Abstract
Enterohemorrhagic Escherichia coli (EHEC) strains, of which E. coli O157:H7 is the best-studied serotype, are an important group of foodborne pathogens causing severe illness in humans worldwide. The main reservoirs for EHEC are ruminants, mostly cattle, which harbor the bacteria in their intestinal tracts without showing clinical symptoms. In this study, we used bovine lactoferrin, a natural occurring bactericidal and immunomodulating protein, as an antibacterial agent against EHEC infection in cattle. Nine 3-month-old Holstein-Friesian calves were experimentally infected with EHEC (strain NCTC12900). Three animals received a daily rectal spray treatment with bovine lactoferrin, three animals received an oral treatment, and three animals served as a control group. Blood samples were collected weekly and fecal samples twice weekly to monitor antibody responses and fecal excretion, respectively. Animals in the rectal group ceased shedding within 26 days of the experimental treatment and remained negative. This beneficial effect of bovine lactoferrin was not observed in the oral group, where animals were still shedding at the time of euthanasia (day 61). All groups developed serum responses, but no clear differences could be observed between the groups. However, the results indicate that the use of bovine lactoferrin as a rectal treatment can be a useful strategy to preclude further transmission of EHEC infections from cattle to humans.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 is a foodborne pathogen which causes human illness ranging from self-limited watery or bloody diarrhea and hemorrhagic colitis to acute renal failure and hemolytic uremic syndrome (HUS) (1). Infection in humans is typically acquired through the ingestion of contaminated food or water, direct contact with infected animals, or person-to-person transmission. The main reservoirs for E. coli O157:H7 are ruminants, mostly cattle, which, in contrast to humans, harbor the bacteria in the gastrointestinal tract without showing apparent illness (2, 3). The persistence of E. coli O157:H7 and the duration of shedding in naturally and experimentally infected cattle are highly variable and vary from days to months (2, 4, 5).
Mechanisms leading to persistence of E. coli O157:H7 in ruminants are unknown, which impedes the search for a successful approach to combat E. coli O157:H7 zoonotic transfer (6). Furthermore, the current treatment for EHEC-related diseases in humans is limited to supportive care, as antibiotic treatment is contraindicated because it might increase the frequency of HUS occurrence due to the release of bacterial Shiga toxins (7). Different strategies to reduce the EHEC carriage rates in ruminants, including vaccination (8), probiotic treatment (9), bacteriophages (1), and modification of diet (10), have been tested. However, these strategies show only limited results. Currently, no methods that can substantially reduce EHEC carriage rates in ruminants are available. Thus, innovative strategies which could effectively reduce E. coli O157:H7 carriage in ruminants are urgently required for diminishing the risk to public health.
Emerging multiple-antimicrobial-resistant pathogens and the full ban on in-feed antibiotics in Europe have increased research on natural antimicrobial proteins such as transferrins. Lactoferrin, a member of the transferrin protein family, is an iron-binding glycoprotein that is found in many exocrine secretions, including milk, tears, saliva, and serum. Lactoferrin exhibits antibacterial activity against Gram-negative and Gram-positive bacteria in a variety of ways. First, it sequesters iron, which is an essential growth factor for microorganisms (11). Second, lactoferrin is capable of destabilizing the outer membranes of Gram-negative bacteria, which results in the release of bacterial lipopolysaccharides (LPS) from the bacterial membrane (12).
Several studies have identified the epithelium of the recto-anal junction, located above the gut-associated lymphoid tissue, as the most important colonization site of EHEC in cattle (13, 14). The present study evaluated the curative effect of orally or rectally administered bovine lactoferrin (bLF) in an experimental EHEC infection model in calves, which we established in our laboratory (unpublished results, 2013). Three major questions were addressed. (i) Can orally administered lactoferrin sufficiently reach the predilection site for E. coli O157:H7, the rectal mucosa, to clear EHEC colonization? (ii) Does rectal administration of lactoferrin result in EHEC clearance? (iii) Does lactoferrin treatment influence the antibody responses against EHEC virulence proteins, and might these responses be used in the future to monitor the infection status of a cattle herd?
MATERIALS AND METHODS
EHEC strain.
E. coli O157:H7 strain NCTC12900, a well-characterized Shiga toxin-negative EHEC strain of human origin (15), was used for experimental infections in calves. We used this Shiga toxin-negative strain for biosafety reasons. Bacteria were grown overnight in Luria-Bertani broth (LB) with aeration (200 rpm) at 37°C, harvested by centrifugation (11,337 × g, 5 min), resuspended in sterile phosphate-buffered saline (PBS), to a concentration of 1010 CFU/10 ml, and subsequently used for experimental infections.
bLF.
Bovine lactoferrin (bLF) with 92% purity and 16% iron saturation (manufacturer [Ingredia Nutritional, France] information), derived from bovine milk, was used in this study. Using a bovine Ig enzyme-linked immunosorbent assay (ELISA), the absence of bovine antibodies (AbD Serotec) in the bovine lactoferrin was confirmed.
Experimental infection of calves and sampling.
