Abstract
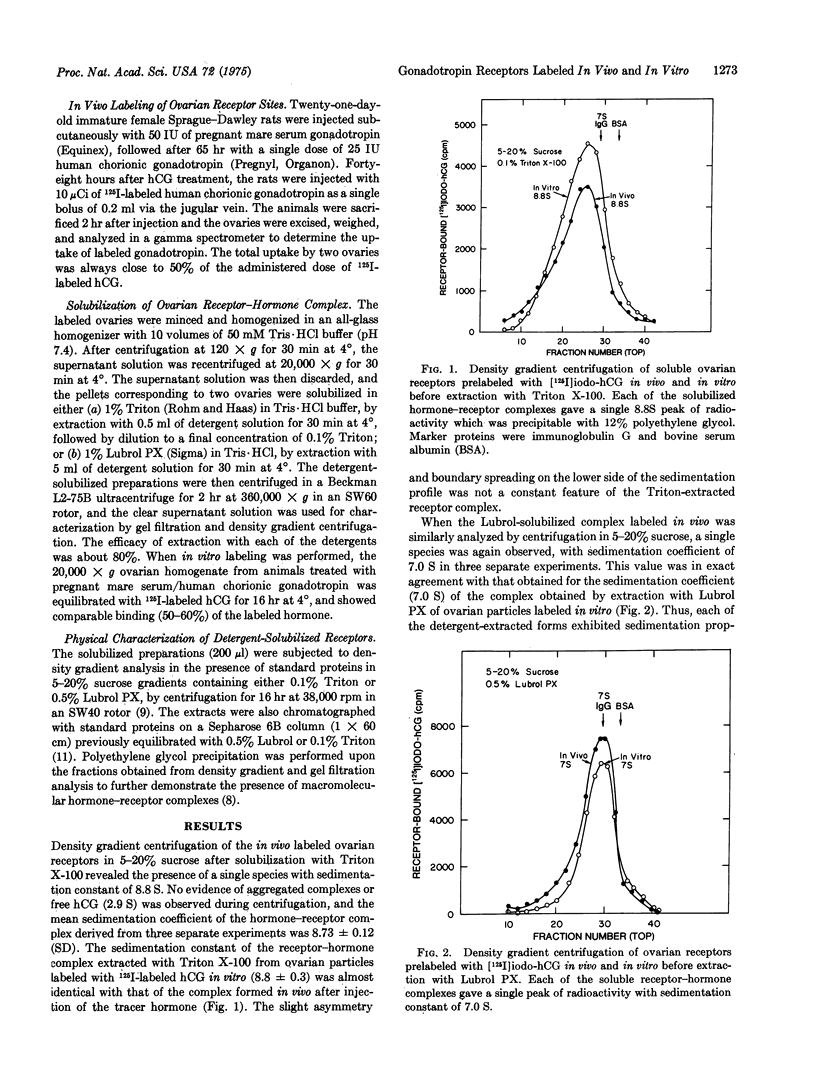
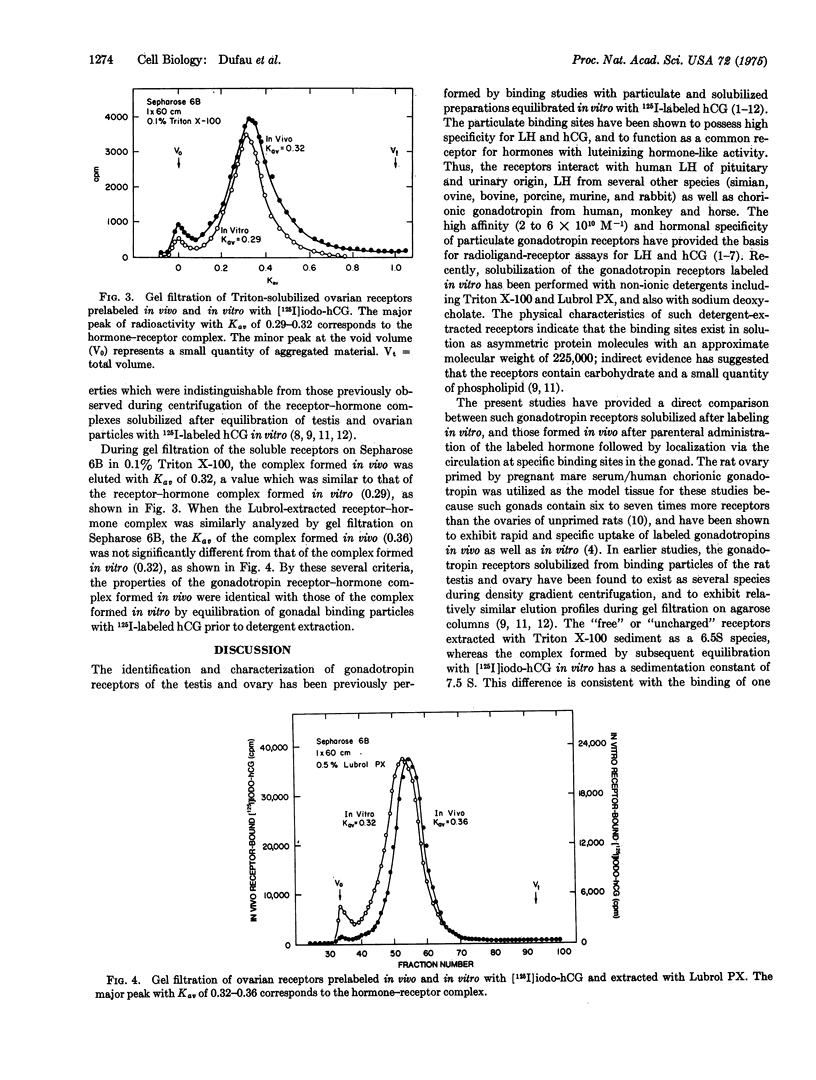
The physical properties of detergent-solubilized gonadotropin receptor-hormone complexes, determined by density gradient centrifugation and gel filtration, were compared after in vivo and in vitro labeling of specific ovarian binding sites with radioiodinated human chorionic gonadotropin (hCG). Following intravenous administration of biologically active 125I-labeled hCG, up to 50% of the gonadotropin tracer was bound to the luteinized ovaries of immature female rats treated with pregnant mare serum/human chorionic gonadotropin. Comparable binding of 125I-labeled hCG was observed after equilibration of ovarian particles with the labeled hormone in vitro. The sedimentation properties of the solubilized receptor-hormone complexes formed in vivo were identical with those derived for the corresponding complexes formed in vitro and extracted with Triton X-100 and Lubrol PX, with sedimentation constants of 8.8 S for the Triton-solubilized complex and 7.0 S for the complex extracted with Lubrol PX. During analytical gel filtration of the Triton-solubilized receptor-hormone complex on Sepharose 6B in 0.1% Triton X-100, the partition coefficient (Kav) of the "in vivo" complex (0.32) was not significantly different from that of the complex formed in vitro (0.29). Gel filtration of the Lubrol-solubilized ovarian particles on Sepharose 6B in 0.5% Lubrol PX gave Kav values for the "in vivo" and "in vitro" labeled complexes of 0.36 and 0.32, respectively. These findings demonstrate that the physical properties of size and shape which determine the partition coefficient and sedimentation characteristics of detergent-solubilized gonadotropin receptor-hormone complexes formed in vitro are not distinguishable from those of the complexes extracted after specific interaction of the ovarian gonadotropin receptors with radioiodinated hCG in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catt K. J., Dufau M. L. Interactions of LH and hCG with testicular gonadotropin receptors. Adv Exp Med Biol. 1973;36(0):379–418. doi: 10.1007/978-1-4684-3237-4_18. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L., Tsuruhara T. Radioligand-receptor assay of luteinizing hormone and chorionic gonadotropin. J Clin Endocrinol Metab. 1972 Jan;34(1):123–132. doi: 10.1210/jcem-34-1-123. