Abstract
Aerobic methanotrophs oxidize methane at ambient temperatures and pressures and are therefore attractive systems for methane-based bioconversions. In this work, we developed and validated genetic tools for Methylomicrobium buryatense, a haloalkaliphilic gammaproteobacterial (type I) methanotroph. M. buryatense was isolated directly on natural gas and grows robustly in pure culture with a 3-h doubling time, enabling rapid genetic manipulation compared to many other methanotrophic species. As a proof of concept, we used a sucrose counterselection system to eliminate glycogen production in M. buryatense by constructing unmarked deletions in two redundant glycogen synthase genes. We also selected for a more genetically tractable variant strain that can be conjugated with small incompatibility group P (IncP)-based broad-host-range vectors and determined that this capability is due to loss of the native plasmid. These tools make M. buryatense a promising model system for studying aerobic methanotroph physiology and enable metabolic engineering in this bacterium for industrial biocatalysis of methane.
INTRODUCTION
Methane, the principal component of natural gas, is a promising feedstock for industrial biotechnology. Domestic natural gas supplies are increasing, and natural gas prices have remained lower than oil prices over the last decade (1, 2). Methane has also been identified as a prime mitigation target for reducing greenhouse gas emissions (3). Aerobic methanotrophs oxidize methane under ambient temperatures and pressures and are genetically tractable, making these bacteria potentially useful biocatalysts for converting natural gas into chemicals and liquid fuels via metabolic engineering (1, 4).
Previous industrial interest in aerobic methanotrophs has included attempts to produce biomass for use as single-cell protein or carotenoids in animal feed (5, 6). However, production of biomass from natural gas is complicated by the fact that nonmethane components of natural gas can be toxic to methanotrophs and can also provide substrates for growth of contaminating nonmethanotrophic bacterial species (5). Methylomicrobium buryatense is a gammaproteobacterial (type I) methanotroph that was isolated directly on natural gas from a soda lake in the Transbaikal region of Russia, a harsh environment with fluctuating temperatures, salinity, and pH levels (7). M. buryatense is naturally adapted to this environment and moderately haloalkaliphilic and consequently grows robustly in pure culture on natural gas, with increased resistance to contamination. The genome of this species was also recently sequenced and annotated by the Joint Genome Institute (8), enabling genomic manipulation for metabolic engineering. These characteristics make M. buryatense a strain of interest for industrial biotechnology.
Genetic manipulation of methanotrophic bacteria is often a slow process with marker exchange mutagenesis typically taking weeks per mutation, making it difficult to construct multiple mutations in a timely manner (9, 10). Counterselection protocols can accelerate the process of creating multiple mutations in a strain by eliminating the need for antibiotic marker recycling and can enable other unmarked genomic manipulations such as point mutations and promoter swaps. While there have been reports of successful unmarked allelic exchange using sucrose counterselection in Methylomonas sp. strain 16a (6) and Methylococcus capsulatus (Bath) (11), these methods are not widely used and have not been validated in most methanotroph species.
Replicable plasmids are another valuable genetic tool that can be used to express multiple copies of a gene of interest and to rapidly test different genetic constructs for metabolic engineering. A plasmid-based gene expression system has not yet been validated for M. buryatense. Broad-host-range replicons that have been successfully used in other methanotrophs include the RP4/RK2 replicon that defines incompatibility group P (IncP) (12) as well as the RSF1010 (IncQ) (13) and pBBR1 (11) replicons.
We report a set of genetic tools and strain modifications for genetic manipulation of M. buryatense strain 5GB1, including a sucrose counterselection system and small replicable vector. We validated these tools by knocking out glycogen production and analyzing relative promoter strengths in this methanotroph. This work provides a platform for metabolic engineering of M. buryatense for future biotechnological applications.
MATERIALS AND METHODS
Plasmid construction.