Nine 3-month-old calves (Holstein-Friesian; Hindryckx N.V., Ichtegem) were randomly assigned to three groups (oral, rectal, and control) of 3 animals, each reared in a separate pen. The calves were fed grain-based pellets and were allowed free access to hay and water. All animals were screened prior to experimental infection to be seronegative for intimin, EspA, and EspB. The feces of these animals were free of E. coli O157:H7 as demonstrated by direct plating and isolation (the procedure is described below). Experimental procedures and animal management procedures were undertaken in accordance with the requirements of the animal care and ethics committee of the Faculty of Veterinary Medicine, Ghent University, Belgium (EC2012/170).
The experimental infection took place 1 week after arrival of the animals. First, using a nursing bottle, calves were given 300 ml of a 10% NaHCO3 solution in order to close the esophageal groove and to allow the bacterial inoculum to drain directly into the abomasum. Each animal was infected with 1010 CFU E. coli NCTC12900 for 2 consecutive days (days 0 and 1 of the experiment) and reinfected on days 7 and 8. Treatment with bLF started on day 11 and continued until day 61 for the oral and control groups and until day 43 for the rectal group. The oral group received bLF orally (1.5 g twice a day) suspended in a volume of 10 ml sodium bicarbonate buffer (10%). The rectal group was treated with bLF powder, which was dispersed by air blast onto the rectum (300 mg per day) using a 20-cm-long polyvinyl chloride tube (diameter, 1 cm). The control group received only the bicarbonate buffer orally.
Serum samples (taken from the jugular vein) were collected to detect EHEC-specific antibody titers before the primary infection on day 0, before reinfection on day 7, and subsequently on a weekly basis for the duration of the experiment. In all groups, fecal excretion of E. coli O157:H7 was monitored twice a week from day 1 onwards until EHEC was no longer present in the feces. Animals in the rectal group were euthanized at day 43, while animals in the oral and the control groups were euthanized at day 61. At euthanasia, tissues and fecal contents of the jejunum, ileum, cecum, colon, and rectum were sampled for enumeration of the inoculated strain.
Excretion and intestinal presence of E. coli O157:H7.
Fecal samples were analyzed immediately after sampling, as described by Vande Walle et al. (16). Briefly, 10 g of feces was homogenized in 90 ml sterile modified tryptone soy broth (Oxoid Ltd., Hanst, United Kingdom) supplemented with 20 mg/liter novobiocin (Sigma Aldrich, St. Louis, MO, USA). Enumeration of E. coli O157 was performed by plating 10-fold serial dilutions onto MacConkey agar supplemented with sorbitol, cefixime, tellurite, and nalidixic acid (NalCT-SMAC) (Merck, Darmstadt, Germany) and incubating the plates at 37°C for 18 h. The remaining broth was enriched for 6 h at 42°C and subjected to immunomagnetic separation (IMS) using Dynabeads (Invitrogen, Merelbeke, Belgium), according to the manufacturer's instructions. Finally, 100 μl was plated onto NalCT-SMAC agar and incubated for 18 h at 37°C. Selected sorbitol-negative colonies were confirmed by the O157-specific latex agglutination assay (Oxoid Ltd., Basingstoke, United Kingdom).
At euthanasia, 10 g of intestinal content from the jejunum, ileum, cecum, colon, and rectum and 10 g of tissues (washed with sterile PBS) from the jejunum with and without Peyer's patches (PP), ileum with and without PP, cecum, colon, and recto-anal junction were tested for the presence of NCTC12900 by direct plate counts, as described for the fecal samples.
Serum antibody response against virulence factors of E. coli O157:H7.
Blood samples were processed directly after collection. Briefly, sera were heat inactivated (30 min at 56°C) and kaolin treated. Polysorb 96-well plates (Polysorb Immuno Plates; Nunc, Roskilde, Denmark) were coated (200 ng/well) with recombinant intimin, EspA, or EspB in PBS and incubated overnight at 4°C. Nonspecific binding sites were blocked for 1 h at 37°C by adding PBS plus 0.2% Tween 80. After washing with PBS plus 0.2% Tween 20, the plates were incubated with 2-fold dilution series of serum in PBS plus 0.05% Tween 20, followed by washing and incubating (1 h at 37°C) with affinity chromatography-purified horseradish peroxidase (HRP)-conjugated sheep anti-cattle IgA and IgG antibodies (AbD Serotec, United Kingdom). After a final washing step, 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) diammonium salt (ABTS) (Roche Diagnostics, Vilvoorde, Belgium) was added. The optical density at 405 nm (OD405) was measured. Positive- and negative-control sera were kindly provided by the Laboratory of Veterinary Public Health and Food Safety (Merelbeke) (17). The cutoff value was determined as mean of the value for the negative-control sera ± three times the standard deviation (SD). For the calculation of the titers, the maximum dilution that exceeded the cutoff value was used. This values were subsequently log2 transformed.
Statistical analysis.
