Abstract
Microbiologically influenced corrosion (MIC) of metallic materials imposes a heavy economic burden. The mechanism of MIC of metallic iron (Fe0) under anaerobic conditions is usually explained as the consumption of cathodic hydrogen by hydrogenotrophic microorganisms that accelerates anodic Fe0 oxidation. In this study, we describe Fe0 corrosion induced by a nonhydrogenotrophic nitrate-reducing bacterium called MIC1-1, which was isolated from a crude-oil sample collected at an oil well in Akita, Japan. This strain requires specific electron donor-acceptor combinations and an organic carbon source to grow. For example, the strain grew anaerobically on nitrate as a sole electron acceptor with pyruvate as a carbon source and Fe0 as the sole electron donor. In addition, ferrous ion and l-cysteine served as electron donors, whereas molecular hydrogen did not. Phylogenetic analysis based on 16S rRNA gene sequences revealed that strain MIC1-1 was a member of the genus Prolixibacter in the order Bacteroidales. Thus, Prolixibacter sp. strain MIC1-1 is the first Fe0-corroding representative belonging to the phylum Bacteroidetes. Under anaerobic conditions, Prolixibacter sp. MIC1-1 corroded Fe0 concomitantly with nitrate reduction, and the amount of iron dissolved by the strain was six times higher than that in an aseptic control. Scanning electron microscopy analyses revealed that microscopic crystals of FePO4 developed on the surface of the Fe0 foils, and a layer of FeCO3 covered the FePO4 crystals. We propose that cells of Prolixibacter sp. MIC1-1 accept electrons directly from Fe0 to reduce nitrate.
INTRODUCTION
Metallic iron (Fe0) and stainless steel corrosion causes a severe economic burden. The annual cost of corrosion worldwide has been estimated to exceed 3% of the world's GDP (http://www.nace.org/uploadedFiles/Publications/ccsupp.pdf). Under aerobic conditions, molecular-oxygen-mediated corrosion may be predominant, whereas under anaerobic conditions, microbiologically influenced corrosion (MIC) is believed to be a major cause of corrosion-related failures (1). Specifically, sulfate-reducing bacteria (SRB) are considered to be major causative microorganisms of MIC in anaerobic environments because FeS has frequently been observed as a major corrosion product (2).
The mechanism underlying Fe0 corrosion by SRB has been explained on the basis of the cathodic-depolarization theory: Fe0 oxidation occurs at the anode (Fe0 → Fe2+ + 2e−), while protons are reduced to molecular hydrogen at the cathode (2e− + 2H+ → H2). In the presence of SRB, a hydrogenase of the organism consumes either molecular hydrogen or electrons at the cathode, thereby accelerating the anodic Fe0 oxidation (3, 4). Recently, a methanogenic archaeon and an iron-oxidizing bacterium were shown to cause Fe0 corrosion (5–7). These reports suggest that diverse microorganisms contribute to metal corrosion.
MIC of metallic materials imposes a heavy economic burden, and technologies for MIC control are being actively investigated. However, the process of MIC is still poorly understood, and further research is required. The characterization of Fe0-corroding microorganisms and a clear understanding of the mechanism of MIC are required for effective MIC prevention and control. In this study, we isolated a novel Fe0-corroding bacterium from a crude-oil sample collected at an oil well and investigated its Fe0 corrosion activity under a variety of culture conditions.
MATERIALS AND METHODS
Sampling of crude oil.
Crude oil was sampled from an oil well in Akita Prefecture, Japan, on 16 December 2004. The oil sample was kept in a sealed nylon bag with an O2-absorbing agent and a CO2-generating agent (Anaero-Pack; Mitsubishi Gas Chemical, Tokyo, Japan) until inoculation on fresh culture medium.
Enrichment, isolation, and cultivation of a bacterial strain from a crude-oil sample.
Sw medium was composed of (liter−1) 0.54 g NH4Cl, 0.14 g KH2PO4, 0.20 g MgCl2·6H2O, 0.15 g CaCl2·2H2O, 2.5 g NaHCO3, and 1.0 ml of a modified trace element solution used by Touzel and Albagnac (8) lacking NaCl and supplemented with 4.0 mg/liter of Na2WO4·H2O. The pH of the medium was adjusted to 7.0 with 6 N HCl, and 20 ml of the medium was dispensed into each 50-ml serum bottle. Dissolved air was removed by flushing with N2-CO2 (4:1 [vol/vol]), and the bottles were sealed with butyl rubber stoppers. Prior to inoculation, 0.2 ml of vitamin solution (9) and 0.2 ml of a reductant solution containing 0.5 g/liter of Na2S and 0.5 g/liter of l-cysteine–HCl were added to each bottle after the filtration of the solutions through 0.2-μm-pore-size membrane filters.
Sw medium was supplemented with filter-sterilized 10 mM sulfate, 10 mM sodium pyruvate, and 0.01% (wt/vol) yeast extract (Becton Dickinson, Franklin Lakes, NJ, USA) to prepare SPYSw medium. The SPYSw medium (20 ml) was inoculated with 0.5 ml of the crude oil sampled from an oil well and cultivated at 25°C for 3 weeks. After the transfer of the culture into fresh medium, it was further cultivated for 3 weeks. This process was repeated several times, and strain MIC1-1 was purified on slants of SPYSw medium solidified with 1.5% (wt/vol) agar. Purification on agar slants was repeated several times until the cultures were deemed pure and a uniformly shaped axenic culture, designated strain MIC1-1, was obtained.
Bacterial strain.
Prolixibacter bellariivorans JCM 13498T was obtained from the Japan Collection of Microorganisms of the RIKEN Bioresource Center (RIKEN-BRC JCM) and maintained in JCM medium no. 512 (http://www.jcm.riken.jp/JCM/catalogue.shtml).
Microscopy.