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L., Tsuruhara T. Studies on a radioligand-receptor assay system for luteinizing hormone and chorionic gonadotropin. J Clin Endocrinol Metab. 1971 Jun;32(6):860–863. doi: 10.1210/jcem-32-6-860. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Tsuruhara T., Dufau M. L. Gonadotrophin binding sites of the rat testis. Biochim Biophys Acta. 1972 Aug 18;279(1):194–201. doi: 10.1016/0304-4165(72)90254-1. [DOI] [PubMed] [Google Scholar]
- Charreau E. H., Dufau M. L., Catt K. J. Multiple forms of solubilized gonadotropin receptors from the rat testis. J Biol Chem. 1974 Jul 10;249(13):4189–4195. [PubMed] [Google Scholar]
- Dufau M. L., Catt K. J. Extraction of soluble gonadotrophin receptors from rat testis. Nat New Biol. 1973 Apr 25;242(121):246–248. doi: 10.1038/newbio242246a0. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Charreau E. H., Catt K. J. Characteristics of a soluble gonadotropin receptor from the rat testis. J Biol Chem. 1973 Oct 25;248(20):6973–6982. [PubMed] [Google Scholar]
- Dufau M. L., Charreau E. H., Ryan D., Catt K. J. Soluble gonadotropin receptors of the rat ovary. FEBS Lett. 1974 Feb 15;39(2):149–153. doi: 10.1016/0014-5793(74)80038-4. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Tsuruhara T., Catt K. J. Interaction of glycoprotein hormones with agarose-concanavalin A. Biochim Biophys Acta. 1972 Sep 29;278(2):281–292. doi: 10.1016/0005-2795(72)90233-4. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Properties of the luteinizing hormone receptor of isolated bovine corpus luteum plasma membranes. J Biol Chem. 1973 Jul 25;248(14):5042–5049. [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. Interaction of ovarian receptors with human luteinizing hormone and human chorionic gonadotropin. Biochemistry. 1973 Nov 6;12(23):4609–4615. doi: 10.1021/bi00747a011. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. Luteinizing hormone receptors: specific binding of human luteinizing hormone to homogenates of luteinized rat ovaries. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3520–3523. doi: 10.1073/pnas.69.12.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tsuruhara T., Van Hall E. V., Dufau M. L., Catt K. J. Ovarian binding of intact and desialytated hcg in vivo and in vitro. Endocrinology. 1972 Aug;91(2):463–469. doi: 10.1210/endo-91-2-463. [DOI] [PubMed] [Google Scholar]