Statistical analysis was performed based on the linear fixed-effects model with treatment as the categorical fixed effect and the area under a curve (AUC) as the response variable. The data represent mean ± standard deviation. A P value of <0.025 (Bonferroni adjusted) was considered statistically significant.
Colony counts were log10 transformed for data analysis. If E. coli O157:H7 could not be detected by direct plating but could be detected only by enrichment, a concentration of 10 CFU/g was assigned to the sample (16).
RESULTS
Excretion of E. coli O157:H7.
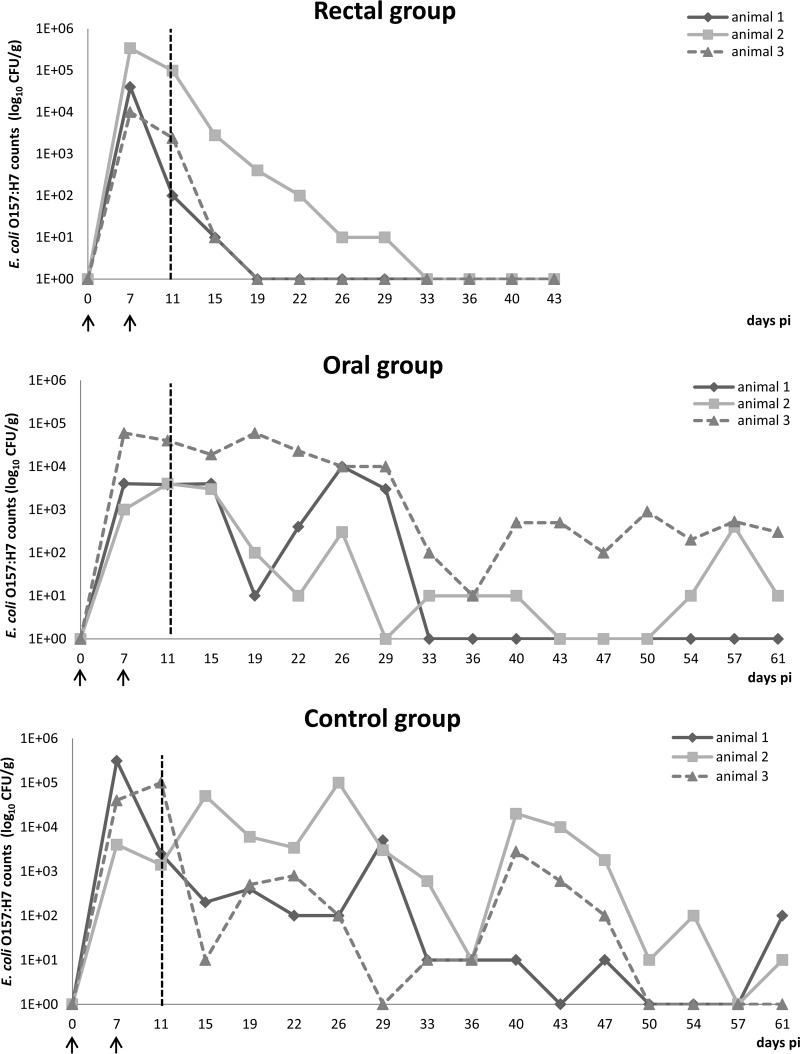
Animals in the control group excreted between 101 and 105.5 CFU E. coli O157:H7/g feces when positive (Fig. 1). For all 3 animals, fecal shedding heavily fluctuated from day 1 until euthanasia at day 61. The feces of animals 1, 2, and 3 were E. coli O157:H7 negative on 4 (25%), 1 (6.2%), and 5 (31%) of 16 sampling time points, respectively. The feces of only 1 of 3 (33%) animals (animal 3) became E. coli O157:H7 negative by the end of the experiment (day 61). Shedding ceased at day 50.
FIG 1.
Excretion of E. coli O157:H7 in calves. Results are represented as real values of CFU/g feces for individual animals of the three groups on a logarithmic scale. A value of 1E+00 corresponds to negative sampling after enrichment and IMS. The vertical dashed line at day 11 indicates the start of the treatment. The arrows indicate the starts of the first and second infections.
Animal 1, 2, and 3 in the oral group excreted between 101 and 104, 101 and 103.5, and 101 and 105 CFU E. coli O157:H7/g feces, respectively, when positive (Fig. 1). As in the control group, the fecal E. coli excretion fluctuated heavily. For animal 1, E. coli O157:H7 shedding was no longer detected from day 33 onwards. For animal 2, feces was negative on 4 of 16 (25%) sampling time points. On the other hand, the feces of animal 3 remained E. coli O157:H7 positive from day 1 until day 61. Thus, as for the control group, the feces of only 1 of 3 (33%) animals (animal 1) became E. coli O157:H7 negative by the end of the experiment (day 61).
Rectal administration of bovine lactoferrin gave a completely different result (Fig. 1). As in the oral and control groups, at day 1 large amounts of E. coli O157:H7 were found in all 3 animals in the rectal group (104.5,105, and 104 CFU, respectively). However, E. coli O157:H7 shedding constantly declined afterwards, resulting in 2 of 3 (66%) negative animals (animal 1 and 3) by day 12. The remaining animal (animal 2) became negative by day 26. Importantly, once they became negative, E. coli O157:H7 was never found again in the feces of these animals.