Routine microscopic observation was performed with an IX-81 microscope (Olympus Co., Tokyo, Japan). Transmission electron micrographs were made with an H-7600 electron microscope (Hitachi High-Technologies Co., Tokyo, Japan) operated at 80 kV. Cells were stained with 1% (wt/vol) phosphotungstic acid (pH 6.5 to 7.0) and 0.04% (wt/vol) lead citrate.
Growth tests with possible electron donors and acceptors.
The use of various compounds as electron acceptors or donors by strain MIC1-1 was determined by growth tests and measurement of the consumption of the compounds. For the identification of electron acceptors, the growth of strain MIC1-1 was tested in a screw-cap tube (18 by 180 mm; Sanshin Industrial Co., Yokohama, Japan) containing 10 ml of Sw medium (pH 7.0) lacking NH4Cl and supplemented with 0.1 ml of the vitamin solution (9), 2 mM l-cysteine-HCl, 10 mM sodium pyruvate, 0.01% (wt/vol) yeast extract, and various concentrations of potential electron acceptors, including sulfate (10 mM), sulfite (2 mM), thiosulfate (5 mM), elemental sulfur (1%, wt/vol), nitrate (10 mM), nitrite (2 mM), ferric oxide (2 mM), ferric chloride (2 mM), molecular oxygen (5% [vol/vol]), and fumarate (10 mM). Potential electron donors, including H2-CO2 (1%, 5%, and 10% [vol/vol]), sulfide (2 mM), elemental sulfur (1% [wt/vol]), thiosulfate (5 mM), sulfite (2 mM), ammonium (10 mM), nitrite (2 mM), Fe0 granules (diameter, 1 to 2 mm; Fe0 purity, 99.98%; Alfa Aesar, Lancashire, United Kingdom) (10% [wt/vol]), ferrous chloride (2 mM), and l-cysteine–HCl (2 mM), were also screened by growth tests in 10 ml of Sw medium (pH 7.0) lacking NH4Cl and supplemented with 0.1 ml of the vitamin solution (9), 10 mM sodium nitrate, 10 mM sodium pyruvate, and 0.01% (wt/vol) yeast extract. To a 10-ml volume of each of these Sw-based media, 0.01 ml of a preculture of strain MIC1-1 was added, followed by incubation at 25°C for 30 days. Bacterial growth was determined by measurement of the optical density at 660 nm with a Genesys 20 spectrophotometer (Thermo Scientific, MA, USA). The concentrations of electron acceptors and donors and their oxidoreduction products in each of the cultures were quantified with a high-performance liquid chromatography (HPLC) system (model HIC-20Asuper; Shimadzu Corp., Kyoto, Japan) equipped with a conductivity detector (model CDD-10ADsp), a Shim-Pack cation column (IC-C4), and a Shim-Pack anion column (IC-SA2).
Preparation of DNA, PCR amplification of the 16S rRNA gene, and DNA sequencing.
Harvested cells (approximately 1 mg fresh weight) of strain MIC1-1 were lysed using 0.1 ml of a cell lysis solution (10 mg of lysozyme [Wako Pure Chemical Industries, Ltd., Osaka, Japan]/ml of Tris-EDTA [TE] buffer [pH 8.0]) at 37°C for 1 h, and the genomic DNA was extracted and purified by the method of Saito and Miura (10). The 16S rRNA gene was amplified by PCR with a forward primer (27F, 5′-AGAGTTTGATCCTGGCTCAG-3′; positions 8 to 27 in the Escherichia coli numbering system) and a reverse primer (1492R, 5′-GGTTACCTTGTTACGACTT-3′; positions 1510 to 1492). The PCR mixture (50 μl) contained 1× PCR buffer, 3.5 mM MgCl2, 10 mM deoxynucleoside triphosphates (dNTPs), 1.25 U AmpliTaq Gold (Applied Biosystems, CA, USA), and 0.4 μM (each) forward and reverse primers. Approximately 100 ng of genomic DNA was used as a template under the following cycling conditions: initial AmpliTaq Gold activation at 95°C for 9 min, followed by 25 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 30 s, extension at 72°C for 60 s, and a final extension step at 72°C for 10 min. The PCR product was purified with the QIAquick PCR purification kit (Qiagen, Venro, Netherlands), and an almost-complete 16S rRNA gene sequence (1,444 bp) was determined with a 3130xl genetic analyzer (Applied Biosystems), the BigDye Terminator v3.1 cycle-sequencing kit, and one of the following six primers: 27F, 520F (5′-GTGCCAGCAGCCGCGG-3′), 920F (5′-AAACTCAAAGGAATTGAC-3′), 520R (5′-ACCGCGGCTGCTGGC-3′), 920R (5′-GTCAATTCCTTTGAGTTT-3′), and 1492R.
Phylogenetic analyses.
According to the method previously described (11), the 16S RNA gene sequences of 55 phylogenetically related bacteria in the order Bacteroidales were selected. Phylogenetic trees were constructed by the neighbor-joining (NJ) method with CLUSTAL_X (12, 13) and the maximum-likelihood (ML) method with MORPHY version 2.3b3 (14, 15).
Fe0 corrosion test.
For Fe0 corrosion tests, 20 ml of sulfide-free artificial-seawater medium supplemented with 100 mM HEPES buffer (pH 7.0) was prepared according to the method of Uchiyama et al. (7) and added anaerobically to a 50-ml serum bottle containing either 1.5 g of Fe0 granules or an Fe0 foil (purity, >99.99%; 10 by 10 by 0.1 mm; Sigma-Aldrich, St. Louis, MO, USA). Air in the medium was removed by flushing with N2-CO2 (4:1 [vol/vol]), and the bottle was sealed with a butyl rubber stopper. If necessary, filtered solutions of 10 mM nitrate, 10 mM acetate, 10 mM citrate, 10 mM lactate, and/or 10 mM pyruvate were added to the medium as described above before inoculation with 0.2 ml of a preculture of strain MIC1-1. The culture was incubated at 25°C for 30 days.
Chemical analyses.