All plasmids were constructed via Gibson assembly (14) (Table 1). Select plasmids and their sequences have been deposited with the nonprofit plasmid repository Addgene (http://www.addgene.org/). The dTomato gene was amplified by PCR from Addgene plasmid 48688. A list of primers used for plasmid backbone amplification and construction of knockout vectors can be found in Table S1 in the supplemental material. A list of promoter sequences for dTomato promoter probe vectors can be found in Table S2 in the supplemental material.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| E. coli S17-1 λpir | Donor strain. Tpr Smr recA thi pro hsd(r− m+)RP4-2-Tc::Mu::Km Tn7 λpir | 28 |
| M. buryatense 5GB1 | Variant of M. buryatense 5G. Genotype differences are listed in Table S1 in the supplemental material. | This study |
| M. buryatense 5GB1S | Selected variant of of M. buryatense 5GB1 capable of being conjugated with small IncP-based plasmids. Missing native plasmid. | This study |
| M. buryatense 5GB1C | Variant of M. buryatense 5GB1 intentionally cured of native plasmid by knocking out repA (METBUDRAFT_0076/MBURv2_10076) to repB (METBUDRAFT_0086/MBURv2_10086) native plasmid locus. Capable of being conjugated with small IncP-based plasmids. | This study |
| M. buryatense ΔglgA1 | M. buryatense 5GB1 ΔglgA1 (METBUDRAFT_3833/MBURv2_210177) | This study |
| M. buryatense ΔglgA2 | M. buryatense 5GB1 ΔglgA2 (METBUDRAFT_3841/MBURv2_210185) | This study |
| M. buryatense ΔglgA1 ΔglgA2 | M. buryatense 5GB1 ΔglgA1 ΔglgA2 | This study |
| Plasmids | ||
| pCM66 | IncP-based broad-host-range plasmid | 22 |
| pVK100 | IncP-based cosmid | 23 |
| pCM433kanT | Minimized, Kanr version of pCM433 (19) with bla, tet, and cat antibiotic resistance genes removed. | This study |
| pAWP45 | Kanr variant of pCM433 containing flanks to knock out glgA1 | This study |
| pAWP47 | Kanr variant of pCM433 containing flanks to knock out glgA2 | This study |
| pAWP93 | pCM433kanT containing flanks to knock out repA-repB locus of native plasmid | This study |
| pAWP57 | pVK100 derivative used for selection of 5GB1S. Contains RP4/RK2 oriT and oriV as well as the trfA, kor, kfr, and krf operons, but not the trb and tra operons. | This study |
| pAWP78 | Minimized version of pCM66 with bla and tet antibiotic resistance gene fragments removed | This study |
| pAWP86 | pAWP78 containing dTomato with no promoter | This study |
| pAWP87 | pAWP78 containing dTomato driven by M. buryatense mxaF promoter | This study |
| pAWP88 | pAWP78 containing dTomato driven by lac promoter | This study |
| pAWP89 | pAWP78 containing dTomato driven by tac promoter | This study |
| pAWP92 | pAWP78 containing dTomato driven by M. buryatense rpoD promoter | This study |
Methylomicrobium buryatense culture and genetic manipulation.
For these studies, we used a variant of M. buryatense 5G that arose during laboratory culture, which we have named strain 5GB1 due to several differences identified by genome sequencing compared to the published draft genome of strain 5G (see Table S3 in the supplemental material) (7, 8).
M. buryatense strains were cultured in an atmosphere of 25% methane in air at 30°C. Plates were incubated in sealed jars (Oxoid Limited, Hampshire, United Kingdom), while liquid cultures were grown in 250-ml glass serum bottles (Kimble Chase, Vineland, NJ, USA) or 18- by 150-mm tubes (Bellco Glass, Vineland, NJ, USA) sealed with rubber stoppers and aluminum seals (Wheaton, Millville, NJ, USA). Cultures were grown in modified nitrate mineral salts medium (NMS2), which contains 0.2 g/liter MgSO4 · 7H2O, 0.02 g/liter CaCl2 · 6H2O, 1 g/liter KNO3, and 7.5 g/liter NaCl, as well as 1× trace elements (500× trace elements contains 1.0 g/liter Na2-EDTA, 2.0 g/liter FeSO4 · 7H2O, 0.8 g/liter ZnSO4 · 7H2O, 0.03 g/liter MnCl2 · 4H2O, 0.03 g/liter H3BO3, 0.2 g/liter CoCl2 · 6H2O, 0.6 g/liter CuCl2 · 2H2O, 0.02 g/liter NiCl2 · 6H2O, and 0.05 g/liter Na2MoO4 · 2H2O). Final concentrations of 50 mM sodium carbonate buffer, pH 9.5, and 2.3 mM phosphate buffer, pH 6.8, were added immediately before use.
All genetic manipulation was performed via conjugation on NMS2 mating plates, which contain less NaCl than the standard medium (2 g/liter) and are supplemented with 15% (vol/vol) nutrient broth. The final concentrations of sodium carbonate buffer and phosphate buffer were also adjusted to 5 mM and 5.8 mM, respectively. Attempts to introduce vectors via electroporation were unsuccessful. For conjugation, M. buryatense strains were spread onto an NMS2 mating plate and grown overnight. An equal volume of Escherichia coli S17-1 λpir donor biomass containing the vector of interest was then added to the plate, and the resulting mixture was incubated at 30°C for 2 days. This biomass was then spread onto NMS2 plates containing kanamycin (50 μg/ml) to select for transconjugants, which were subsequently purified from the donor via passaging on NMS2 plates containing rifamycin (50 μg/ml). For sucrose counterselection, double crossovers were selected for by plating kanamycin-resistant transconjugants (single crossovers) on NMS2 containing 2.5% (mass/vol) sucrose and rifamycin. Colonies were then checked for kanamycin sensitivity and genotyped via PCR, using either colony biomass as the PCR template or genomic DNA extracted with DNeasy blood and tissue kit number 69504 (Qiagen, Hilden, Germany).
Genome resequencing.