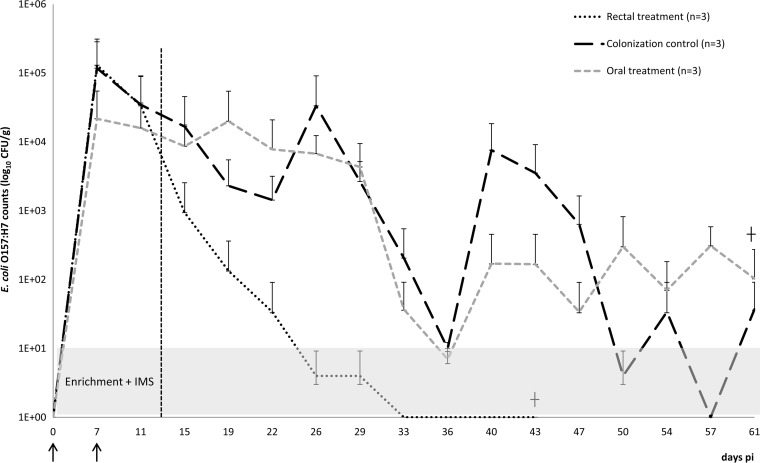
The treatment groups did not have a significantly lower mean E. coli O157:H7 CFU/g feces (AUC) than the control group (Fig. 2) However, the P value for the comparison between the rectal and the control groups equaled 0.0414, slightly above the Bonferroni-adjusted significance level of 0.05.
FIG 2.
Results are presented as the mean real values of CFU/g feces + SD on a logarithmic scale. A value of 1E+00 corresponds to negative sampling after enrichment and IMS. The vertical dashed line at day 11 indicates the start of the treatment. The arrows indicate the starts of the first and second infections.
Presence of E. coli O157:H7 in intestinal tissues at euthanasia.
E. coli O157:H7 could not be isolated from the intestinal contents or from intestinal tissues of animals in the rectal group (Table 1). On the other hand, the bacteria could be isolated from the intestinal contents of 2 of 3 (66%) animals in the oral and control groups. In the control group, E. coli O157:H7 was found in contents of the small intestines and rectums of 1 of 3 (33%) and 2 of 3 (66%) animals, respectively, with the largest amount in the ileum (6 × 102). Large intestinal contents were negative. For the oral group, E. coli O17:H7 was found in contents of the large intestines and rectums of 1 of 3 (33%) and 2 of 3 (66%) animals, respectively. The largest amount of E. coli O157:H7 was found in the rectum (3 × 102). Small intestinal contents were negative. Overall, E. coli O157:H7 was isolated most often from rectal contents (in 4 of 9 [44%] samples).
TABLE 1.
Number of E. coli O157:H7 CFU/g of intestinal content at euthanasia on day 43 (rectal group) or day 61 (oral and control groups)
| Group | Animal no. |
E. coli O157:H7 CFU in intestinal contents ofa: |
||||
|---|---|---|---|---|---|---|
| Jejunum | Ileum | Cecum | Colon | Rectum | ||
| Oral | 1 | 0 | 0 | 0 | 0 | 1 × 101 |
| 2 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 2 × 103 | 1.1 × 103 | 3 × 102 | |
| Rectal | 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | |
| Control | 1 | 0 | 0 | 0 | 0 | 1 × 101 |
| 2 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 1 × 102 | 6 × 102 | 0 | 0 | 1 × 102 | |
The number of CFU in gut contents at different locations was determined by direct plating.
At euthanasia, E. coli O157:H7 was never found in tissues of the jejunum and colon and was absent in all intestinal tissue suspensions of animals in the rectal group (Table 2). The bacteria were present in recto-anal tissue suspensions of 1 of 3 (33%) animals of the oral and of the control group. In addition, for the oral group, tissue suspensions of ileum without PP were found positive in 2 of 3 (66%) animals.
TABLE 2.
Number of E. coli O157:H7 CFU/g of tissue at euthanasia on day 43 (rectal group) or day 61 (oral and control group)
| Group | Animal no. |
E. coli O157:H7 CFU in tissue suspensions ofa: |
|
|---|---|---|---|
| Ileum without PP | Recto-anal junction | ||
| Oral | 1 | 4 × 102 | 0 |
| 2 | 0 | 0 | |
| 3 | 3 × 102 | 1 × 102 | |
| Rectal | 1 | 0 | 0 |
| 2 | 0 | 0 | |
| 3 | 0 | 0 | |
| Control | 1 | 0 | 0 |
| 2 | 0 | 1 × 102 | |
| 3 | 0 | 0 | |
The number of CFU in tissue was determined by direct plating. Jejunum with Peyer's patches (PP), jejunum without PP, ileum with PP, and colon were negative in all groups.
Serum antibody responses against virulence factors of E. coli O157:H7.
