Abstract
Two populations of Trichoplusia ni that had developed resistance to Bacillus thuringiensis sprays (Bt sprays) in commercial greenhouse vegetable production were tested for resistance to Bt cotton (BollGard II) plants expressing pyramided Cry1Ac and Cry2Ab. The T. ni colonies resistant to Bacillus thuringiensis serovar kurstaki formulations were not only resistant to the Bt toxin Cry1Ac, as previously reported, but also had a high frequency of Cry2Ab-resistant alleles, exhibiting ca. 20% survival on BollGard II foliage. BollGard II-resistant T. ni strains were established by selection with BollGard II foliage to further remove Cry2Ab-sensitive alleles in the T. ni populations. The BollGard II-resistant strains showed incomplete resistance to BollGard II, with adjusted survival values of 0.50 to 0.78 after 7 days. The resistance to the dual-toxin cotton plants was conferred by two genetically independent resistance mechanisms: one to Cry1Ac and one to Cry2Ab. The 50% lethal concentration of Cry2Ab for the resistant strain was at least 1,467-fold that for the susceptible T. ni strain. The resistance to Cry2Ab in resistant T. ni was an autosomally inherited, incompletely recessive monogenic trait. Results from this study indicate that insect populations under selection by Bt sprays in agriculture can be resistant to multiple Bt toxins and may potentially confer resistance to multitoxin Bt crops.
INTRODUCTION
Evolution of pesticide resistance in insect populations is a continuing threat to the efficacy of both synthetic and biological insecticides. Resistance of insects to the biological insecticide Bacillus thuringiensis (Bt), a naturally occurring soil bacterium, was first reported in the 1980s for a pest of stored grains, Plodia interpunctella (1). Resistance to Bt in Plutella xylostella and Trichoplusia ni has been identified following frequent applications of sprayable Bt formulations in agricultural situations (2–4). Since the mid-1990s, crops genetically engineered to contain insecticidal toxins from Bt have increasingly been adopted worldwide (5). With the wide adoption of Bt crops, insect resistance is a serious threat to their continuing success. Field populations of several major pest species have been reported to have developed resistance after Bt crop adoption, leading to increased crop damage (6, 7). More insect species have been reported to have increased frequencies of resistant alleles in field populations after the adoption of Bt crops (8–11).
Selective application of pesticides with different modes of action has been a principal strategy for insecticide resistance management. Similarly, Bt gene pyramiding, i.e., simultaneous expression of multiple toxins that have different binding sites in the target insects, is an effective strategy to delay the development of resistance to Bt crops. This has been promoted for Bt crops currently deployed in the United States and some other regions of the world (7, 12). Bt toxins Cry1Ac and Cry2Ab do not share the same binding sites in target Lepidoptera insects (13, 14) and have therefore become two primary Bt toxins pyramided in the new generation of Bt crops. This has been effective at slowing the development of resistance to Bt plants (15, 16). For continuing success of the pyramids of Cry1Ac and Cry2Ab, it is important to understand the development of resistance in insect pests to pyramided Cry1Ac-Cry2Ab crops.
While Bt crops with pyramided Bt toxins have been deployed in the field since 2002, sprayable Bt formulations that contain multiple toxins have been applied in the field for over half a century worldwide. B. thuringiensis serovar kurstaki (Btk) is the most commonly used strain in commercial sprayable formulations for lepidopteran pest control. Btk contains both Cry1A and Cry2A toxins (17, 18), so Btk formulations are virtually a bioinsecticide with pyramided Bt Cry1A and Cry2A toxins. Therefore, Btk-resistant insect populations may have developed resistance to multiple Bt toxins and offer opportunities to study resistance of insect populations to multiple Bt toxins selected in agriculture. To date, field-evolved resistance to Btk products has occurred in P. xylostella populations in vegetable fields and in T. ni populations in vegetable production greenhouses (4), and these Btk-resistant populations are known to be highly resistant to Cry1Ac (19–21), the major toxin in Btk.
T. ni is a major crucifer crop pest, but its host range covers more than 160 plant species from 36 families, including cotton and many other major crops (22). Resistance to Btk in greenhouse T. ni populations is polygenic in inheritance (23), suggesting the possibility that multiple resistance mechanisms are involved in resistance to multiple toxins. The trait of resistance to Cry1Ac in the Btk-resistant greenhouse T. ni population has been isolated and introgressed into a susceptible laboratory T. ni strain for analysis of Cry1Ac resistance selected in greenhouse populations (24). The resistance to Cry1Ac in Btk-resistant greenhouse T. ni populations is characteristic of the “mode 1” type of Bt resistance and is monogenically inherited and controlled by a single locus mapped to the region where the ABCC2 gene resides (21, 24–26). The underlying biochemical resistance mechanism is the loss of Cry1Ac binding sites in the midgut epithelium in resistant T. ni, associated with downregulation of the Cry1Ac receptor aminopeptidase N1 (APN1) in the midgut brush border membrane (27). Previous studies have confirmed that the high-level resistance to Cry1Ac in T. ni does not confer significant cross-resistance to Cry2Ab (24). In this study, two greenhouse-originated Btk-resistant T. ni strains (4) were examined for resistance to pyramided Cry1Ac-Cry2Ab-expressing BollGard II cotton plants, and BollGard II-resistant strains were established from the Btk-resistant populations. The levels of Cry2Ab resistance were determined, and the Cry2Ab resistance trait in T. ni was characterized to be incompletely recessive and genetically independent of Cry1Ac resistance by use of combinations with a Cry1Ac-resistant T. ni strain from the same original greenhouse population and biological assays with Cry2Ab toxin and cotton plants expressing no toxin, a single Cry1Ac toxin, and pyramided Cry1Ac-Cry2Ab dual toxins.