Culture fluids (100 μl) containing dissolved iron were either acidified with 50 μl of 6 M HCl and reduced with 100 μl of 1 M ascorbic acid for the quantification of total iron (ferrous and ferric ions) or acidified with 150 μl of sulfuric acid, pH 2.5, for the quantification of ferrous ions. The iron ion concentration in each of the acidified solutions was determined colorimetrically using o-phenanthroline, as described by Sandell (16). The concentration of ferric ions in the culture fluid was calculated by subtraction of the concentration of ferrous ions from that of total iron ions. Molecular hydrogen concentrations in the headspaces of serum bottles were quantified on a gas chromatograph equipped with a thermal conductivity detector and a molecular-sieve 60/80 mesh column (Shimadzu Corp., Kyoto, Japan). The column, injector, and detector temperatures were set at 95°C, 150°C, and 120°C, respectively. Nitrate, nitrite, and ammonium ions in the culture were quantified with a TRAACS 2000 autoanalyzer (BLTEC K. K., Osaka, Japan). Organic acids in culture fluids were quantified on an ICS-2000 ion chromatography system (Dionex, CA, USA).
Scanning electron microscopy of the surfaces and cross sections of Fe0 foils.
Scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM/EDX) was used for surface analyses of corroded Fe0 foils, as previously described (7).
Accession numbers.
Prolixibacter sp. MIC1-1 has been deposited in the RIKEN-BRC JCM and National Institute of Technology and Evaluation (NITE) Biological Resource Center (NBRC) of NITE under the culture collection accession numbers JCM 18694 and NBRC 102688, respectively. The 16S rRNA gene sequence of Prolixibacter sp. MIC1-1 has been deposited in the DDBJ/EMBL/GenBank nucleotide sequence database under accession number AB986195.
RESULTS
Growth properties of strain MIC1-1.
As shown in Table 1, an appropriate electron donor, electron acceptor, and organic carbon compound must be supplied for growth of strain MIC1-1. When nitrate and pyruvate were provided under anaerobic conditions as an electron acceptor and a carbon source, respectively, Fe0, ferrous ion, and l-cysteine served as sole electron donors, whereas molecular hydrogen (Fig. 1), sulfide, elemental sulfur, thiosulfate, sulfite, ammonium, and nitrite did not (Table 1). Pyruvate did not serve as an electron donor, indicating that the strain is lithotrophic.
TABLE 1.
Growth of Prolixibacter sp. MIC1-1 on various media under anaerobic and aerobic conditionsa
| Component or characteristic |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaerobic conditions (condition no.) |
Aerobic conditions (condition no.) |
||||||||||
| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 |
| Electron acceptor | Nitrate | Nitrate | Nitrate | Nitrate | Nitrate | Nitrate | Nitrate | Othersb | Molecular oxygen | Molecular oxygen | Molecular oxygen |
| Electron donor | Iron(0) | Iron(II) | l-Cysteine | Iron(0) | Iron(II) | l-Cysteine | Othersc | l-Cysteine | l-Cysteine | l-Cysteine | Othersc |
| Carbon source | Pyruvate | Pyruvate | Pyruvate | None | None | None | Pyruvate | Pyruvate | Pyruvate | None | Pyruvate |
| Growthd | + | + | + | − | − | − | − | − | + | − | − |
The medium composition and detailed growth conditions are described in Materials and Methods.
Ten millimolar sulfate, 2 mM sulfite, 5 mM thiosulfate, 1% (wt/vol) elemental sulfur, 2 mM nitrite, 2 mM ferric oxide, 2 mM ferric chloride, 10 mM fumarate, and none.
H2-CO2 (4:1 [vol/vol], N2-CO2-H2 (79:20:1 [vol/vol/vol]; 75:20:5 [vol/vol/vol]; 70:20:10 [vol/vol/vol]), 2 mM sulfide, 1% (wt/vol) elemental sulfur, 5 mM thiosulfate, 2 mM sulfite, 10 mM ammonium, 2 mM nitrite, and none.
+, growth; −, no growth.
FIG 1.
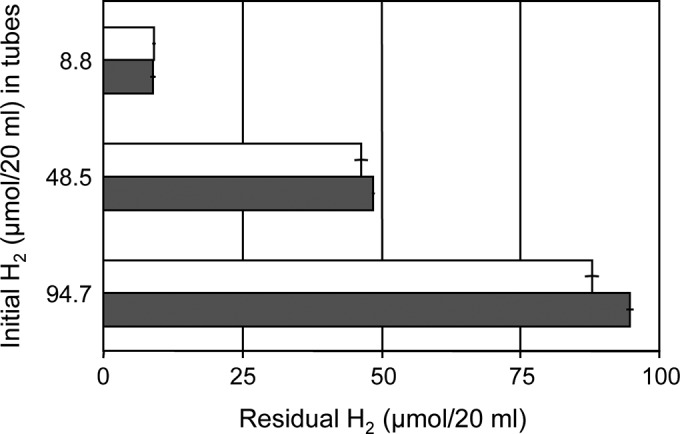
Use of molecular hydrogen as a sole electron donor by Prolixibacter sp. MIC1-1. Experiments were performed under three different initial concentrations of molecular hydrogen (8.8, 48.5, and 94.7 μmol/20 ml) with nitrate as the electron acceptor, and the concentration of residual molecular hydrogen in the headspace (20 ml) of each bottle was determined at the end of the experiment. Solid bars, Prolixibacter sp. MIC1-1 cultures; open bars, aseptic control. The data points and error bars represent means and standard deviations, respectively (n = 3).
The strain grew with molecular oxygen as an electron acceptor in the presence of l-cysteine as an electron donor and pyruvate as a carbon source. The roles of Fe0 and ferrous ion as electron donors could not be confirmed under aerobic conditions because of insoluble ferric compound formation that interfered with growth measurement. Aside from nitrate and molecular oxygen, other compounds, including sulfate, sulfite, thiosulfate, elemental sulfur, nitrite, ferric oxide, ferric chloride, and fumarate, did not serve as sole electron acceptors.