Genomic DNA was prepared via phenol-chloroform extraction. The genome sequencing was performed on an Illumina MiSeq with Illumina's 300-bp paired-end (PE) protocol (2 × 300 bp) by Genewiz (South Plainfield, NJ, USA) with multiplexing. The paired-end reads for each condition were pooled and processed with breseq version 0.24rc6 (15) against the MaGE (16) annotation of M. buryatense 5G from 26 November 2013 known as MBURv2. In addition, to identify gene duplications and deletions in an assembly-free approach, we mapped the reads to the 5G draft genome from MaGE using BWA version 0.7.8-r455 (17) using the BWA-MEM algorithm with eight threads and default options, followed by postprocessing of the alignments with SAMtools version 0.1.19-44428cd (18). The reads were attributed to genes with our in-house tool BAM2ORF to compute the reads per kilobase of gene per millions of reads in the sample (RPKMs). The RPKM ratios for different conditions were computed and examined manually.
Glycogen quantification.
Fifty milliliters of stationary-phase cultures was pelleted for 15 min at 5,000 × g. Bacterial pellets were lyophilized overnight, and dry cell weight was recorded for normalization. Cell pellets were then dissolved in 30% KOH and heated for 30 min at 95°C. The mixtures were then cooled on ice, and glycogen was precipitated with 2 volumes ethanol at −20°C overnight. Samples were spun at 21,000 × g for 10 min at room temperature, and the resulting pellets were resuspended in Milli-Q H2O. Samples were then acidified to pH 3 with 5 N HCl, reprecipitated with 1 volume ethanol at room temperature, and repelleted as before. The pellets were then washed with ethanol and resuspended in Abcam (Cambridge, MA, USA) hydrolysis buffer for glycogen quantification using the Abcam glycogen assay kit (ab65620).
dTomato reporter data.
Fifty milliliters of exponential-phase cultures was pelleted for 15 min at 5,000 × g and resuspended in 3 ml 50 mM Tris, pH 7.5. Cell extracts were then created by lysing the samples by French press and clearing them at 15,000 × g for 30 min. Total protein concentrations were measured by bicinchoninic acid (BCA; Pierce, Rockford, IL, USA) for normalization. One hundred microliters of cell extracts was assayed in a 96-well plate (Nunc black optical bottom) after incubation for 4 h at 37°C by measuring fluorescence by plate reader (Tecan Infinite F500) with excitation/emission of 535/590 nm.
Nucleotide sequence accession numbers.
The raw sequencing reads from M. buryatense 5GB1 and 5GB1S have been deposited with the NCBI as PRJNA265098 and PRJNA265105, respectively.
RESULTS AND DISCUSSION
Development and validation of a counterselection vector for unmarked allelic exchange.
In order to rapidly make multiple deletions in M. buryatense 5GB1, we sought to validate a counterselection system for unmarked allelic exchange. We started with the vector pCM433 (19), which contains the sacB gene to confer sucrose sensitivity and has been used for allelic exchange in nonmethanotrophic methylotrophs. pCM433 contains antibiotic resistance markers for tetracycline, chloramphenicol, and ampicillin; however, tetracycline is less stable at elevated pH, M. buryatense is resistant to chloramphenicol, and ampicillin resulted in predominantly false positives following conjugation. We therefore replaced the tetracycline and chloramphenicol resistance markers with one for kanamycin, which can be used for selection of transconjugants in M. buryatense 5GB1.
As a proof of concept, we used this vector to knock out glycogen production in M. buryatense 5GB1. Methylomicrobium species have been reported to accumulate very large quantities of intracellular glycogen (upwards of 30% cell dry weight) when grown on methanol or under nitrogen limitation (20, 21). This may significantly impact the carbon conversion efficiency of methane biocatalysis in an industrial process.
We assembled constructs and subsequently knocked out the genes encoding two glycogen synthases in M. buryatense 5GB1, glgA1 and glgA2, both individually and in combination. We found that 2.5% sucrose effectively selected for double crossovers during counterselection, while 5% sucrose resulted almost exclusively in false positives (sucrose resistant, kanamycin resistant) (data not shown). Neither the single glgA mutants nor the double mutant had a detectable growth defect (Fig. 1A). The single mutants showed intracellular glycogen content similar to that of the wild type in stationary-phase cultures, suggesting that these glycogen synthases may have redundant roles. No glycogen was detectable in the double mutant, confirming the elimination of glycogen synthesis in this strain (Fig. 1B). While M. buryatense 5GB1 produces low quantities of glycogen under our batch growth conditions, it is possible that larger quantities may be produced in an industrial process wherein fluctuations in nutrient availability may occur.
FIG 1.