MATERIALS AND METHODS
T. ni strains and cotton plants.
A laboratory inbred strain of T. ni (Cornell strain) (21), which has been maintained in the laboratory for over 30 years and has never been exposed to Bt toxins, was used as a susceptible strain. Two T. ni strains resistant to the Bt formulation DiPel (Abbot Laboratories), namely, GLEN-DiPel and GIPN-DiPel, were used in this study. Both of the DiPel-resistant strains originated from Bt-resistant greenhouse populations (4). The strain GLEN-DiPel (21) was obtained from a Bt-resistant population on pepper plants in a commercial greenhouse and selected with DiPel in the laboratory (23). GIPN-DiPel was from a Bt-resistant greenhouse population on tomato plants, which was designated T2c in an earlier publication by Janmaat and Myers (4). GIPN-DiPel was selected with DiPel in the laboratory, in parallel to the selection of the GLEN-DiPel strain (23), to remove susceptible alleles in the colonies. A T. ni strain resistant to Cry1Ac, namely, GLEN-Cry1Ac-BCS (24), was also used in this study. GLEN-Cry1Ac-BCS was generated from GLEN-DiPel by a cross and multiple backcrosses with the susceptible laboratory strain and selections with Cry1Ac (24). The three resistant strains had stable resistance, to DiPel for the two Btk-resistant strains and to Cry1Ac for the GLEN-Cry1Ac-BCS strain, and were routinely maintained on a high-wheat-germ-based artificial diet (21), without further selection with DiPel or Bt toxins to minimize the likelihood of occurrence of laboratory-selected Bt resistance mechanisms in the strains.
Seeds of transgenic cotton BollGard I (event 531) (expresses Cry1Ac) and BollGard II (event 15985) (expresses Bt toxins Cry1Ac and Cry2Ab) plants, as well as their closely related non-Bt toxin-producing variety (Stoneville 474), were provided by the Monsanto Company (St. Louis, MO). Cotton plants were potted in formulated potting soil (Cornell mix) (28) and grown in a greenhouse.
Assay for response of T. ni larvae to BollGard II plants and selection of T. ni with BollGard II leaves.
The 3rd and 4th leaves of cotton plants were collected when the plants were 7 to 8 weeks old, at the stage of 6 or 7 leaves. The leaves were cut longitudinally into 3 or 4 pieces so that bioassays of T. ni larvae from different strains received cotton foliage from the same leaves. In addition to BollGard II plants, non-Bt cotton and BollGard I plants were also included in the assays as controls.
For bioassays to determine the susceptibility of T. ni larvae to non-Bt and Bt cotton plants, a piece of cotton leaf and newly hatched T. ni larvae were placed in 30-ml insect rearing cups, which were closed with lids and placed in an insect rearing chamber at 28°C with a relative humidity of 50% and a photoperiod of 16 h of light and 8 h of dark. For assays of the T. ni larvae on control non-Bt cotton leaves, 10 larvae were placed in each cup, with 5 replications. For assays of the T. ni larvae on BollGard I cotton leaves, 20 larvae were used per cup, with 8 to 10 replications. Larval mortality was examined 3 days after feeding on the cotton leaves.
For selection of T. ni strains resistant to BollGard II plants, neonate larvae of the GLEN-DiPel and GIPN-DiPel strains were reared on BollGard II leaves. Larval mortality was recorded 3 days after feeding on BollGard II leaves to assess the susceptibility to BollGard II plants, in parallel to the bioassays on non-Bt cotton and BollGard I plants described above. The larvae that survived after feeding on BollGard II leaves for 7 days were transferred to an artificial diet to allow completion of their larval development. Adjusted survival was calculated by dividing survival on Bt cotton leaves by survival on non-Bt cotton leaves (29). These BollGard II-selected colonies were repeatedly selected on BollGard II leaves in the same manner for 5 generations to establish stable BollGard II-resistant strains.
Observation of growth, development, and egg laying of T. ni on BollGard II plants.
To examine the larval growth, development, and egg laying of BollGard II-resistant T. ni larvae on BollGard II plants in comparison with those of BollGard II-resistant T. ni larvae on non-Bt cotton plants, newly hatched neonates were fed detached leaves from BollGard II or control non-Bt cotton plants in 30-ml rearing cups (10 larvae/cup) at 28°C as described above. After being reared in cups for 7 days, the larvae were transferred to BollGard II or control non-Bt cotton plants in the greenhouse to allow the larvae to feed freely on plants. Pupae were collected, weighed, and incubated at 28°C with a relative humidity of 50% and a photoperiod of 16 h of light and 8 h of dark to monitor emergence of adults.
To examine the fecundity of BollGard II-resistant T. ni reared on BollGard II plants, larvae of BollGard II-resistant T. ni were reared on BollGard II and non-Bt cotton plants as described above. Pupae were collected, and single-pair crosses were set up in 237-ml paper food containers with 10% sugar solution as the adults emerged. Eggs were collected and counted daily until the adults ceased egg laying. To determine the rate of egg hatching, 100 eggs were incubated at 28°C with a relative humidity of 50% and a photoperiod of 16 h of light and 8 h of dark, and the number of neonates emerging was counted.
Bioassays of level of resistance of T. ni to Cry2Ab.
To determine the level of resistance of BollGard II-resistant T. ni to Cry2Ab, larvae of the BollGard II-resistant T. ni strain GLEN-BGII, which had been reared on an artificial diet without exposure to Bt toxins for 20 generations, were used. Bioassays for susceptibility of T. ni larvae to Cry2Ab toxin were conducted using a diet surface overlay method (21). Briefly, 5 ml of artificial diet was poured into a 30-ml plastic rearing cup (diet surface area of ca. 7 cm2). Two hundred microliters of Cry2Ab toxin (protoxin; provided by the Monsanto Company) was evenly spread onto the diet surface, and 10 neonate larvae were placed into each rearing cup after the toxin solution had dried. Six to 8 Cry2Ab toxin concentrations were used for each assay, and each treatment contained 5 replications (cups). The rearing cups were placed in an incubator at 27°C with a relative humidity of 50% and a photoperiod of 16 h of light and 8 h of dark. The control was treated with the same procedures, except that no Cry2Ab was added to the diet. Larval mortality was recorded after 7 days, and observed mortality was corrected using Abbott's formula (30). Fifty percent lethal concentrations (LC50s) and 95% confidence intervals (95% CI) were determined by probit analysis using the computer program POLO (31). Resistance ratios were calculated by dividing the LC50 for a tested strain by the LC50 for the Cornell strain assayed under the same conditions.