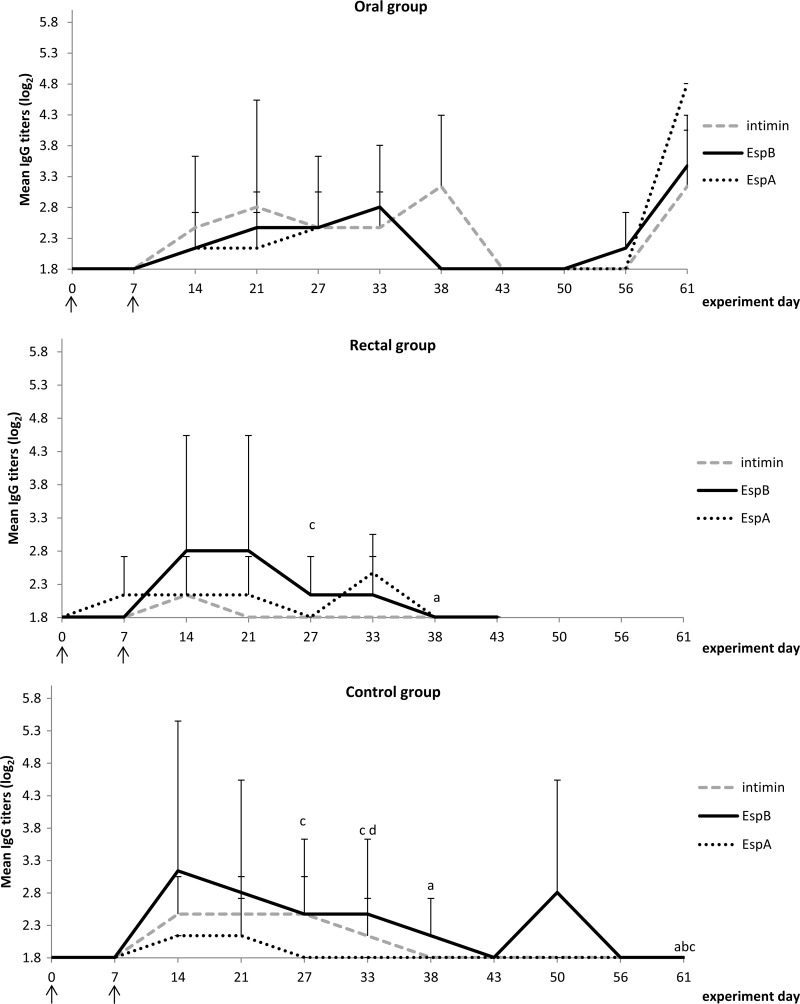
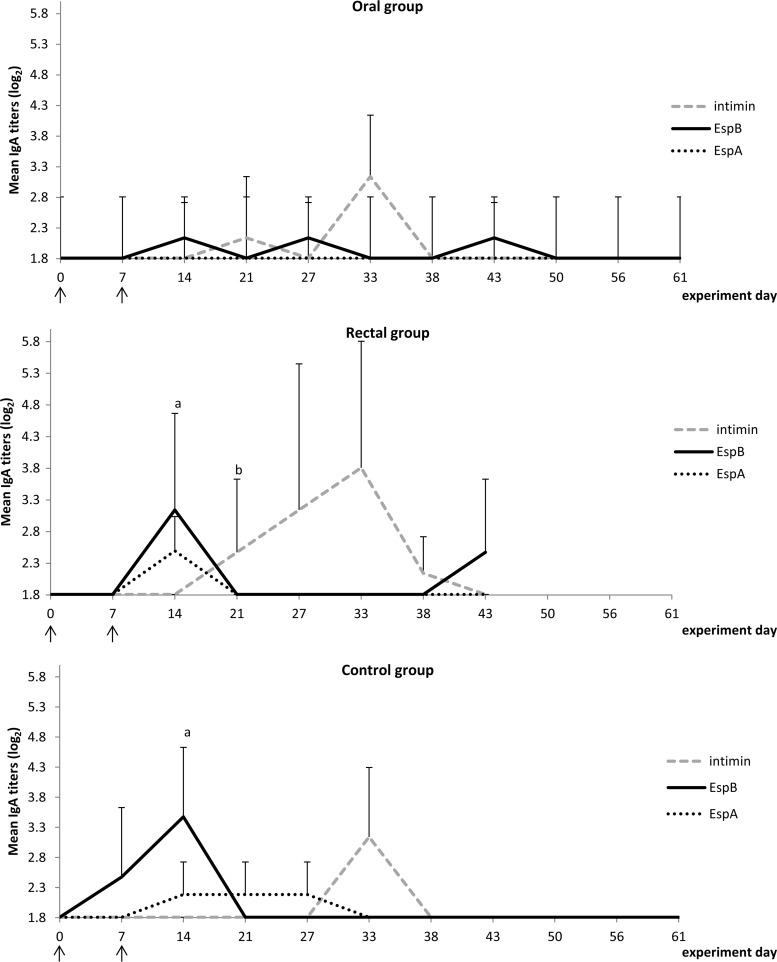
IgG responses against intimin, EspB, and EspA are presented in Fig. 3 and IgA responses in Fig. 4. The responses in all 3 groups showed some similarities, such as (i) the occurrence of low IgG titers against all 3 virulence factors, (ii) the appearance of an IgG response against EspB at 14 days post-primary infection (dppi) until 38 to 43 dppi, (iii) an EspB-specific IgA response which peaked at 14 dppi, and (iv) an intimin-specific IgA response which peaked 33 dppi.