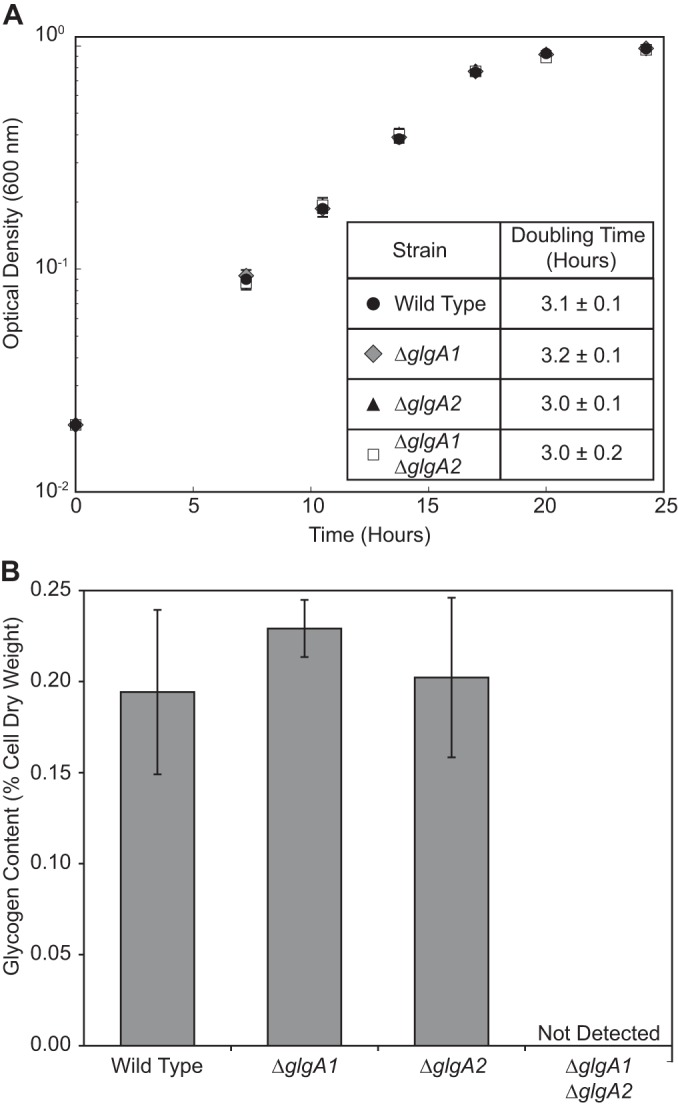
Analysis of M. buryatense 5GB1 glycogen synthase (glgA1, glgA2) mutants. Growth curves and doubling times (A) and intracellular glycogen contents (B) for wild-type and glycogen synthase mutants. Data represent the means from 3 replicates ± standard deviations.
After validating sucrose counterselection in M. buryatense using a kanamycin-resistant derivative of pCM433, we further minimized this vector for future use by removing the ampicillin resistance marker. A smaller vector helps facilitate the assembly of complete allelic exchange constructs in one step from PCR-amplified backbone and target flanking regions using Gibson assembly (14). The resulting vector, pCM433kanT (Fig. 2A), has functionality similar to that of the vector successfully used in Methylomonas sp. strain 16a (6). These results demonstrate that sucrose counterselection can be used to make multiple unmarked deletions in M. buryatense 5GB1.
FIG 2.
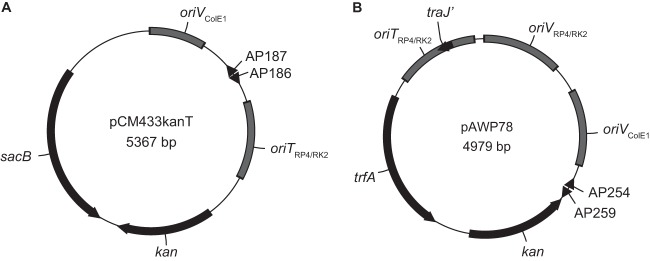
Genetic tools for M. buryatense. (A) Sucrose counterselection vector pCM433kanT for unmarked allelic exchange. (B) Minimized IncP-based broad-host-range vector pAWP78 for plasmid-based gene expression. In both cases, the vector backbone was amplified using the annotated primers to add inserts of interest (AP186 and AP187 for pCM433kanT; AP259 and AP254 for pAWP78; see Table S1 in the supplemental material).
Selection of an M. buryatense 5GB1 variant that can be conjugated with small IncP-based broad-host-range plasmids.
Plasmid-based gene expression systems are useful genetic tools for strain manipulation, as they enable multiple genetic constructs to be rapidly tested. We therefore sought to develop and validate a plasmid system for M. buryatense. The 7.6-kb IncP-based broad-host-range plasmid pCM66 (22) was previously developed for use in nonmethanotrophic methylotrophs, but we were unable to introduce pCM66 into M. buryatense 5GB1. We then tested the much larger IncP-based cosmid pVK100 (23 kb) (23), which successfully resulted in kanamycin-resistant transconjugants, demonstrating that the IncP replicon is functional in this strain.