Inheritance analysis of resistance to Cry2Ab in T. ni.
Genetic inheritance of the resistance of T. ni to Cry2Ab was analyzed using two methods: feeding on foliage of Bt cotton plants and feeding on Cry2Ab toxin in an artificial diet. For analysis of inheritance of resistance to Cry2Ab on BollGard II plants, reciprocal crosses were made between the BollGard II-resistant strain and the Cry1Ac-resistant strain GLEN-Cry1Ac-BCS. BollGard I foliage was used as a non-Cry2Ab control, and non-Bt cotton plant foliage was used as a non-Bt cotton control. To test the responses of F1 larvae from the reciprocal crosses to non-Bt cotton plants and Bt cotton plants (BollGard I and BollGard II plants), 100 or 150 neonate larvae were placed in 30-ml rearing cups (10 larvae/cup) provided with leaves from non-Bt cotton, BollGard I, or BollGard II plants and incubated at 28°C with a photoperiod of 16 h of light and 8 h of dark. Mortality was recorded 3 days after feeding on the leaves. Responses of F2 larvae from the cross between GLEN-Cry1Ac-BCS4 (male) and the BollGard II-resistant strain (female) to non-Bt cotton, BollGard I, and BollGard II plants were examined by the same method. In addition, a backcross was made between the F1 progeny (male) and the BollGard II-resistant strain (female), and the larvae from the backcross family were assayed for responses to non-Bt cotton, BollGard I, and BollGard II plant foliages, also as described above.
For analysis of inheritance of resistance by use of Cry2Ab toxin in an artificial diet, reciprocal crosses were made between the GLEN-BGII and Cornell strains to produce F1 progeny, and a backcross was made between the GLEN-BGII strain and the F1 larvae, as described above. The diet overlay bioassay method described above was used to determine the toxicity of Cry2Ab to the neonates from the two parental strains (GLEN-BGII and the Cornell strain), their F1 progeny, and the backcross progeny from the cross between the F1 progeny and the GLEN-BGII strain. The degree of dominance (D) for resistance was calculated based on the method described by Stone (32), as follows: D = (2 log LC50F1 − log LC50RR − log LC50SS)/(log LC50RR − log LC50SS), where log LC50RR is the log LC50 for the GLEN-BGII strain and log LC50SS is the log LC50 for the Cornell strain.
RESULTS
Survival of larvae of GLEN-DiPel and GIPN-DiPel strains on BollGard II leaves.
After feeding on BollGard II leaves for 7 days, larvae of the DiPel-resistant T. ni strain GLEN-DiPel had 21% ± 3% survival (mean ± standard error [SE]; n = 25, for 750 larvae in total), and larvae of GIPN-DiPel had 19% ± 3% survival (mean ± SE; n = 25, for 750 larvae in total). There were no surviving larvae of the susceptible Cornell strain or the Cry1Ac-resistant GLEN-Cry1Ac-BCS strain after 7 days on BollGard II leaves. The T. ni individuals that survived on BollGard II leaves were further selected for 4 additional generations on BollGard II leaves to establish BollGard II-resistant strains. The BollGard II-resistant strain from GLEN-DiPel was named GLEN-BGII, and the strain from GIPN-DiPel was named GIPN-BGII. After 4 generations of selection on BollGard II leaves, 50% ± 3% and 72% ± 3% of the larvae of the two selected strains survived on BollGard II leaves for 7 days (n = 25, for 500 larvae in total). In the parallel bioassay with non-Bt cotton leaves, the survival rates of the larvae of these two selected strains were 89% ± 3% and 92% ± 2%, respectively (n = 10, for 100 larvae in total). Therefore, the adjusted survival values (survival on Bt cotton leaves/survival on non-Bt cotton leaves) for the BollGard II-resistant larvae on BollGard II leaves after 7 days were 0.56 and 0.78, respectively, which are typical for most Bt-resistant insect strains and represent incomplete resistance (29). After 7 generations of selection, the adjusted survival values were 0.70 and 0.56 for the GLEN-BGII and GIPN-BGII strains, respectively. After 8 rounds of selection of the two resistant strains on BollGard II leaves performed in October 2008, these BollGard II-resistant strains were routinely maintained on an artificial diet without further exposure to Bt toxins. Selection of the resistant strains on BollGard II foliage was occasionally performed to examine and ensure the resistance in the strains (3 selections were performed in the period from October 2008 to July 2014).
Larval growth and development and adult fecundity of the GIPN-BGII strain feeding on BollGard II and non-Bt cotton plants.
On-plant rearing assays to examine the larval growth and development of the BollGard II-resistant strain GIPN-BGII on BollGard II plants showed that the larval growth and development from neonates to pupae on BollGard II plants (mean ± 95% CI, 36 ± 1 day) were slower than those on non-Bt cotton plants (28 ± 2 days) (Table 1). However, the pupal recovery rates from larvae that remained on BollGard II and non-Bt cotton plants in the greenhouse during their entire larval stage were not statistically different, with rates of 28% and 36%, respectively, for one experiment (Table 1) and 60% and 48%, respectively, for another independent replicate experiment (Table 2).
TABLE 1.
Larval development and pupal weights of T. ni strain GIPN-BGII on BollGard II and control non-Bt cotton plantsa
| Cotton plant strain | No. of larvae | No. (%) of pupae recovered | Days to pupation (mean ± 95% CI) | Pupal wt (mg) (mean ± 95% CI) |
|---|---|---|---|---|
| Control | 50 | 18 (36) | 28 ± 2 | 139 ± 14 (female), 182 ± 14 (male) |
| BollGard II | 98 | 27 (28) | 36 ± 1 | 148 ± 13 (female), 186 ± 16 (male) |
| P value | 0.35b | <0.01c | 0.38 (female), 0.73 (male)c |
One hundred percent emergence of pupae was observed for both groups.