FIG 3.
Mean IgG titers (log2) for the 3 treatment groups. Letters indicate a significant difference between groups for a designated time point: a, significant differences in intimin responses compared to the oral group; b, significant differences in EspB responses compared to the oral group; c and d, significant differences in EspA responses compared to the oral group (c) and to the rectal group (d). Arrows indicate the time points of oral inoculation with bacteria.
FIG 4.
Mean IgA titers (log2) for the 3 treatment groups. Letters indicate a significant difference between groups for a designated time point: a, significant differences in EspB responses compared to the oral group; b, significant differences in EspA responses compared to the oral group. Arrows indicate the time points of oral inoculation with bacteria.
The EspA-specific antibody responses were different between groups. EspA-specific IgG was observed at one time point only in the control group (21 dppi), at 4 time points in the rectal group (7, 14, 21, and 33 dppi), and at 5 time points in the oral group (14, 21, 27, 33, and 62 dppi), whereas the opposite was seen for the EspA-specific IgA response, which was observed at 3 time points in the control group (21, 27, and 33 dppi), at 1 time point in the rectal group (21 dppi), and not in the oral group. The intimin-specific IgG response resembled the EspB response in the control group and the oral group but not in the rectal group, where intimin-specific IgG was detected at 14 dppi only. Another important difference was the reappearance of an IgG response against all three type III secretion system (TTSS) proteins at the end of the experiment (61 dppi) in the oral group and not in other groups, as if a reinfection occurred in the oral group. Furthermore, EspB-specific IgA appeared and disappeared 3 times in the oral group during the observation period but was absent at the end of the experiment.
Overall, all groups developed IgG responses against EspA, EspB, and intimin, with IgG responses against EspB being comparable between groups. However, the anti-intimin response was less pronounced in the rectal group than in the control and oral groups, while on the other hand, the anti-EspA response was more pronounced in both lactoferrin groups.
The rectal and control groups mounted an IgA response against all three EHEC proteins, while the oral group did not produce IgA against EspA. Interestingly, intimin-specific IgA responses appeared 1 week earlier upon reinfection in both lactoferrin-treated groups than in the nontreated control group.
DISCUSSION
Bullen et al. (18) first demonstrated the in vivo effects of lactoferrin by using milk against enteropathogenic E. coli in the small intestines of guinea pigs. They found that the bacteriostatic effect of milk was mainly due to lactoferrin. Later, Wada et al. (19) showed that the administration of bovine lactoferrin to Helicobacter pylori-infected mice resulted in a marked decrease of bacterial colonization and attachment to the gastric epithelium. More recently, we demonstrated clearance of E. coli O157:H7 excretion from the intestine of sheep by oral administration of lactoferrin (20). Since cattle are the main reservoir of E. coli O157:H7, we investigated in the present study the effects of rectal and oral administration of lactoferrin on fecal shedding and intestinal clearance of E. coli O157:H7 in experimentally infected calves. As in our previous sheep experiment, lactoferrin was administered orally in a 10% bicarbonate buffer. This buffer closes the esophageal groove, allowing lactoferrin to pass the rumen, reticulum, and omasum and be directly transported to the abomasum (21). Delivering lactoferrin directly to the abomasum could prevent lactoferrin being consumed by ruminal bacteria and enhance its degradation by pepsin in the abomasum. Pepsin can cut lactoferrin into different peptides, of which one peptide, lactoferricin, is believed to be involved in many lactoferrin functions. It has been shown that lactoferricin can be a more potent antibacterial compound than the native protein (22, 23).
Whereas in a previous study, oral lactoferrin treatment of sheep reduced the amount and duration of E. coli O157 fecal shedding, such an effect was not seen in the orally treated calves in this study. An explanation for this difference could be the different tissue tropisms of E. coli O157:H7 in sheep and calves. In calves, the terminal rectal mucosa, which is rich in lymphoid follicles, has been identified as the major site of E. coli O157:H7 colonization (13, 24). In sheep, the bacteria seem to colonize the entire intestinal tract (16, 20, 25), even though slightly higher numbers could be seen in the ileum and large intestine (26) or at the recto-anal junction (6, 27). It is likely that the antimicrobial activity of orally administered lactoferrin decreases from cranial to caudal in the gut so that an effect can be seen on O157:H7 colonization in the orally inoculated sheep, with a more equal colonization throughout the gut, compared to calves, in which the bacterium has a predilection for the recto-anal junction. This is supported by the following observations: (i) only in the absence of lactoferrin treatment could the bacteria be isolated from the jejunal contents; (ii) in the orally treated calves, the infection seems to be aggravated again near the end of the observation period, as evidenced by an increase in the number of excreted bacteria in two calves with a secondary antibody response; and (iii) rectal administration of lactoferrin to calves not only caused fecal shedding to cease but also seemed to clear the bacteria from the intestine. To our knowledge, a total clearance of EHEC in cattle using a rectal treatment had not yet been described. Naylor et al. (28) and Rozema et al. (29) reported different strategies for rectal treatment that could reduce but not clear the bacterial colonization.