The strain did not use CO2 as a sole carbon source, even in the presence of l-cysteine and nitrate (Table 2), but used pyruvate (10 mM) as a carbon source under aerobic (with molecular oxygen as an electron acceptor) or anaerobic (with nitrate as an electron acceptor) conditions in the presence of l-cysteine (Table 2), indicating that the bacterium is lithoheterotrophic.
TABLE 2.
Growth of Prolixibacter sp. MIC1-1 on various carbon sources under anaerobic and aerobic conditions
| Component or characteristic |
||||
|---|---|---|---|---|
| Anaerobic conditions with indicated carbon source |
Aerobic conditions with indicated carbon source |
|||
| Parameter | CO2 | Pyruvate | CO2 | Pyruvate |
| Electron acceptor | Nitrate | Nitrate | Molecular oxygen | Molecular oxygen |
| Electron donor | l-Cysteine | l-Cysteine | l-Cysteine | l-Cysteine |
| Growtha | − | + | − | + |
+, growth; −, no growth.
Taxonomic characterization of strain MIC1-1.
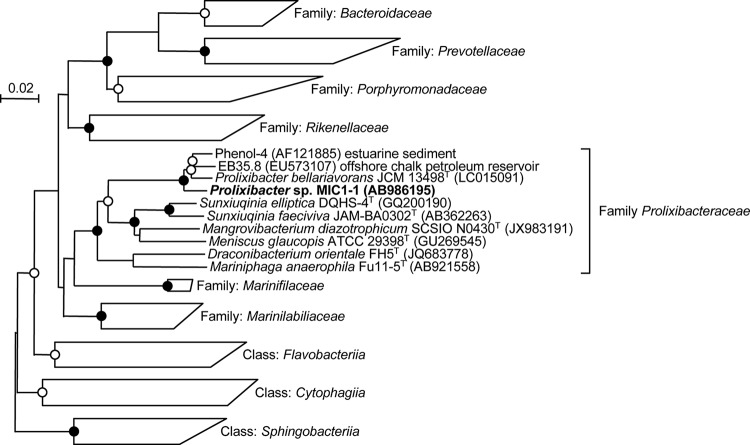
Phylogenetic analysis based on the 16S rRNA gene sequence showed that strain MIC1-1 belonged to the family Prolixibacteraceae in the order Bacteroidales and formed an independent phylogenetic lineage with P. bellariivorans JCM 13498T with high bootstrap values by two phylogenetic analysis methods (Fig. 2). The 16S rRNA gene sequence similarity of strain MIC1-1 to P. bellariivorans JCM 13498T was 97.5%. Therefore, strain MIC1-1 was provisionally identified as Prolixibacter sp.
FIG 2.
Phylogenetic tree of Prolixibacter sp. MIC1-1, related bacteria, and environmental clones based on the 16S rRNA gene sequences. The tree was inferred from an alignment of 1,308 bp of 16S rRNA gene sequences and constructed by NJ. The solid circles at branching nodes indicate supporting probabilities of >95% by bootstrap analyses of both the NJ and ML methods, whereas the open circles indicate probabilities of >85% by either of the analyses. The numbers at nodes are bootstrap percentages derived from 1,000 replications (NJ/ML methods). Bar, 0.02 substitution per nucleotide position.
Cells of Prolixibacter sp. MIC1-1 were rods 0.3 to 0.5 μm wide and 3.4 to 6.3 μm long (Fig. 3). Motility, flagellation, and spore formation were not observed under phase-contrast and electron microscopy. In summary, Prolixibacter sp. MIC1-1 is a facultatively aerobic, obligately heterotrophic, iron-oxidizing and nitrate-reducing bacterium (NRB) belonging to the order Bacteroidales.
FIG 3.
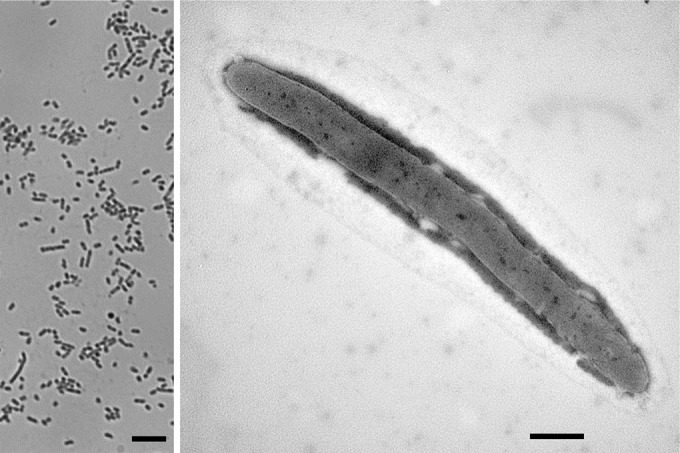
Phase-contrast (left) and transmission electron (right) micrographs of cells of Prolixibacter sp. MIC1-1. Bars, 5.0 μm (left) and 0.5 μm (right).
Fe0 corrosion by the bacterial isolate.
Prolixibacter sp. MIC1-1 oxidized Fe0 granules in the presence of 10 mM nitrate when artificial-seawater medium was supplemented with 10 mM acetate, 10 mM lactate, or 10 mM pyruvate as a carbon source (Table 3). The surfaces of the Fe0 granules in these cultures turned dull gray to grayish black, and the culture fluids assumed a light-yellow color. The ratio of ferrous ion to ferric ion in these cultures ranged from approximately 3:2 to 2:3, whereas it was approximately 4:1 in the aseptic control. Slight Fe0 granule oxidation by Prolixibacter sp. MIC1-1 was detected in the presence of citrate or in the absence of an electron donor, but the metal surface did not lose its luster. Prolixibacter sp. MIC1-1 did not oxidize Fe0 granules in the absence of nitrate (Table 3).
TABLE 3.