Calculated by the chi-square test.
Calculated by the t test.
TABLE 2.
Egg laying of T. ni strain GIPN-BGII fed on BollGard II and control non-Bt cotton plantsa
| Cotton plant strain | No. of pupae recoveredb | Egg laying (no. of eggs/pair [mean ± SE]) (n) | % of hatching (mean ± SE) (n) |
|---|---|---|---|
| Control | 19 | 285 ± 143 (3) | 64 ± 15 (3) |
| BollGard II | 24 | 356 ± 84 (5) | 51 ± 18 (3) |
| P value | 0.37b | 0.80c | 0.32c |
Forty larvae were fed on each type of plant.
Calculated by the χ2 test.
Calculated by the t test.
Although the duration of development from neonates to pupae on BollGard II plants was 8 days longer than that on non-Bt cotton, the pupal weights of both males and females from BollGard II and non-Bt cotton plants were not statistically different, and the pupae had 100% emergence regardless of the host plant (Table 1). The adults of the BollGard II-resistant T. ni strain from BollGard II plants and from non-Bt cotton plants laid similar numbers of eggs, and the eggs had similar hatching rates (Table 2).
Independence of resistance of BollGard II-resistant T. ni to Cry1Ac and Cry2Ab in BollGard II plants.
Bioassays of larvae from the Cry1Ac-resistant strain GLEN-Cry1Ac-BCS, the BollGard II-resistant strain GLEN-BGII, and the F1 progeny from their reciprocal crosses on non-Bt, BollGard I, and BollGard II cotton leaves showed that larvae of the GLEN-Cry1Ac-BCS strain survived well on leaves from non-Bt cotton and BollGard I plants (mortalities of 0 to 8% and 2 to 7%, respectively), but they could not survive on leaves from BollGard II cotton plants (mortality of 100%) (Table 3). Larvae of the GLEN-BGII strain, however, not only could survive on non-Bt cotton and BollGard I plants (mortalities of 1 to 7% and 4 to 5%, respectively), but they also had high survival rates (61 to 67%) on BollGard II plant leaves (Table 3). The larvae from the F1 progeny from the reciprocal crosses of GLEN-Cry1Ac-BCS and GLEN-BGII could survive on the leaves from non-Bt cotton and BollGard I plants (mortalities of 1% to 7%), but they had very poor survival on leaves from BollGard II plants after 3 days (95% and 99% mortalities for larvae from the reciprocal crosses) (Table 3). However, these F1 survivors died within 7 days on BollGard II leaves. These results indicate that the genetic mechanism conferring resistance to Cry1Ac in the GLEN-BGII strain is the same as that in the GLEN-Cry1Ac-BCS strain and that the resistance to Cry2Ab in BollGard II-resistant T. ni is independent of the resistance to Cry1Ac. The resistance of T. ni to Cry2Ab in BollGard II leaves is autosomal and recessive.
TABLE 3.
Inheritance analysis of resistance to Cry2Ab in T. ni strain GLEN-BGII on cotton plants
| Expt | T. ni larvae | % survival on cotton plant leaves for 3 days |
||
|---|---|---|---|---|
| Non-Bt cotton control (100 larvae) | BollGard I (100 larvae) | BollGard II (150 larvae) | ||
| I | GLEN-Cry1Ac-BCS | 92 | 93 | 0 |
| GLEN-BGII | 93 | 95 | 61 | |
| F1a (GLEN-Cry1Ac-BCS ♂ × GLEN-BGII ♀) | 99 | 99 | 1a | |
| F1b (GLEN-Cry1Ac-BCS ♀ × GLEN-BGII ♂) | 93 | 98 | 5a | |
| II | GLEN-Cry1Ac-BCS | 100 | 98 | 1a |
| GLEN-BGII | 99 | 96 | 67 | |
| F2 (from GLEN-Cry1Ac-BCS ♂ × GLEN-BGII ♀) | 98 | 100 | 21 (expected value, 19; P = 0.61)b | |
| BCS (F1a ♀ × GLEN-BGII ♂) | 96 | 97 | 44 (expected value, 36; P = 0.10)c | |
These survivors all died within 7 days on BollGard II leaves.
Expected % survival = (% of average survival of F1a and F1b progeny)/2 + (% of survival of GLEN-BGII)/4. Statistical significance was determined with the χ2 test, using percentage data.
Expected % survival = (% of survival of F1b progeny)/2 + (% of survival of GLEN-BGII)/2. Statistical significance was performed with the χ2 test, using percentage data.
Level of Cry2Ab resistance and inheritance of the resistance in BollGard II-resistant T. ni.
In diet surface overlay bioassays, the LC50 of Cry2Ab for the susceptible strain of T. ni was 0.30 μg/ml (Table 4). However, in the parallel bioassays, the BollGard II-resistant strain, GLEN-BGII, had low mortalities at Cry2Ab concentrations of up to 440 μg/ml (Table 4). Therefore, the LC50 of Cry2Ab in GLEN-BGII is >1,467-fold higher than that of the susceptible strain. The LC50s for the F1 progeny from the reciprocal crosses between GLEN-BGII and the susceptible strains were similar (0.95 and 1.16 μg/ml) and were only minimally higher than that for the susceptible parent strain (3.2- and 3.9-fold) (Table 4). The degrees of dominance (D) of the resistance for the F1 progeny from the two reciprocal crosses were calculated to be −1 < D < −0.72 and −1 < D < −0.76. The autosomal and incomplete recessive resistance to Cry2Ab is also clearly shown in the dose-response (mortality) plot (Fig. 1). The dose-response lines for the two F1 populations are shifted to the right but are close to that of the susceptible strain. The dose-response curve for the larvae from the backcross population shows 2 phases: mortality increased with increasing doses of Cry2Ab from 0 to 4.4 μg/ml and then plateaued at ca. 50% mortality (5 probit units), showing characteristic monogenic inheritance of resistance.
