.F., Chou S.P., Goldstein R.B., Huang B., Stinson F.S. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry. 2008;69(4):533–545. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J.E., Correia S., Brennan-Krohn T., Malloy P.F., Laidlaw D.H. Frontal white matter integrity in borderline personality disorder with self-injurious behavior. J. Neuropsychiatry Clin. Neurosci. 2007;19(4):383–390. doi: 10.1176/jnp.2007.19.4.383. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett E.A., New A.S., Newmark R., Haznedar M.M., Lo J.N. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biol. Psychiatry. 2005;58(8):614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Hazlett E.A., Zhang J., New A.S., Zelmanova Y., Goldstein K.E. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol. Psychiatry. 2012;72(6):448–456. doi: 10.1016/j.biopsych.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz S.C., Dietrich T.M., Wenning B., Krings T., Erberich S.G. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol. Psychiatry. 2001;50(4):292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Jacob G.A., Zvonik K., Kamphausen S., Sebastian A., Maier S. Emotional modulation of motor response inhibition in women with borderline personality disorder: an fMRI study. J. Psychiatry Neurosci. 2013;38(3):164–172. doi: 10.1503/jpn.120029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kamphausen S., Schröder P., Maier S., Bader K., Feige B. Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. World J. Biol. Psychiatry. 2013;14(4):307–318. doi: 10.3109/15622975.2012.665174. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Diamond D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Koenigsberg H.W., Denny B.T., Fan J., Liu X., Guerreri S. The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. Am. J. Psychiatry. 2014;171(1):82–90. doi: 10.1176/appi.ajp.2013.13070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann A., Bertsch K., Schmidinger I., Thomann P.A., Herpertz S.C. Morphometric differences in central stress-regulating structures between women with and without borderline personality disorder. J. Psychiatry Neurosci. 2013;38(2):129–137. doi: 10.1503/jpn.120039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lenzenweger M.F., Lane M.C., Loranger A.W., Kessler R.C. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol. Psychiatry. 2007;62(6):553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Wei Z., Gao W., Wu W., Liao M. White matter integrity alterations in young healthy adults reporting childhood trauma: a diffusion tensor imaging study. Aust N ZJ. Psychiatry. 2013;47(12):1183–1190. doi: 10.1177/0004867413508454. [DOI] [PubMed] [Google Scholar]
- Maier-Hein K.H., Brunner R., Lutz K., Henze R., Parzer P. Disorder-specific white matter alterations in adolescent borderline personality disorder. Biol. Psychiatry. 2014;75(1):81–88. doi: 10.1016/j.biopsych.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Fan J., New A.S., Tang C.Y., Siever L.J. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;155(3):231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg M.J., Poole J.H., Vinogradov S. A neurocognitive model of borderline personality disorder: effects of childhood sexual abuse and relationship to adult social attachment disturbance. Dev. Psychopathol. 2008;20(1):341–368. doi: 10.1017/S0954579408000163. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moeller F.G., Barratt E.S., Dougherty D.M., Schmitz J.M., Swann A.C. Psychiatric aspects of impulsivity. Am. J. Psychiatry. 2001;158(11):1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Nelson H.E. National Adult Reading Test. Test Manual. National Adult; Windsor: 1982. [Google Scholar]
- New A.S., Carpenter D.M., Perez-Rodriguez M.M., Ripoll L.H., Avedon J. Developmental differences in diffusion tensor imaging parameters in borderline personality disorder. J. Psychiatr. Res. 2013;47(8):1101–1109. doi: 10.1016/j.jpsychires.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill A., Frodl T. Brain structure and function in borderline personality disorder. Brain Struct. Funct. 2012;217(4):767–782. doi: 10.1007/s00429-012-0379-4. [DOI] [PubMed] [Google Scholar]