Fe0 corrosion by Prolixibacter sp. MIC1-1 in the presence of various carbon sources
| Strain | Electron acceptor (10 mM) | Carbon source (10 mM) | Dissolved iron (μmol/20 ml)a |
||
|---|---|---|---|---|---|
| Total | Fe2+ | Fe3+ | |||
| Prolixibacter sp. MIC-1 | Nitrate | Acetate | 156.7 ± 3.6 | 85.9 ± 2.0 | 70.7 ± 1.6 |
| Nitrate | Citrate | 99.5 ± 0.3 | ND | ND | |
| Nitrate | Lactate | 235.3 ± 4.1 | 106.9 ± 1.9 | 128.5 ± 2.3 | |
| Nitrate | Pyruvate | 224.3 ± 0.1 | 92.9 ± 0.1 | 131.4 ± 0.1 | |
| Nitrate | None | 60.0 ± 1.4 | ND | ND | |
| None | Lactate | 27.8 ± 4.2 | ND | ND | |
| Aseptic control | Nitrate | Lactate | 64.8 ± 9.5 | 49.6 ± 7.3 | 15.3 ± 2.2 |
ND, not determined.
Importantly, P. bellariivorans JCM 13498T, a close relative of Prolixibacter sp. MIC1-1, did not oxidize Fe0 granules under any of the culture conditions described above (data not shown). Thus, the ability of Prolixibacter sp. MIC1-1 to use Fe0 as an electron donor is not a common trait of the genus Prolixibacter.
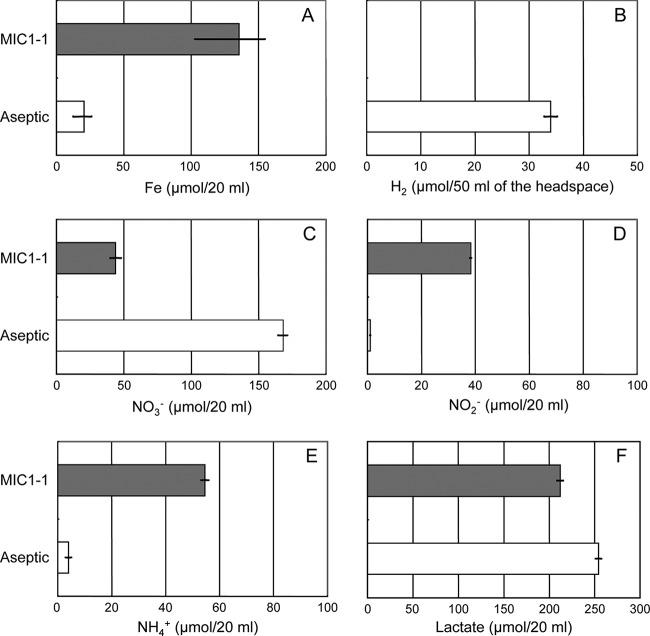
The amount of dissolved iron in Prolixibacter sp. MIC1-1 cultures in the presence of nitrate and lactate was approximately six times greater than that in the aseptic control (Fig. 4A). Molecular hydrogen was detected in the aseptic control, but not in the culture of Prolixibacter sp. MIC1-1 grown in the presence of nitrate and lactate (Fig. 4B). A significant amount of nitrate was transformed to nitrite or ammonium in the Prolixibacter sp. MIC1-1 culture. The reduction of nitrate to nitrite or ammonium, but in much smaller amounts, was also observed in the aseptic control (Fig. 4C, D, and E). Nitrite was the sole metabolic product of nitrate in Prolixibacter sp. MIC1-1 when l-cysteine was used as the sole electron donor (data not shown). This result indicated that ammonium was formed by a chemical reaction between nitrate/nitrite and Fe0, as reported previously (17, 18). The amount of lactate in the Prolixibacter sp. MIC1-1 culture decreased compared with that in the aseptic control (Fig. 4F).
FIG 4.
Fe0 dissolution (A), molecular-hydrogen production (B), nitrate consumption (C), nitrite (D) and ammonium (E) production, and lactate consumption (F) in Prolixibacter sp. MIC1-1 cultures and in aseptic controls. All experiments were performed with Fe0 granules as the sole source of electrons. Solid bars, Prolixibacter sp. MIC1-1; open bars, aseptic control. The data points and error bars represent means and standard deviations, respectively (n = 3). The ammonium produced was calculated by subtracting the amount of ammonium initially added from the amount of ammonium detected at the end of the experiment.
Electron microscopic analyses of corroded Fe0 foils.
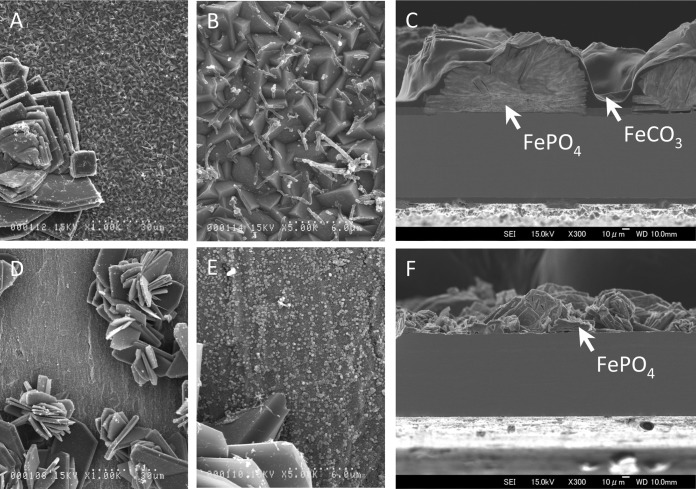
Prolixibacter sp. MIC1-1 was cultivated in a sulfide-free artificial seawater medium supplemented with nitrate and lactate in which an Fe0 foil was submerged. After incubation for 30 days, the surfaces of the Fe0 foils were observed with a scanning electron microscope, together with Fe0 foils incubated in aseptic media. As shown in Fig. 5A and B, crystal structures developed on the surfaces of Fe0 foils that had been submerged in a culture of Prolixibacter sp. MIC1-1. Rod-like extrusions 3 to 6 μm long were also observed (Fig. 5B), with the size corresponding to that of Prolixibacter sp. MIC1-1. On the surfaces of Fe0 foils incubated under aseptic conditions, smaller crystal structures developed (Fig. 5D and E). X-ray photoelectron spectroscopy and X-ray diffraction analyses of cross-sectional samples revealed that the crystals that developed on the Fe0 foil surface mainly consisted of FePO4 (Fig. 5C and F). The crystals on the Fe0 foils from a Prolixibacter sp. MIC1-1 culture were covered by a layer of FeCO3 (Fig. 5C). Such a layer was not observed in Fe0 foils from aseptic controls (Fig. 5F).