TABLE 4.
Inheritance of Cry2Ab resistance in the GLEN-BGII strain of T. nib
| Strain | n | Mean slope (SE) | LC50 (95% CI) (μg/ml) | χ2 (df) | Resistance ratio | Da |
|---|---|---|---|---|---|---|
| Cornell strain (SS) | 350 | 3.69 (0.37) | 0.30 (0.26–0.33) | 3.31 (4) | 1.0 | |
| GLEN-BGII (RR) | 200 | NA | >440 | NA | >1,467 | |
| F1a (SS ♂ × RR ♀) | 300 | 2.71 (0.29) | 1.16 (0.96–1.38) | 2.46 (3) | 3.9 | −1 < D < −0.72 |
| F1b (RR ♂ × SS ♀) | 300 | 2.18 (0.26) | 0.95 (0.60–1.32) | 3.09 (3) | 3.2 | −1 < D < −0.76 |
The degree of dominance was calculated using the Stone formula (32).
NA, not available.
FIG 1.
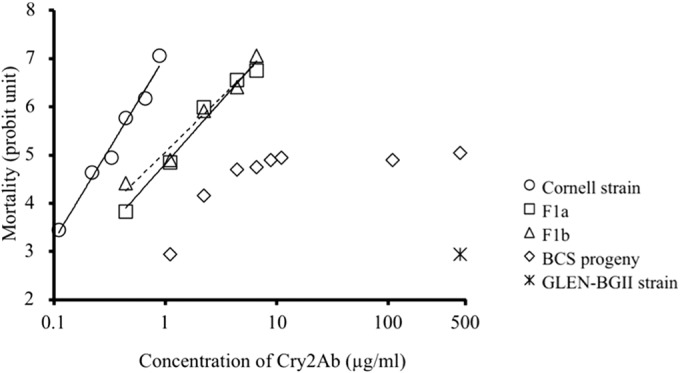
Responses of T. ni neonates of the susceptible strain (Cornell strain), the BollGard II-resistant strain (GLEN-BGII), the F1 progeny from the reciprocal crosses (F1a and F1b), and the progeny from the backcross (BCS progeny) to Cry2Ab.
In the examination of the inheritance of Cry2Ab resistance in cotton plants, bioassays of T. ni larvae on leaves from non-Bt cotton and BollGard I and BollGard II cotton plants showed that larvae of the F2 generation from the cross between GLEN-Cry1Ac-BCS and GLEN-BGII could survive on leaves from non-Bt cotton and BollGard I plants (mortalities of 2% and 0%, respectively) and also had 21% survival on leaves from BollGard II plants. This is statistically consistent with the expectation under the assumption that Cry2Ab resistance is a monogenic recessive trait (Table 3, data for experiment II). The larvae from a backcross between the F1 progeny of GLEN-Cry1Ac-BCS × GLEN-BGII and GLEN-BGII could survive on leaves from non-Bt cotton (4% mortality) and BollGard I (3% mortality) plants (Table 3), and 44% of these larvae also survived on leaves from BollGard II plants, which is also statistically consistent with Cry2Ab resistance being a monogenic recessive trait (Table 3). These results are consistent with the results of the diet overlay bioassays with Cry2Ab toxin described above (Fig. 1).
DISCUSSION
Bt strains that are used for microbial control of insect pests commonly produce multiple Cry toxins that are responsible for their insecticidal properties. The T. ni strains used in this study originated from greenhouse populations in British Columbia, Canada (4). These greenhouse populations were exposed to multiple Cry toxins in Btk sprays and could have been selected for resistance to multiple Bt toxins. The polygenic inheritance of Btk resistance in T. ni (23) suggests that these T. ni populations may have evolved multiple resistance mechanisms to the different Cry toxins from Btk. This study confirmed that T. ni populations resistant to a commercial product, DiPel, not only were highly resistant to Cry1Ac (21), the major Cry toxin in Btk, but also had a high frequency of a resistant allele(s) to Cry2A, another Cry toxin in Btk. These T. ni populations had ca. 20% survival on foliage of BollGard II cotton plants, which express both Cry1Ac and Cry2Ab. The Cry2Ab-resistant alleles were evidently selected in both the GLEN-DiPel and GIPN-DiPel strains by Btk, as resistance to Cry1Ac alone does not confer resistance to BollGard II plants (Table 3). However, the frequencies of the Cry2Ab-resistant allele in these two strains were not 100%, likely due to the relatively low Cry2A content in Btk (33). After 4 rounds of selection on BollGard II leaves, 50 to 72% of the larvae survived on BollGard II leaves in 7-day feeding assays, compared to 89 to 92% survival on non-Bt cotton leaves. Therefore, the results from this study show that Btk-resistant T. ni from greenhouse populations had developed resistance to Cry1Ac and Cry2Ab, indicating that application of sprayable Bt formulations in agriculture may select for multiple resistance alleles for multiple Cry toxins in insect populations.
Cry1Ac and Cry2Ab are known to bind to different receptors in the midguts of lepidopteran larvae (13, 14). Therefore, Cry1Ac and Cry2Ab have become two primary Bt toxins pyramided in new Bt cotton varieties. The Btk strain is commonly used in Bt formulations for lepidopteran pest control and is known to harbor genes coding for the toxins Cry1Aa, Cry1Ab, Cry1Ac, Cry2Aa, and Cry2Ab. Although the Cry2Ab gene in Btk is cryptic (34), Cry2Aa is produced and shares the same binding site with Cry2Ab in the insect midgut (13). Therefore, insect populations highly resistant to Btk formulations may already be resistant to Bt crops with pyramided Cry1A and Cry2A toxins. This study confirms such a selection of resistance to both Cry1Ac and Cry2Ab by the application of Bt sprays in agriculture.