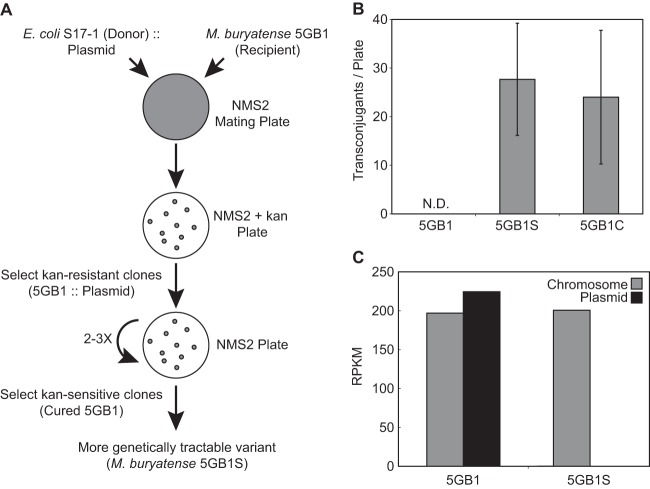
We took advantage of the fact that M. buryatense 5GB1 can be conjugated with pVK100 to select for a more genetically tractable variant strain (Fig. 3A). To do this, we conjugated a smaller derivative of pVK100 (called pAWP57) into M. buryatense 5GB1 and then isolated a strain cured of this plasmid by passaging multiple times on medium without kanamycin. We then repeated this mating and curing selection one more time. The resulting strain, 5GB1S, can be conjugated with the smaller IncP plasmid pCM66 with a frequency of roughly 10−9 per donor (Fig. 3B).
FIG 3.
Loss of M. buryatense 5GB1 native plasmid results in more genetically tractable variants capable of being conjugated with small IncP-based plasmids. (A) Selection scheme used to obtain the more genetically tractable variant strain 5GB1S. This selection was repeated twice. (B) Number of transconjugants containing the small IncP-based plasmid pCM66 for M. buryatense 5GB1, selected strain 5GB1S, and a strain intentionally cured of the native plasmid, 5GB1C. N.D., not detected. (C) Genome sequence reads mapped to chromosome versus native plasmid for strains 5GB1 and 5GB1S. RPKM, reads per kilobase of gene per millions of reads in the sample.
One potential explanation for this low conjugation frequency is that the plasmid is integrating into the M. buryatense chromosome. However, we can reliably reisolate the plasmid and there are no stretches of sequence homology between pCM66 and the published M. buryatense 5G genome (8), suggesting that integration is unlikely. Nevertheless, this draft genome sequence remains gapped, so we cannot rule out homology definitively. The genome sequence also contains multiple annotated restriction/modification (R/M) systems (24) that may explain the low mating frequencies. The fact that small plasmids can now be introduced into M. buryatense 5GB1S provides a good readout for further improvement of the genetic tractability of this strain, such as by deletion of these R/M systems.
In order to determine why M. buryatense 5GB1S can be conjugated with pCM66 but the 5GB1 strain cannot, we sequenced the 5GB1S genome. Notably, we discovered that 5GB1S no longer contains the native 80-kb plasmid found in M. buryatense 5GB1 (Fig. 3C). Despite this plasmid loss, there is no difference in growth rate under normal laboratory conditions between the 5GB1 and 5GB1S strains (see Fig. S1 in the supplemental material).
To determine if loss of this endogenous plasmid is necessary and sufficient to confer the pCM66 conjugation phenotype, we removed the plasmid from M. buryatense 5GB1 by knocking out a locus in the native plasmid containing annotated repA and repB genes with pCM433kanT using sucrose counterselection. The resulting strain, 5GB1C, no longer contains the endogenous plasmid as assessed by PCR at three separate loci (see Fig. S2 in the supplemental material). Strain 5GB1C can be conjugated with pCM66 at the same frequency and has no change in growth rate compared to strains 5GB1 and 5GB1S (Fig. 3B; see also Fig. S1 in the supplemental material).
These results demonstrate that strains 5GB1S and 5GB1C are able to replicate small IncP-based vectors due to curing of the native 80-kb plasmid found in M. buryatense 5GB1 and suggest that there is competition between small IncP-based vectors and the native M. buryatense plasmid. The native plasmid does not contain the canonical RP4/RK2 IncP replicon but may share other stability and maintenance functions that result in incompatibility between these plasmids. For example, the native plasmid contains korB, which suppresses expression of trfA, a gene necessary for replication of IncP vectors (25). The lack of growth phenotype also suggests that the native plasmid does not contain genes essential for growth under our conditions. Loss of this plasmid in M. buryatense strains 5GB1S and 5GB1C leads to a more genetically tractable strain capable of being conjugated with smaller vectors that are amenable to modern cloning techniques.
A trimmed IncP-based vector serves as a backbone for promoter probing.
After confirming that small IncP-based vectors can be successfully used for gene expression in M. buryatense 5GB1S, we sought to improve the vector backbone, pCM66. Smaller vectors are advantageous because they do not contain unnecessary genetic elements that may have unforeseen effects and they enable rapid plasmid construction from PCR products. pCM66 confers only kanamycin resistance but contains fragments of the bla and tetA genes as well as a complete tetR gene left over from restriction-based cloning methods (see Fig. S3 in the supplemental material). We removed these elements using Gibson assembly, resulting in the 5-kb plasmid pAWP78 (Fig. 2B).