FIG 5.
Scanning electron micrographs of the surfaces and cross sections of Fe0 foils submerged in a Prolixibacter sp. MIC1-1 culture and an aseptic medium. (A and B) Surface of an Fe0 foil incubated in the Prolixibacter sp. MIC1-1 culture. (C) Cross section of an Fe0 foil incubated in the Prolixibacter sp. MIC1-1 culture. (D and E) Surface of an Fe0 foil incubated in the aseptic medium. (F) Cross section of an Fe0 foil incubated in the aseptic medium.
DISCUSSION
This study reports the isolation and characterization of the Fe0-corroding facultatively aerobic bacterium Prolixibacter sp. MIC1-1, which is a member of the order Bacteroidales (Fig. 2). The genus Prolixibacter may be widespread in the marine environment, because P. bellariivorans and its phylogenetic relatives have previously been cultured from estuarine and marine sediments and an offshore oil reservoir (19–21). To date, methanogens belonging to the phylum Euryarchaeota and SRB and iron-oxidizing bacteria, both belonging to the phylum Proteobacteria, have frequently been studied as causative microorganisms of MIC (3, 6, 7, 22, 23). To our knowledge, Prolixibacter sp. MIC1-1 is the first Fe0-corroding representative belonging to the phylum Bacteroidetes.
The molar amount of iron dissolved by Prolixibacter sp. MIC1-1 cultivated for 30 days corresponded to 2/3 of that dissolved by Methanococcus maripaludis KA1 (7) and 1.8 times that dissolved by Mariprofundus sp. strain GSB2 (6).
The finding that the major corrosion products of Fe0 formed in anaerobic cultures of Prolixibacter sp. MIC1-1 were FePO3 and FeCO3 indicates that Fe0 was oxidized to both ferrous and ferric ions. The oxidation to ferric ion by MIC under anaerobic conditions was unusual because ferrous compounds have generally been detected as corrosion products in SRB- and methanogen-mediated MIC (7, 22). We propose the following mechanisms for the production of ferric ion from Fe0 by Prolixibacter sp. MIC1-1. The strain may oxidize Fe0 primarily to ferrous ion, similarly to other corrosive microorganisms:
| (1) |
Given that Prolixibacter sp. MIC1-1 was able to use ferrous ion as an electron donor, ferrous ion formed by equation 1 may be further oxidized to ferric ion:
| (2) |
In addition, chemical reactions that oxidize Fe0 to ferrous ion concomitant with the reduction of nitrate to either nitrite (equation 1) or ammonium (equation 3) are known (17, 18).
| (3) |
Chemical reactions that oxidize ferrous ion to ferric ion concomitant with the reduction of nitrate either to nitrite (equation 2) or to ammonium (equation 4) have also been reported (24).
| (4) |
Thus, the reduction of nitrate to either nitrite or ammonium coupled with the oxidation of Fe0 to Fe3+ is described as follows:
| (5) |
| (6) |
The stoichiometric relationship between Fe0 oxidation and nitrate reduction was investigated in an aseptic medium (Fig. 4). In this experiment, 21 μmol of dissolved iron was detected in the culture fluid, 34 μmol of molecular hydrogen was produced in the headspace, and 1 μmol of nitrite and 4 μmol of ammonium were detected in the culture fluid.
Two-step chemical reactions that generate molecular hydrogen from Fe0 are known. As shown in equation 7, Fe0 undergoes ionization at the anode by a reaction whose rate is low under anaerobic conditions at neutral pH:
| (7) |
At the anode, electrons generated in reaction 7 are consumed to produce molecular hydrogen if no other electron acceptor is present:
| (8) |
The generation of 34 μmol of molecular hydrogen, 1 μmol of nitrite, and 4 μmol of ammonium in the aseptic medium required 106 μmol of electrons. Given that the ratio of ferrous and ferric ions present in the aseptic culture fluid was approximately 4:1, 1 μmol of Fe0 was estimated to generate 2.2 μmol of electrons. Thus, 48 μmol of Fe0 was calculated to be dissolved, whereas the experimentally determined value was 21 μmol. Given that the acid extraction of iron ions from crystal structures of FePO4 developed on the surface of Fe0 (Fig. 5) may be inefficient, the experimentally determined iron ion concentrations may be underestimated.
In the culture of Prolixibacter sp. MIC1-1, approximately 124 μmol of nitrate was transformed and 38 μmol of nitrite and 55 μmol of ammonium were produced. The inconsistency between the amount of nitrate reduced and the amounts of nitrite and ammonium formed could be the result of experimental error. For the production of 38 μmol of nitrite and 55 μmol of ammonium, 571 μmol of electrons was required. Given that the ratio of ferrous and ferric ions present in Prolixibacter sp. MIC1-1 culture was approximately 1:1, 1 μmol of Fe0 was estimated to generate 2.5 μmol of electrons. Thus, 228 μmol of Fe0 was calculated to have been dissolved, whereas the experimentally determined value was 136 μmol (Fig. 4). As proposed above, the discrepancy could be due to the inefficiency of extraction of iron ions from crystal structures of FePO4 developed on the surface of Fe0 (Fig. 5).
Molecular hydrogen, which accumulated in the aseptic control, was not detected in the Prolixibacter sp. MIC1-1 culture. We speculate, but did not show, that electrons and molecular hydrogen generated in the reactions shown in equations 7 and 8 were largely consumed by the chemical reduction of nitrite to ammonium. In the aseptic medium, molecular hydrogen accumulated because the speed of nitrite formation was lower than that of molecular hydrogen formation, whereas in the Prolixibacter sp. MIC1-1 culture, the speed of nitrite formation greatly exceeded that of molecular hydrogen formation.