Genetic complementation tests that assayed the survival of the progeny from reciprocal crosses between the Cry1Ac-resistant strain (GLEN-Cry1Ac-BCS) and the BollGard II-resistant strain (GLEN-BGII) on leaves of BollGard I and BollGard II plants (Table 3) indicated that (i) BollGard II-resistant T. ni shares a genetic mechanism with the GLEN-Cry1Ac-BCS strain for resistance to BollGard I and (ii) resistance to Cry2Ab in BollGard II-resistant T. ni is autosomal and recessive. Further bioassays of the F2 and backcross populations generated between the GLEN-Cry1Ac-BCS and GLEN-BGII strains (Table 3, data for experiment II) indicated that resistance to Cry2Ab in T. ni is genetically independent of resistance to Cry1Ac, and the resistance to Cry2Ab appears to be a monogenic recessive trait. Cry1Ac resistance in T. ni is associated with downregulation of APN1 gene expression, and the resistance gene has been localized to the ABCC2 gene locus region (25, 27). This Cry1Ac resistance-conferring mechanism in T. ni does not confer cross-resistance to Cry2Ab (24). The bioassay data (Table 3) confirm that the Cry1Ac resistance mechanism in T. ni does not confer cross-resistance to Cry2Ab.
Bioassays of GLEN-BGII larvae on an artificial diet with Cry2Ab toxin further demonstrated the high LC50 of Cry2Ab for GLEN-BGII (>1,467-fold higher than that of the susceptible strain) (Table 4). Inheritance analysis of the Cry2Ab resistance in BollGard II-resistant T. ni on an artificial diet with Cry2Ab toxin (Table 4) further confirmed that the resistance to Cry2Ab in the T. ni larvae was autosomal and incompletely recessive, and the resistance trait appears to be monogenic (Fig. 1).
Resistance to Cry2Ab has been studied in laboratory strains of Heliothis virescens and Pectinophora gossypiella (35–37) and, recently, other cotton pest species, including Helicoverpa zea, Helicoverpa armigera, and Helicoverpa punctigera (8, 9, 38). The resistance to Cry2Ab in H. armigera and H. punctigera has been reported to be recessive (39, 40) and conferred by reduced binding of the toxin to the midgut (14). The molecular genetic mechanisms for Cry2Ab resistance are currently unknown for any insects. It will be important to understand how diverse or how conserved the molecular genetic mechanisms of Cry2Ab resistance are in these insects in order to understand the development of insect resistance to Cry2Ab in the field and to develop technology for management of insect resistance to Bt toxins.
T. ni is a highly polyphagous species, and its distribution covers all cotton production areas in the United States. Although T. ni is normally considered to be a secondary pest on cotton (41), if it is uncontrolled, T. ni may cause severe yield losses of over 90% (42). The Bt-resistant T. ni populations used in this study initially evolved resistance in commercial vegetable greenhouses in British Columbia, Canada, where no cotton is planted. However, wild populations of T. ni migrate annually from California to British Columbia. Although the overwintering populations may be exposed to Bt cotton, they were susceptible compared to resistant greenhouse T. ni populations (43).
Examination of the performance of BollGard II-resistant T. ni on BollGard II plants in comparison with that on non-Bt cotton plants showed similar levels of development to pupation (36% on control plants and 28% on BollGard II plants) (Table 1). A larger sample size may be needed to further evaluate survival of BollGard II-resistant T. ni on BollGard II plants. On BollGard II plants, the BollGard II-resistant larvae developed significantly more slowly than on control non-Bt plants. The slower larval development of resistant T. ni on BollGard II plants indicates that, in the field, there may be asynchrony of mating between the T. ni populations from non-Bt cotton and BollGard II cotton plants. Slower larval development on Bt plants than on non-Bt plants has been reported for several insect species, based on both laboratory and field observations (44–48). Asynchrony of mating between insects from non-Bt plants and Bt plants in the field has been reported to be a factor that may affect the refuge strategy for resistance management (44, 49).
Although the larval growth and development of BollGard II-resistant T. ni were slower on BollGard II plants, there was no significant difference in pupal weight and pupal emergence rate between the two groups (Table 1). Furthermore, no significant difference was observed between the two groups in terms of egg laying, measured as the number of eggs laid per pair of adults, or the egg hatching rate (Table 2). This suggests that larvae resistant to Bt cotton may cause similar levels of damage to Bt cotton and non-Bt cotton. Therefore, the potential of resistance development of T. ni in field populations cannot be ignored given the high adoption rate of Bt cotton in the United States (12).
ACKNOWLEDGMENTS
This project was supported by Cornell University Agricultural Experiment Station federal formula funds received from the USDA Cooperative State Research, Education, and Extension Service, by Agriculture and Food Research Initiative competitive grant 2008-35302-18806, and by Biotechnology Risk Assessment Grant Program competitive grant 2012-33522-19791, from the USDA National Institute of Food and Agriculture and Agricultural Research Service.