Knowledge of relative promoter strengths is important for expressing genes in a given organism. To probe promoter strengths in M. buryatense 5GB1S, we constructed a series of vectors using the red fluorescent protein dTomato (26) as a reporter in the pAWP78 backbone. Fluorescent protein expression in methanotrophs is generally assayed in cell extracts (12). We found that cell extracts are also necessary to obtain maximal fluorescence from dTomato expression in M. buryatense 5GB1S, as can be seen by the large increase in fluorescence observed over a 200-min incubation at 37°C following cell disruption (Fig. 4). The ratio of fluorescent signals from different promoter probes also changes during incubation, making it crucial to allow the fluorescent signal to mature before comparing different promoter strengths. We were also able to use catechol 2,3-dioxygenase (encoded by xylE) successfully as a reporter in cell extracts but not beta-galactosidase (encoded by lacZ) (data not shown).
FIG 4.
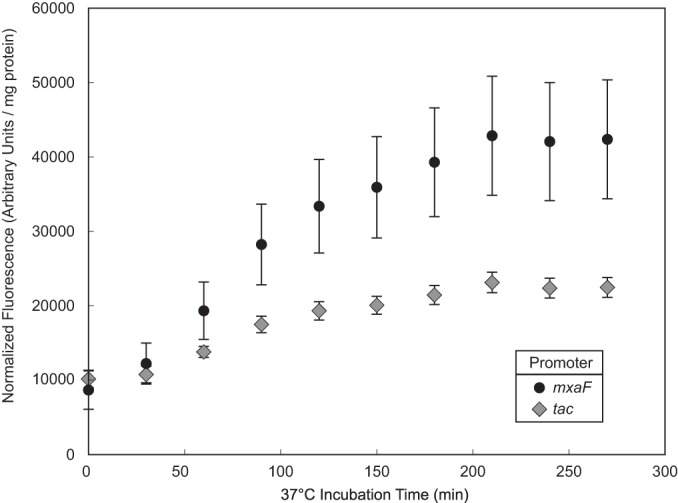
dTomato fluorescence increases over time after cell breakage. Cell extract was incubated at 37°C, and fluorescence (excitation, 535 nm; emission, 590 nm) was measured with a fixed gain at the time points indicated. Data represent the means from 3 replicates ± standard deviations.
In order to assay a wide range of gene expression levels in M. buryatense, we tested the commonly used lac and tac promoters, as well as endogenous promoters that drive expression of the M. buryatense methanol dehydrogenase gene mxaF and sigma factor gene rpoD (Fig. 5). To assess the heterogeneity of plasmid-based gene expression, we selected three different transconjugants after mating each promoter probe into M. buryatense 5GB1S. While we observed substantial heterogeneity between transconjugants harboring the same promoter probe, overall the four selected promoters provided distinct levels of expression that spanned 3 orders of magnitude.
FIG 5.
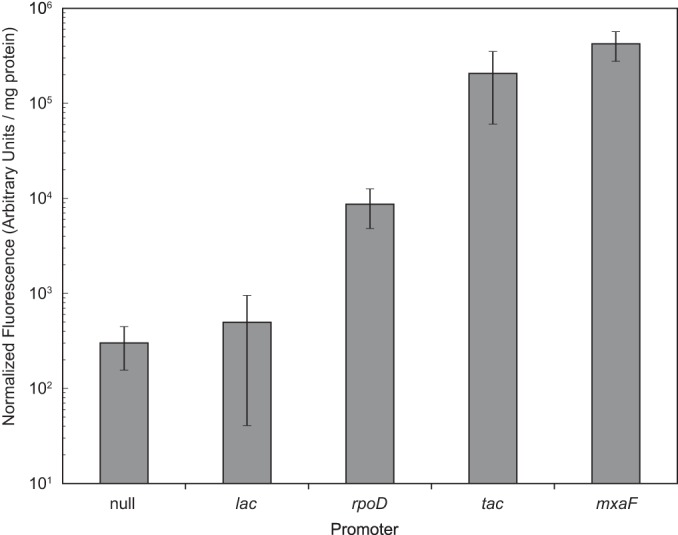
Relative promoter strengths in M. buryatense. Fluorescence (excitation, 535 nm; emission, 590 nm) was measured in cell extracts of M. buryatense 5GB1S containing probe vectors with promoter-dTomato fusions and normalized to protein content. Data represent the mean results for 3 distinct transconjugants ± standard deviations.
We also tested the relative strengths of these promoters in E. coli whole cells (see Fig. S4 in the supplemental material). We did not detect any activity for the endogenous mxaF promoter in E. coli, although this was the strongest of the promoters tested in M. buryatense. This promoter therefore provides a potentially useful tool for cloning genes that are toxic to E. coli for expression in M. buryatense, such as the genes that encode the particulate methane monooxygenase (27), the primary methane oxidation system in most aerobic methanotrophs.