Given that Prolixibacter sp. MIC1-1 reduced nitrate only to nitrite when the electron donor was l-cysteine, we infer that the contribution of biotic activity in Fe0 corrosion was limited to Fe0 oxidation coupled to the reduction of nitrate to nitrite. Assuming that the amount of biologically reduced nitrate was 88 [38 + 55 − (1 + 4)], it was approximately 18 times [88/(4 + 1)] higher than that of chemically reduced nitrate in the aseptic control. As described above, the Fe0 corrosion activity in the Prolixibacter sp. MIC1-1 culture was expressed as 571 μmol of electrons, whereas the biological activity generated only 176 μmol of electrons, or 30% of the total corrosion activity.
NRB-assisted Fe0 corrosion has been reported mainly in association with the remediation of nitrate-contaminated groundwater using granular Fe0. The biological denitrification of groundwater by a combination of a hydrogenotrophic NRB and Fe0 granules has been studied, and the addition of an NRB, such as Paracoccus denitrificans ATCC 17741, or an NRB-containing microbial consortium generally resulted in faster nitrate removal and faster Fe0 oxidation than in aseptic controls (25–30). The bioelectrochemical mechanisms of the NRB-assisted Fe0 corrosion in these studies are largely unknown. One explanation of the Fe0 corrosion by hydrogenophilic NRB is the classic cathodic depolarization mechanism. However, given that nitrite was shown to be much more corrosive than nitrate (31), another possibility is that NRB indirectly stimulate Fe0 corrosion through the production of nitrite.
Unlike these NRB, Prolixibacter sp. MIC1-1 was not hydrogenophilic and thus may directly stimulate Fe0 corrosion by abstracting cathodic electrons from the surface of Fe0. The model of Fe0 corrosion by Prolixibacter sp. MIC1-1 discussed above is summarized in Fig. 6.
FIG 6.
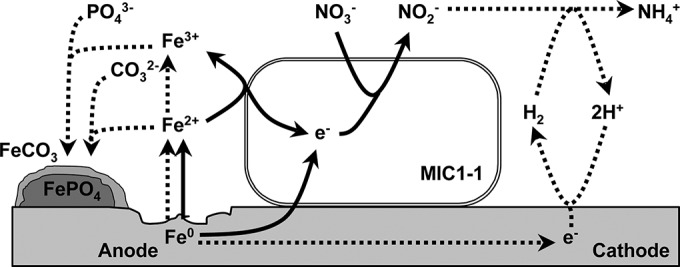
Hypothetical model of Fe0 corrosion by the nitrate-reducing Prolixibacter sp. MIC1-1. Solid arrows, biotic steps; dotted arrows, abiotic chemical steps.
Nitrate injection into oil reservoirs has been used to mitigate SRB-assisted MIC, with the idea that it promotes NRB growth and in turn inhibits SRB growth (32–34). However, it should be realized that amendment with nitrate can lead to either hydrogenophilic-NRB-assisted or MIC1-1-like bacterium-assisted MIC by the mechanisms described above.
ACKNOWLEDGMENTS
We thank Takahiro Iwami (National Institute of Technology and Evaluation) for technical support and Koji Mori (NITE) for helpful discussions of several aspects of the paper.
This study was partly supported by the New Energy and Industrial Technology Development Organization (NEDO) (grant no. P05032).
REFERENCES
- 1.Javaherdashti R. 2008. Microbiologically influenced corrosion: an engineering insight. Springer-Verlag, New York, NY. [Google Scholar]
- 2.Enning D, Garrelfs J. 2014. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol 80:1226–1236. doi: 10.1128/AEM.02848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venzlaff H, Enning D, Srinivasan J, Mayrhofer KJJ, Hassel AW, Widdel F, Stratmann M. 2013. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros Sci 66:88–96. doi: 10.1016/j.corsci.2012.09.006. [DOI] [Google Scholar]
- 4.Widdel F, Bak F. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p 3352–3378. In Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (ed), The prokaryotes, 2nd ed, vol 6 Springer, New York, NY. [Google Scholar]
- 5.Kato S, Yumoto I, Kamagata Y. 2015. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl Environ Microbiol 81:67–73. doi: 10.1128/AEM.02767-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBeth JM, Little BJ, Ray RI, Farrar KM, Emerson D. 2011. Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments. Appl Environ Microbiol 77:1405–1412. doi: 10.1128/AEM.02095-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchiyama T, Ito K, Mori K, Tsurumaru H, Harayama S. 2010. Iron-corroding methanogen isolated from a crude-oil storage tank. Appl Environ Microbiol 76:1783–1788. doi: 10.1128/AEM.00668-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touzel JP, Albagnac G. 1983. Isolation and characterization of Methanococcus mazei strain MC3. FEMS Microbiol Lett 16:241–245. doi: 10.1111/j.1574-6968.1983.tb00295.x. [DOI] [Google Scholar]
- 9.Wolin EA, Wolin MJ, Wolfe RS. 1963. Formation of methane by bacterial extracts. J Biol Chem 238:2882–2886. [PubMed] [Google Scholar]
- 10.Saito H, Miura K. 1963. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim Biophys Acta 72:619–629. doi: 10.1016/0926-6550(63)90386-4. [DOI] [PubMed] [Google Scholar]
- 11.Iino T, Mori K, Uchino Y, Nakagawa T, Harayama S, Suzuki K. 2010. Ignavibacterium album gen. nov., sp. nov., a moderately thermophilic anaerobic bacterium isolated from microbial mats at a terrestrial hot spring and proposal of Ignavibacteria classis nov., for a novel lineage at the periphery of green sulfur bacteria. Int J Syst Evol Microbiol 60:1376–1382. doi: 10.1099/ijs.0.012484-0. [DOI] [PubMed] [Google Scholar]