REFERENCES
- 1.McGaughey WH. 1985. Insect resistance to the biological insecticide Bacillus thuringiensis. Science 229:193–195. doi: 10.1126/science.229.4709.193. [DOI] [PubMed] [Google Scholar]
- 2.Tabashnik BE, Cushing NL, Finson N, Johnson MW. 1990. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 83:1671–1676. [Google Scholar]
- 3.Shelton AM, Robertson JL, Tang JD, Perez C, Eigenbrode SD, Preisler HK, Wilsey WT, Cooley RJ. 1993. Resistance of diamondback moth (Lepidoptera: Plutellidae) to Bacillus thuringiensis subspecies in the field. J Econ Entomol 86:697–705. [Google Scholar]
- 4.Janmaat AF, Myers J. 2003. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Proc Biol Sci 270:2263–2270. doi: 10.1098/rspb.2003.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James C. 2013. Global status of commercialized biotech/GM crops: 2013. ISAAA brief 46. ISAAA, Ithaca, NY. [Google Scholar]
- 6.Sumerford DV, Head GP, Shelton A, Greenplate J, Moar W. 2013. Field-evolved resistance: assessing the problem and ways to move forward. J Econ Entomol 106:1525–1534. doi: 10.1603/EC13103. [DOI] [PubMed] [Google Scholar]
- 7.Tabashnik BE, Brevault T, Carriere Y. 2013. Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31:510–521. doi: 10.1038/nbt.2597. [DOI] [PubMed] [Google Scholar]
- 8.Downes S, Parker T, Mahon R. 2010. Incipient resistance of Helicoverpa punctigera to the Cry2Ab Bt toxin in Bollgard II cotton. PLoS One 5:e12567. doi: 10.1371/journal.pone.0012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabashnik BE, Van Rensburg JB, Carriere Y. 2009. Field-evolved insect resistance to Bt crops: definition, theory, and data. J Econ Entomol 102:2011–2025. doi: 10.1603/029.102.0601. [DOI] [PubMed] [Google Scholar]
- 10.Tabashnik BE, Wu K, Wu Y. 2012. Early detection of field-evolved resistance to Bt cotton in China: cotton bollworm and pink bollworm. J Invertebr Pathol 110:301–306. doi: 10.1016/j.jip.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Wan P, Huang Y, Wu H, Huang M, Cong S, Tabashnik BE, Wu K. 2012. Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS One 7:e29975. doi: 10.1371/journal.pone.0029975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Cornejo J, Wechsler S, Livingston M, Mitchell L. 2014. Genetically engineered crops in the United States. Economic research report no. 162. US Department of Agriculture, Washington, DC. [Google Scholar]
- 13.Hernandez-Rodriguez CS, Van Vliet A, Bautsoens N, Van Rie J, Ferre J. 2008. Specific binding of Bacillus thuringiensis Cry2A insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl Environ Microbiol 74:7654–7659. doi: 10.1128/AEM.01373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caccia S, Hernandez-Rodriguez CS, Mahon RJ, Downes S, James W, Bautsoens N, Van Rie J, Ferre J. 2010. Binding site alteration is responsible for field-isolated resistance to Bacillus thuringiensis Cry2A insecticidal proteins in two Helicoverpa species. PLoS One 5:e9975. doi: 10.1371/journal.pone.0009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao JZ, Cao J, Li Y, Collins HL, Roush RT, Earle ED, Shelton AM. 2003. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat Biotechnol 21:1493–1497. doi: 10.1038/nbt907. [DOI] [PubMed] [Google Scholar]
- 16.Zhao JZ, Cao J, Collins HL, Bates SL, Roush RT, Earle ED, Shelton AM. 2005. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc Natl Acad Sci U S A 102:8426–8430. doi: 10.1073/pnas.0409324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koziel MG, Carozzi NB, Currier TC, Warren GW, Evola SV. 1993. The insecticidal crystal protein of Bacillus thuringiensis: past, present and future uses. Biotechnol Genet Eng Rev 11:171–228. doi: 10.1080/02648725.1993.10647901. [DOI] [Google Scholar]
- 19.Cao J, Zhao JZ, Tang D, Shelton M, Earle D. 2002. Broccoli plants with pyramided cry1Ac and cry1C Bt genes control diamondback moths resistant to Cry1A and Cry1C proteins. Theor Appl Genet 105:258–264. doi: 10.1007/s00122-002-0942-0. [DOI] [PubMed] [Google Scholar]
- 20.Tabashnik BE, Malvar T, Liu YB, Finson N, Borthakur D, Shin BS, Park SH, Masson L, de Maagd RA, Bosch D. 1996. Cross-resistance of the diamondback moth indicates altered interactions with domain II of Bacillus thuringiensis toxins. Appl Environ Microbiol 62:2839–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kain WC, Zhao JZ, Janmaat AF, Myers J, Shelton AM, Wang P. 2004. Inheritance of resistance to Bacillus thuringiensis Cry1Ac toxin in a greenhouse-derived strain of cabbage looper (Lepidoptera: Noctuidae). J Econ Entomol 97:2073–2078. doi: 10.1603/0022-0493-97.6.2073. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland DWS, Greene GL. 1984. Cultivated and wild host plants, p 1–13. In Lingren PD, Greene GL (ed), Suppression and management of cabbage looper populations. USDA technical bulletin 1684. US Department of Agriculture, Washington, DC. [Google Scholar]
- 23.Janmaat AF, Wang P, Kain W, Zhao JZ, Myers J. 2004. Inheritance of resistance to Bacillus thuringiensis subsp. kurstaki in Trichoplusia ni. Appl Environ Microbiol 70:5859–5867. doi: 10.1128/AEM.70.10.5859-5867.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang P, Zhao JZ, Rodrigo-Simon A, Kain W, Janmaat AF, Shelton AM, Ferre J, Myers J. 2007. Mechanism of resistance to Bacillus thuringiensis toxin Cry1Ac in a greenhouse population of the cabbage looper, Trichoplusia ni. Appl Environ Microbiol 73:1199–1207. doi: 10.1128/AEM.01834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baxter SW, Badenes-Perez FR, Morrison A, Vogel H, Crickmore N, Kain W, Wang P, Heckel DG, Jiggins CD. 2011. Parallel evolution of Bacillus thuringiensis toxin resistance in Lepidoptera. Genetics 189:675–679. doi: 10.1534/genetics.111.130971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Tiewsiri K, Kain W, Huang L, Wang P. 2012. Resistance of Trichoplusia ni to Bacillus thuringiensis toxin Cry1Ac is independent of alteration of the cadherin-like receptor for Cry toxins. PLoS One 7:e35991. doi: 10.1371/journal.pone.0035991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiewsiri K, Wang P. 2011. Differential alteration of two aminopeptidases N associated with resistance to Bacillus thuringiensis toxin Cry1Ac in cabbage looper. Proc Natl Acad Sci U S A 108:14037–14042. doi: 10.1073/pnas.1102555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boodley J, Sheldrake R Jr. 1982. Cornell peat-lite mixes for commercial plant growing. N Y Agric Exp Station Agric Info Bull 43. [Google Scholar]