Although antibody responses were detected in the three groups of animals, we do not believe that they play a major role in the clearance of bacteria, since a study by Joris et al. (17) showed that responses against EspA, intimin, and Tir did not always occur during infection with EHEC. When responses against EspB occurred, the feces were positive for EHEC and the response lasted for several months. Therefore, responses against EspB might be used in the future as an indication of the EHEC-positive status of animals. Nevertheless, interpretation of these serum responses should be performed carefully, as we saw in this experiment that responses against EspB can disappear although animals are still shedding. Furthermore, we could not observe a clear effect of lactoferrin on the antibody responses against EspA, EspB, and intimin. In a study by Yekta et al. (20) with oral lactoferrin treatment in sheep, there was no observed effect of lactoferrin on the intimin responses. The EspA and EspB responses were significantly augmented by the high lactoferrin dose. This result was not seen in our experiment with calves, although the same dose of lactoferrin was used in our oral treatment group.
Although we were able to use only a limited number of laboratory animals, the results of this preliminary study are promising since we observed a complete clearance of EHEC after rectal administration of lactoferrin. Future investigations should address the limitations of this study related to sample size and could therefore be performed on the farm level.
Conclusions.
To our knowledge, this is the first study demonstrating a reduction of E. coli O157:H7 shedding by rectal administration of lactoferrin. Moreover, the administration of this antimicrobial protein resulted in the clearance of rectal colonization. However, when lactoferrin was administered orally, no clearance could be observed. This could indicate that orally administered lactoferrin might not reach the recto-anal junction in a sufficient way. No clear influence of lactoferrin on the serum responses could be observed, suggesting that lactoferrin could have other effects on the immune system which affect the clearance of the bacteria. Lactoferrin might be an interesting tool to reduce the number of E. coli O157:H7-excreting cattle, thereby reducing the exposure of humans to this zoonotic pathogen. However, field experiments for further validation of this new strategy have to be performed.
ACKNOWLEDGMENTS
We thank M. J. Woodward for providing E. coli strain NCTC12900.
This research was funded by the Belgian Federal Public Service of Health, the Food Chain Safety and Environmentthe (RF10/6233).
REFERENCES
- 1.Sheng H, Knecht HJ, Kudva IT, Hovde CJ. 2006. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl Environ Microbiol 72:5359–5366. doi: 10.1128/AEM.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hancock DD, Besser TE, Rice DH, Herriotts DE, Tarr PI. 1997. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol Infect 118:193–195. doi: 10.1017/S0950268896007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman PA, Siddons CA, Wright J, Norman P, Fox J, Crick E. 1993. Cattle as a possible source of verocytotoxin-producing Escherichia coli O157 infections in man. Epidemiol Infect 111:439–447. doi: 10.1017/S0950268800057162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shere JA, Kaspar CW, Bartlett KJ, Linden SE, Norell B, Francey S, Schaefer DM. 2002. Shedding of Escherichia coli O157:H7 in dairy cattle housed in a confined environment following waterborne inoculation. Appl Environ Microbiol 68:1947–1954. doi: 10.1128/AEM.68.4.1947-1954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobbaut K, Berkvens D, Houf K, De Deken R, De Zutter L. 2009. Escherichia coli O157 prevalence in different cattle farm types and identification of potential risk factors. J Food Prot 9:1848–1853. [DOI] [PubMed] [Google Scholar]
- 6.Yekta MA. 2011. New strategies for prevention of E. coli O157:H7 infection in sheep. Ph.D. thesis Faculty of Veterinary Medicine, Ghent University, Ghent, Belgium. [Google Scholar]
- 7.Grif K, Dierich MP, Karch H, Allerberger F. 1998. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur J Clin Microbiol Infect Dis 17:761–766. doi: 10.1007/s100960050181. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovitz BC, Gerhardt E, Tironi Farinati C, Abdala A, Galarza R, Vilte DA, Ibarra C, Cataldi A, Mercado EC. 2012. Vaccination of pregnant cows with EspA, EspB, gamma-intimin, and Shiga-toxin 2 proteins from Escherichia coli O157:H7 induces high levels of specific colostral antibodies that are transferred to newborn calves. J Dairy Sci 95:3318–3326. doi: 10.3168/jds.2011-5093. [DOI] [PubMed] [Google Scholar]