These plasmids were conjugated into M. buryatense 5GB1S via the commonly used E. coli donor strain S17-1 (28). S17-1 contains a chromosomally integrated copy of the IncP plasmid RP4 and has been reported to modify transferred plasmids by inserting DNA from the S17-1 chromosome (29). To determine if our vectors are modified after conjugation into M. buryatense, we reisolated plasmids from the 15 transconjugants used to compare promoter strengths. We detected insertions of RP4 DNA downstream of the promoter probe plasmid origin of transfer in 10 cases (data not shown). These modifications presumably arose via recombination between the promoter probe and RP4 DNA cotransferred from the S17-1 genome. However, the presence or absence of these insertions did not correlate with differences in expression between transconjugants.
Together, our results show that genes can be reproducibly expressed at distinct levels using the plasmid system and different promoters (Fig. 5). Plasmids also enable genes to be transiently expressed in M. buryatense, as a plasmid can later be removed by curing. Transient gene expression is useful for developing additional genetic tools for metabolic engineering in this methanotroph, including expressing the Cre recombinase for unmarking of transposon mutants via the cre-lox system (30) or the Cas9 endonuclease for CRISPR/Cas genome editing (31).
The tools described here enable genetic modification of M. buryatense, an aerobic methanotroph with robust growth characteristics in pure culture. M. buryatense has the potential to serve as a promising system for studying aerobic methanotroph physiology and for industrial biocatalysis of methane via metabolic engineering. These streamlined plasmids may also serve as useful tools for genetic manipulation of additional methanotroph species as well as other Gram-negative bacteria.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Lidstrom Lab for helpful discussions.
This work was supported by ARPA-E (DE-AR0000350).
Plasmid pCM433 was generously provided by C. Marx (University of Idaho). We thank M. Moore (MKSCC) for providing the dTomato PCR template via Addgene. This work was facilitated through the use of advanced computational storage and networking infrastructure provided by the Hyak supercomputer system supported in part by the University of Washington eScience Institute.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03795-14.
REFERENCES
- 1.Fei Q, Guarnieri MT, Tao L, Laurens LM, Dowe N, Pienkos PT. 2014. Bioconversion of natural gas to liquid fuel: opportunities and challenges. Biotechnol Adv 32:596–614. doi: 10.1016/j.biotechadv.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Haynes CA, Gonzalez R. 2014. Rethinking biological activation of methane and conversion to liquid fuels. Nat Chem Biol 10:331–339. doi: 10.1038/nchembio.1509. [DOI] [PubMed] [Google Scholar]
- 3.Shindell D, Kuylenstierna JC, Vignati E, van Dingenen R, Amann M, Klimont Z, Anenberg SC, Muller N, Janssens-Maenhout G, Raes F, Schwartz J, Faluvegi G, Pozzoli L, Kupiainen K, Hoglund-Isaksson L, Emberson L, Streets D, Ramanathan V, Hicks K, Oanh NT, Milly G, Williams M, Demkine V, Fowler D. 2012. Simultaneously mitigating near-term climate change and improving human health and food security. Science 335:183–189. doi: 10.1126/science.1210026. [DOI] [PubMed] [Google Scholar]
- 4.Hanson RS, Hanson TE. 1996. Methanotrophic bacteria. Microbiol Rev 60:439–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bothe H, Moller Jensen K, Mergel A, Larsen J, Jorgensen C, Bothe H, Jorgensen L. 2002. Heterotrophic bacteria growing in association with Methylococcus capsulatus (Bath) in a single cell protein production process. Appl Microbiol Biotechnol 59:33–39. doi: 10.1007/s00253-002-0964-1. [DOI] [PubMed] [Google Scholar]
- 6.Ye RW, Yao H, Stead K, Wang T, Tao L, Cheng Q, Sharpe PL, Suh W, Nagel E, Arcilla D, Dragotta D, Miller ES. 2007. Construction of the astaxanthin biosynthetic pathway in a methanotrophic bacterium Methylomonas sp. strain 16a. J Ind Microbiol Biotechnol 34:289–299. doi: 10.1007/s10295-006-0197-x. [DOI] [PubMed] [Google Scholar]
- 7.Kaluzhnaya M, Khmelenina V, Eshinimaev B, Suzina N, Nikitin D, Solonin A, Lin J-L, McDonald I, Murrell C, Trotsenko Y. 2001. Taxonomic characterization of new alkaliphilic and alkalitolerant methanotrophs from soda lakes of the southeastern Transbaikal region and description of Methylomicrobium buryatense sp. nov. Syst Appl Microbiol 24:166–176. doi: 10.1078/0723-2020-00028. [DOI] [PubMed] [Google Scholar]