- Pandya D.N., Van Hoesen G.W., Mesulam M.M. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp. Brain Res. 1981;42(3–4):319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Pawliczek C.M., Derntl B., Kellermann T., Gur R.C., Schneider F. Anger under control: neural correlates of frustration as a function of trait aggression. PLOS One. 2013;8(10):e78503. doi: 10.1371/journal.pone.0078503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J., Brunner R., Parzer P., Resch F., Stieltjes B. Reduced cortical and subcortical volumes in female adolescents with borderline personality disorder. Psychiatry Res. 2014;221(3):179–186. doi: 10.1016/j.pscychresns.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Rüsch N., Bracht T., Kreher B.W., Schnell S., Glauche V. Reduced interhemispheric structural connectivity between anterior cingulate cortices in borderline personality disorder. Psychiatry Res. 2010;181(2):151–154. doi: 10.1016/j.pscychresns.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Salinas J.A., White N.M. Contributions of the hippocampus, amygdala, and dorsal striatum to the response elicited by reward reduction. Behav. Neurosci. 1998;112(4):812–826. doi: 10.1037//0735-7044.112.4.812. [DOI] [PubMed] [Google Scholar]
- Saunders R.C., Aggleton J.P. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus. 2007;17(5):396–411. doi: 10.1002/hipo.20276. [DOI] [PubMed] [Google Scholar]
- Schmahl C.G., Elzinga B.M., Vermetten E., Sanislow C., McGlashan T.H. Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biol. Psychiatry. 2003;54(2):142–151. doi: 10.1016/s0006-3223(02)01720-1. [DOI] [PubMed] [Google Scholar]
- Schmahl C.G., Vermetten E., Elzinga B.M., Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122(3):193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Schmierer K., Wheeler-Kingshott C.A., Boulby P.A., Scaravilli F., Altmann D.R. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35(2):467–477. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig D., Clarkin J.F., Goldstein M., Kernberg O.F., Tuescher O. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am. J. Psychiatry. 2007;164(12):1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Silveri M.M., Rohan M.L., Pimentel P.J., Gruber S.A., Rosso I.M. Sex differences in the relationship between white matter microstructure and impulsivity in adolescents. Magn. Reson. Imaging. 2006;24(7):833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Soloff P., Nutche J., Goradia D., Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Res. 2008;164(3):223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E., Sussmann J.E., Clugston A., Peel A., McKirdy J. White matter integrity in individuals at high genetic risk of bipolar disorder. Biol. Psychiatry. 2011;70(4):350–356. doi: 10.1016/j.biopsych.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L., Hesslinger B., Thiel T., Geiger E., Haegele K. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol. Psychiatry. 2003;54(2):163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Treit S., Chen Z., Rasmussen C., Beaulieu C. White matter correlates of cognitive inhibition during development: a diffusion tensor imaging study. Neuroscience. 2014;276:87–97. doi: 10.1016/j.neuroscience.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Whittle S., Chanen A.M., Fornito A., McGorry P.D., Pantelis C. Anterior cingulate volume in adolescents with first-presentation borderline personality disorder. Psychiatry Res. 2009;172(2):155–160. doi: 10.1016/j.pscychresns.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zanarini M.C., Vujanovic A.A., Parachini E.A., Boulanger J.L., Frankenburg F.R. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J. Pers. Disord. 2003;17(3):233–242. doi: 10.1521/pedi.17.3.233.22147. [DOI] [PubMed] [Google Scholar]
- Zanarini M.C., Williams A.A., Lewis R.E., Reich R.B., Vera S.C. Reported pathological childhood experiences associated with the development of borderline personality disorder. Am. J. Psychiatry. 1997;154(8):1101–1106. doi: 10.1176/ajp.154.8.1101. [DOI] [PubMed] [Google Scholar]
- Zanarini M.C., Yong L., Frankenburg F.R., Hennen J., Reich D.B. Severity of reported childhood sexual abuse and its relationship to severity of borderline psychopathology and psychosocial impairment among borderline inpatients. J. Nerv. Ment. Dis. 2002;190(6):381–387. doi: 10.1097/00005053-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Zetzsche T., Preuss U.W., Frodl T., Schmitt G., Seifert D. Hippocampal volume reduction and history of aggressive behaviour in patients with borderline personality disorder. Psychiatry Res. 2007;154(2):157–170. doi: 10.1016/j.pscychresns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Zhan L., Leow A.D., Zhu S., Baryshev M., Toga A.W. A novel measure of fractional anisotropy based on the tensor distribution function. Med image Comput Comput assist. Intervention. 2009;12(1):845–852. doi: 10.1007/978-3-642-04268-3_104. [DOI] [PubMed] [Google Scholar]