- 9.Ohya T, Marubashi T, Ito H. 2000. Significance of fecal volatile fatty acids in shedding of Escherichia coli O157 from calves: experimental infection and preliminary use of a probiotic product. J Vet Med Sci 62:1151–1155. doi: 10.1292/jvms.62.1151. [DOI] [PubMed] [Google Scholar]
- 10.Callaway TR, Carr MA, Edrington TS, Anderson RC, Nisbet DJ. 2011. Diet, Escherichia coli O157:H7, and cattle: a review after 10 years. Curr Issues Mol Biol 11:67–80. [PubMed] [Google Scholar]
- 11.Otto BR, Verweij-van Vught AM, MacLaren DM. 1992. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol 18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 12.Ellison RT III, Giehl TJ, Laforce FM. 1988. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun 56:2774–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naylor SW, Besser LCTE, Mahajan A, Gunn GJ, Pearce MC, McKendrick IJ, Smith DGE, Gally DL. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect Immun 71:1505–1512. doi: 10.1128/IAI.71.3.1505-1512.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grauke LJ, Kudva IT, Yoon JW, Hunt CW, Williams CJ, Hovde CJ. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl Environ Microbiol 68:2269–2277. doi: 10.1128/AEM.68.5.2269-2277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dibb-Fuller MP, Best A, Stagg DA, Cooley WA, Woodward MJ. 2001. An in-vitro model for studying the interactions of Escherichia coli O157:H7 and other enteropathogens with bovine primary cell cultures. J Med Microbiol 50:759–769. [DOI] [PubMed] [Google Scholar]
- 16.Vande Walle K, Yekta MA, Verdonck F, De Zutter L, Cox E. 2011. Rectal inoculation of sheep with E. coli O157:H7 results in persistent infection in the absence of a protective immune response. Vet Microbiol 147:376–382. doi: 10.1016/j.vetmic.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Joris M-A. 2012. Prevalence, characterization and long-term follow up of enterohemorrhagic Escherichia coli and type III secretion system specific antibodies in cattle. Ph.D. thesis Faculty of Veterinary Medicine, Ghent University, Ghent, Belgium. [Google Scholar]
- 18.Bullen J, Rogers H, Leigh L. 1972. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J 1:69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada T, Aiba Y, Shimizu K, Takagi A, Miwa T, Koga Y. 1999. The therapeutic effect of bovine lactoferrin in the host infected with Helicobacter pylori. Scand J Gastroenterol 34:238–243. doi: 10.1080/00365529950173627. [DOI] [PubMed] [Google Scholar]
- 20.Yekta MA, Cox E, Goddeeris BM, Vanrompay D. 2011. Reduction of Escherichia coli O157:H7 excretion in sheep by oral lactoferrin administration. Vet Microbiol 150:373–378. doi: 10.1016/j.vetmic.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberger G, Dirksen G, Grunder HD, Grunert E, Krause D, Stober M. 1979. Clinical examination of cattle. Parey, Berlin, Germany. [Google Scholar]
- 22.Gifford JL, Hunter HN, Vogel HJ. 2005. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell Mol Life Sci 62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellamy W, Takase M, Kawase K, Shimamura S, Tomita M. 1992. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol 73:472–479. doi: 10.1111/j.1365-2672.1992.tb05007.x. [DOI] [PubMed] [Google Scholar]
- 24.Naylor SW, Roe AJ, Nart P, Spears K, Smith DG, Low JC, Gally DL. 2005. Escherichia coli O157: H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonization requires the LEE4 operon. Microbiology 151:2773–2781. doi: 10.1099/mic.0.28060-0. [DOI] [PubMed] [Google Scholar]
- 25.La Ragione R, Best A, Woodward MJ, Wales AD. 2009. Escherichia coli O157:H7 colonization in small domestic ruminants. FEMS Microbiol Rev 33:394–410. doi: 10.1111/j.1574-6976.2008.00138.x. [DOI] [PubMed] [Google Scholar]
- 26.Aktan IR, La Ragione M, Woodward MJ. 2007. Colonization, persistence, and tissue tropism of Escherichia coli O26 in conventionally reared weaned lambs. Appl Environ Microbiol 73:691–698. doi: 10.1128/AEM.01879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vande Walle K, De Zutter L, Cox E. 2011. Oral infection with a Shiga toxin-negative Escherichia coli O157:H7 strain elicits humoral and cellular responses but does not protect sheep from colonisation with the homologous strain. Vet Microbiol 148:317–322. doi: 10.1016/j.vetmic.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Naylor SW, Nart P, Sales J, Flockhart A, Gally DL, Low JC. 2007. Impact of the direct application of therapeutic agents to the terminal recta of experimentally colonized calves on Escherichia coli O157:H7 shedding. Appl Environ Microbiol 73:1493–1500. doi: 10.1128/AEM.01736-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozema EA, Stephens TP, Bach SJ, Okine EK, Johnson RP, Stanford K, McAllister TA. 2009. Oral and rectal administration of bacteriophages for control of Escherichia coli O157:H7 in feedlot cattle. J Food Prot 72:241–250. doi: 10.1016/j.jprot.2009.01.001. [DOI] [PubMed] [Google Scholar]