- 29.Carrière Y, Crowder DW, Tabashnik BE. 2010. Evolutionary ecology of insect adaptation to Bt crops. Evol Appl 3:561–573. doi: 10.1111/j.1752-4571.2010.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbott WS. 1925. A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267. [Google Scholar]
- 31.LeOra Software. 1997. POLO-PC: probit and logit analysis. LeOra Software, Berkeley, CA. [Google Scholar]
- 32.Stone BF. 1968. A formula for determining degree of dominance in cases of monofactorial inheritance of resistance to chemicals. Bull World Health Organ 38:325–326. [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Fu Z, Ding X, Xia L. 2008. Evaluating the insecticidal genes and their expressed products in Bacillus thuringiensis strains by combining PCR with mass spectrometry. Appl Environ Microbiol 74:6811–6813. doi: 10.1128/AEM.01085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dankocsik C, Donovan WP, Jany CS. 1990. Activation of a cryptic crystal protein gene of Bacillus thuringiensis subspecies kurstaki by gene fusion and determination of the crystal protein insecticidal specificity. Mol Microbiol 4:2087–2094. doi: 10.1111/j.1365-2958.1990.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 35.Jurat-Fuentes JL, Gould FL, Adang MJ. 2003. Dual resistance to Bacillus thuringiensis Cry1Ac and Cry2Aa toxins in Heliothis virescens suggests multiple mechanisms of resistance. Appl Environ Microbiol 69:5898–5906. doi: 10.1128/AEM.69.10.5898-5906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gahan LJ, Ma YT, Coble ML, Gould F, Moar WJ, Heckel DG. 2005. Genetic basis of resistance to Cry1Ac and Cry2Aa in Heliothis virescens (Lepidoptera: Noctuidae). J Econ Entomol 98:1357–1368. doi: 10.1603/0022-0493-98.4.1357. [DOI] [PubMed] [Google Scholar]
- 37.Tabashnik BE, Unnithan GC, Masson L, Crowder DW, Li X, Carriere Y. 2009. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proc Natl Acad Sci U S A 106:11889–11894. doi: 10.1073/pnas.0901351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahon RJ, Olsen KM, Downes S. 2008. Isolations of Cry2Ab resistance in Australian populations of Helicoverpa armigera (Lepidoptera: Noctuidae) are allelic. J Econ Entomol 101:909–914. doi: 10.1603/0022-0493(2008)101[909:IOCRIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Mahon RJ, Young S. 2010. Selection experiments to assess fitness costs associated with Cry2Ab resistance in Helicoverpa armigera (Lepidoptera: Noctuidae). J Econ Entomol 103:835–842. doi: 10.1603/EC09330. [DOI] [PubMed] [Google Scholar]
- 40.Downes S, Parker TL, Mahon RJ. 2010. Characteristics of resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa punctigera (Lepidoptera: Noctuidae) isolated from a field population. J Econ Entomol 103:2147–2154. doi: 10.1603/EC09289. [DOI] [PubMed] [Google Scholar]
- 41.Leonard BR, Graves JB, Ellsworth RC. 1999. Insect and mite pests of cotton, p 489–552. In Smith CW, Cothren JT (ed), Cotton: origin, history, technology and production. John Wiley & Sons Inc, New York, NY. [Google Scholar]
- 42.Schwartz PH. 1983. Losses in yield of cotton due to insects, p 329–358. USDA Agriculture Handbook, 589th ed. US Department of Agriculture, Washington, DC. [Google Scholar]
- 43.Franklin MT, Ritland CE, Myers JH. 2010. Spatial and temporal changes in genetic structure of greenhouse and field populations of cabbage looper, Trichoplusia ni. Mol Ecol 19:1122–1133. doi: 10.1111/j.1365-294X.2010.04548.x. [DOI] [PubMed] [Google Scholar]
- 44.Liu YB, Tabashnik BE, Dennehy TJ, Patin AL, Bartlett AC. 1999. Development time and resistance to Bt crops. Nature 400:519. [DOI] [PubMed] [Google Scholar]
- 45.Liu YB, Tabashnik BE, Dennehy TJ, Patin AL, Sims MA, Meyer SK, Carriere Y. 2001. Effects of Bt cotton and crylac toxin on survival and development of pink bollworm (Lepidoptera: Gelechiidae). J Econ Entomol 94:1237–1242. doi: 10.1603/0022-0493-94.5.1237. [DOI] [PubMed] [Google Scholar]
- 46.Bird LJ, Akhurst RJ. 2004. Relative fitness of Cry1A-resistant and -susceptible Helicoverpa armigera (Lepidoptera: Noctuidae) on conventional and transgenic cotton. J Econ Entomol 97:1699–1709. doi: 10.1603/0022-0493-97.5.1699. [DOI] [PubMed] [Google Scholar]
- 47.Horner TA, Dively GP, Herbert DA. 2003. Development, survival and fitness performance of Helicoverpa zea (Lepidoptera: Noctuidae) in MON810 Bt field corn. J Econ Entomol 96:914–924. doi: 10.1603/0022-0493-96.3.914. [DOI] [PubMed] [Google Scholar]
- 48.Storer NP, Van Duyn JW, Kennedy GG. 2001. Life history traits of Helicoverpa zea (Lepidoptera: Noctuidae) on non-Bt and Bt transgenic corn hybrids in eastern North Carolina. J Econ Entomol 94:1268–1279. doi: 10.1603/0022-0493-94.5.1268. [DOI] [PubMed] [Google Scholar]
- 49.Peck SL, Gould F, Ellner SP. 1999. Spread of resistance in spatially extended regions of transgenic cotton: implications for management of Heliothis virescens (Lepidoptera: Noctuidae). J Econ Entomol 92:1–16. [Google Scholar]