- 8.Khmelenina VN, Beck DAC, Munk C, Davenport K, Daligault H, Erkkila T, Goodwin L, Gu W, Lo C-C, Scholz M, Teshima H, Xu Y, Chain P, Bringel F, Vuilleumier S, Dispirito A, Dunfield P, Jetten MSM, Klotz MG, Knief C, Murrell JC, Op den Camp HJM, Sakai Y, Semrau J, Svenning M, Stein LY, Trotsenko YA, Kalyuzhnaya MG. 2013. Draft genome sequence of Methylomicrobium buryatense strain 5G, a haloalkaline-tolerant methanotrophic bacterium. Genome Announc 1:e00053–13. doi: 10.1128/genomeA.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crombie AT, Murrell JC. 2014. Trace-gas metabolic versatility of the facultative methanotroph Methylocella silvestris. Nature 510:148–151. doi: 10.1038/nature13192. [DOI] [PubMed] [Google Scholar]
- 10.Csáki R, Bodrossy L, Klem J, Murrell JC, Kovács KL. 2003. Genes involved in the copper-dependent regulation of soluble methane monooxygenase of Methylococcus capsulatus (Bath): cloning, sequencing and mutational analysis. Microbiology 149:1785–1795. doi: 10.1099/mic.0.26061-0. [DOI] [PubMed] [Google Scholar]
- 11.Welander PV, Summons RE. 2012. Discovery, taxonomic distribution, and phenotypic characterization of a gene required for 3-methylhopanoid production. Proc Natl Acad Sci U S A 109:12905–12910. doi: 10.1073/pnas.1208255109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali H, Murrell JC. 2009. Development and validation of promoter-probe vectors for the study of methane monooxygenase gene expression in Methylococcus capsulatus Bath. Microbiology 155:761–771. doi: 10.1099/mic.0.021816-0. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd JS, Finch R, Dalton H, Murrell JC. 1999. Homologous expression of soluble methane monooxygenase genes in Methylosinus trichosporium OB3b. Microbiology 145(Part 2):461–470. doi: 10.1099/13500872-145-2-461. [DOI] [PubMed] [Google Scholar]
- 14.Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 15.Deatherage DE, Barrick JE. 2014. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol 1151:165–188. doi: 10.1007/978-1-4939-0554-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallenet D, Belda E, Calteau A, Cruveiller S, Engelen S, Lajus A, Le Fevre F, Longin C, Mornico D, Roche D, Rouy Z, Salvignol G, Scarpelli C, Thil Smith AA, Weiman M, Medigue C. 2013. MicroScope—an integrated microbial resource for the curation and comparative analysis of genomic and metabolic data. Nucleic Acids Res 41:D636–D647. doi: 10.1093/nar/gks1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing Subgroup . 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx CJ. 2008. Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res Notes 1:1. doi: 10.1186/1756-0500-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eshinimaev BT, Khmelenina VN, Sakharovskii VG, Suzina NE, Trotsenko YA. 2002. Physiological, biochemical, and cytological characteristics of a haloalkalitolerant methanotroph grown on methanol. Microbiology 71:512–518. doi: 10.1023/A:1020594300166. [DOI] [PubMed] [Google Scholar]
- 21.Khmelenina VN, Kalyuzhnaya MG, Sakharovsky VG, Suzina NE, Trotsenko YA, Gottschalk G. 1999. Osmoadaptation in halophilic and alkaliphilic methanotrophs. Arch Microbiol 172:321–329. doi: 10.1007/s002030050786. [DOI] [PubMed] [Google Scholar]
- 22.Marx CJ, Lidstrom ME. 2001. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147:2065–2075. [DOI] [PubMed] [Google Scholar]
- 23.Knauf VC, Nester EW. 1982. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid 8:45–54. doi: 10.1016/0147-619X(82)90040-3. [DOI] [PubMed] [Google Scholar]
- 24.Wilson GG, Murray NE. 1991. Restriction and modification systems. Annu Rev Genet 25:585–627. doi: 10.1146/annurev.ge.25.120191.003101. [DOI] [PubMed] [Google Scholar]
- 25.Thomas CM, Smith CA. 1987. Incompatibility group P plasmids: genetics, evolution, and use in genetic manipulation. Annu Rev Microbiol 41:77–101. doi: 10.1146/annurev.mi.41.100187.000453. [DOI] [PubMed] [Google Scholar]
- 26.Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 27.Semrau JD, Chistoserdov A, Lebron J, Costello A, Davagnino J, Kenna E, Holmes AJ, Finch R, Murrell JC, Lidstrom ME. 1995. Particulate methane monooxygenase genes in methanotrophs. J Bacteriol 177:3071–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 29.Strand TA, Lale R, Degnes KF, Lando M, Valla S. 2014. A new and improved host-independent plasmid system for RK2-based conjugal transfer. PLoS One 9:e90372 doi: 10.1371/journal.pone.0090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmeros B, Wild J, Szybalski W, Le Borgne S, Hernandez-Chavez G, Gosset G, Valle F, Bolivar F. 2000. A family of removable cassettes designed to obtain antibiotic-resistance-free genomic modifications of Escherichia coli and other bacteria. Gene 247:255–264. doi: 10.1016/S0378-1119(00)00075-5. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. 2013. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol 31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.