- 12.Saitou N, Nei M. 1987. A neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tool. Nucleic Acids Res 24:4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa M, Fujiwara M. 1993. Relative efficiencies of the maximum likelihood, maximum parsimony, and neighbor-joining methods for estimating protein phylogeny. Mol Phylogenet Evol 2:1–5. doi: 10.1006/mpev.1993.1001. [DOI] [PubMed] [Google Scholar]
- 16.Sandell EB. 1959. Colorimetric determination of trace metals. The chemical analysis monograph series, vol 3 Interscience Publishers, New York, NY. [Google Scholar]
- 17.Choe C, Liljestrand HM, Khim J. 2004. Nitrate reduction by zero-valent iron under different pH regimes. Appl Geochem 19:335–342. doi: 10.1016/j.apgeochem.2003.08.001. [DOI] [Google Scholar]
- 18.Cheng IF, Muftikian R, Fernando Q, Korte N. 1997. Reduction of nitrate to ammonia by zero-valent iron. Chemosphere 35:2689–2695. doi: 10.1016/S0045-6535(97)00275-0. [DOI] [Google Scholar]
- 19.Holmes DE, Nevin KP, Woodard TL, Peacock AD, Lovley DR. 2007. Prolixibacter bellariivorans gen. nov., sp. nov., a sugar-fermenting psychrotolerant anaerobe of the phylum Bacteroidetes, isolated from a marine-sediment fuel cell. Int J Syst Evol Microbiol 57:701–707. doi: 10.1099/ijs.0.64296-0. [DOI] [PubMed] [Google Scholar]
- 20.Kaster KM, Bonaunet K, Berland H, Kjeilen-Eilertsen G, Brakstad OG. 2009. Characterisation of culture-independent and -dependent microbial communities in a high-temperature offshore chalk petroleum reservoir. Antonie Van Leeuwenhoek 96:423–439. doi: 10.1007/s10482-009-9356-1. [DOI] [PubMed] [Google Scholar]
- 21.Knight VK, Kerkhof LJ, Häggblom MM. 1999. Community analyses of sulfidogenic 2-bromophenol-dehalogenating and phenol-degrading microbial consortia. FEMS Microbiol Ecol 29:137–147. doi: 10.1111/j.1574-6941.1999.tb00605.x. [DOI] [Google Scholar]
- 22.Dinh HT, Kuever J, Muβmann M, Hassel AW, Stratmann M, Widdel F. 2004. Iron corrosion by novel anaerobic microorganisms. Nature 427:829–832. doi: 10.1038/nature02321. [DOI] [PubMed] [Google Scholar]
- 23.Enning D, Venzlaff H, Garrelfs J, Dinh HT, Meyer V, Mayrhofer K, Hassel AW, Stratmann M, Widdel F. 2012. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ Microbiol 14:1772–1787. doi: 10.1111/j.1462-2920.2012.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakshit S, Matocha CJ, Haszler GR. 2005. Nitrate reduction in the presence of wüstite. J Environ Qual 34:1286–1292. doi: 10.2134/jeq2004.0459. [DOI] [PubMed] [Google Scholar]
- 25.De Windt W, Boon N, Siciliano SD, Verstraete W. 2003. Cell density related H2 consumption in relation to anoxic Fe(0) corrosion and precipitation of corrosion products by Shewanella oneidensis MR-1. Environ Microbiol 5:1192–1202. doi: 10.1046/j.1462-2920.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 26.Ginner JL, Alvarez PJJ, Smith SL, Scherer MM. 2004. Nitrate and nitrite reduction by Fe0: influence of mass transport, temperature, and denitrifying microbes. Environ Eng Sci 21:219–229. doi: 10.1089/109287504773087381. [DOI] [Google Scholar]
- 27.Kielemoes J, De Boever P, Verstraete W. 2000. Influence of denitrification on the corrosion of iron and stainless steel powder. Environ Sci Technol 34:663–671. doi: 10.1021/es9902930. [DOI] [Google Scholar]
- 28.Till BA, Weathers LJ, Alvarez PJJ. 1998. Fe(0)-supported autotrophic denitrification. Environ Sci Technol 32:634–639. doi: 10.1021/es9707769. [DOI] [Google Scholar]
- 29.Xiaomeng F, Xiaohong G, Jun M, Hengyu A. 2009. Kinetics and corrosion products of aqueous nitrate reduction by iron powder without reaction conditions control. J Environ Sci 21:1028–1035. doi: 10.1016/S1001-0742(08)62378-5. [DOI] [PubMed] [Google Scholar]
- 30.Xu D, Li Y, Song F, Gu T. 2013. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros Sci 77:385–390. doi: 10.1016/j.corsci.2013.07.044. [DOI] [Google Scholar]
- 31.Lin K-S, Chang N-B, Chuang T-D. 2008. Fine structure characterization of zero-valent iron nanoparticles for decontamination of nitrites and nitrates in wastewater and groundwater. Sci Technol Adv Mater 9:0250151. doi: 10.1088/1468-6996/9/2/025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gittel A, Sørensen KB, Skovhus TL, Ingvorsen K, Schramm A. 2009. Prokaryotic community structure and sulfate reducer activity in water from high-temperature oil reservoirs with and without nitrate treatment. Appl Environ Microbiol 75:7086–7096. doi: 10.1128/AEM.01123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwermer CU, Lavik G, Abed RMM, Dunsmore B, Ferdelman TG, Stoodley P, Gieseke A, de Beer D. 2008. Impact of nitrate on the structure and function of bacterial biofilm communities in pipelines used for injection of seawater into oil fields. Appl Environ Microbiol 74:2841–2851. doi: 10.1128/AEM.02027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Telang AJ, Evert S, Foght JM, Westlake DWS, Jenneman GE, Gevertz D, Voordouw G. 1997. Effect of nitrate injection on the microbial community in an oil field as monitored by reverse sample genome probing. Appl Environ Microbiol 63:1785–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]