Abstract
Postweaning diarrhea (PWD) in pigs is a leading cause of economic loss in pork production worldwide. The current practice of using antibiotics and zinc to treat PWD is unsustainable due to the potential of antibiotic resistance and ecological disturbance, and novel methods are required. In this study, an in vitro model was used to test the possibility of producing prebiotic fiber in situ in the gastrointestinal (GI) tract of the piglet and the prebiotic activity of the resulting fiber in the terminal ileum. Soluble fiber was successfully produced from potato pulp, an industrial waste product, with the minimal enzyme dose in a simulated upper GI tract model extracting 26.9% of the initial dry matter. The fiber was rich in galactose and galacturonic acid and was fermented at 2.5, 5, or 10 g/liter in a glucose-free medium inoculated with the gut contents of piglet terminal ileum. Fermentations of 5 g/liter inulin or 5 g/liter of a purified potato fiber were used as controls. The fibers showed high fermentability, evident by a dose-dependent drop in pH and an increase in the organic acid content, with lactate in particular being increased. Deep sequencing showed a significant increase in the numbers of Lactobacillus and Veillonella organisms and an insignificant increase in the numbers of Clostridium organisms as well as a decrease in the numbers of Streptococcus organisms. Multivariate analysis showed clustering of the treatment groups, with the group treated with purified potato fiber being clearly separated from the other groups, as the microbiota composition was 60% Lactobacillus and almost free of Clostridium. For animal studies, a dosage corresponding to the 5-g/liter treatment is suggested.
INTRODUCTION
Postweaning diarrhea (PWD) is a serious condition frequently afflicting piglets after weaning (1–3) and is a leading cause of economic loss in industrial pork production (4, 5). The pathology and etiology of PWD are complex, but the causative agent is believed to be enterotoxigenic Escherichia coli (ETEC) (3, 6, 7). The infective action of ETEC is in turn facilitated by the immature gastrointestinal (GI) system in the piglet, the switch from immunoglobulin-rich maternal milk to solid foods with lower digestibility, increased emotional stress, and the lowered food intake (3, 4, 7).
A substantial amount of antibiotics and the heavy metal zinc are used for prevention and treatment of PWD, as they are presently the most cost-effective means of improving performance in swine production (8, 9). However, due to increasing problems with antibiotic resistance in bacteria of both veterinary and human importance, as well as environmental concerns about the use of heavy metals, alternative strategies for prevention of PWD are needed.
In humans, it is well established that prebiotic fibers, e.g., indigestible fibers fermented by the intestinal microbiota, such as polymers of fructose or galactose, can result in the increased growth of selected beneficial bacteria from the commensal microbiota (10). It is also known that various strains of Lactobacillus and Bifidobacterium act as suppressors or inhibitors of infectious bacteria (11), likely due to production of antimicrobial peptides and organic acids (OAs), as thoroughly reviewed by Liévin-Le Moal and Servin (12). It could then be hypothesized that prebiotic intervention would increase piglet resilience against infection by competitive inhibition of pathogenic bacteria or general improvement of health status. Studies in animals have, however, not been conclusive in establishing if the same effect is possible in weaning piglets (13–17), perhaps because of difficulties in observing an effect in a healthy animal. In contrast, studies using experimental infections along with prebiotic intervention generally show a protective effect against PWD (18–20). In vitro fermentations, using inocula from pig intestines or pig feces, have, however, been fairly successful in showing increases in the numbers of Lactobacillus organisms and, to a much lesser extent, Bifidobacterium organisms and/or increases in the level of production of OAs resulting from bacterial fermentation (21–24).
Potato pulp is a high-volume waste product from the starch industry and is currently produced at ∼1 × 106 tons/year in Europe, where it is used in low-value applications, e.g., in animal feed, and is priced at ∼10 €/ton (25). It has previously been shown that a novel, fermentable, and highly prebiotic fiber could be produced from potato pulp (26, 27) and has the potential for use as a beneficial feed supplement, resulting in a substantial increase in the value of potato pulp. These fibers are tightly bound to the pulp matrix and are insoluble and inaccessible to the gut bacteria in this state. The fibers consist of the rhamnogalacturonan-1 (RG-1) domain of pectin, e.g., an alternating backbone of rhamnose and galacturonic acid substituted with galactose and arabinose chains (28, 29). The pulp is very high in pectin containing this domain, and in 2011 the work of Thomassen et al. showed that a mixture of polygalacturonase (EC 3.2.1.15) and pectin lyase (EC 4.2.2.10) efficiently releases large amounts of RG-1 in an industrially relevant setting (26).
In the pig, peristalsis and body heat provide natural agitation and a constant temperature in the >2 h required for the feed to reach the terminal small intestine. In light of this, it was hypothesized that feeding of enzymes and substrate should allow the enzymatic degradation of highly complex molecules into prebiotic fibers in situ, thereby decreasing the issues of industrial fiber production, such as purification and transport. Release of these fibers from potato pulp by the gut microbiota is not possible, as the bacteria in the pig ileum do not possess genes for pectinolytic enzymes (30).
In this work, the feasibility of executing the enzymatic reaction in an in vitro piglet intestinal system, as well as the effect of these fibers on the microbiota composition in ileum samples obtained from 26-day-old piglets, was evaluated.
MATERIALS AND METHODS
Characterization of pectinolytic enzymes.
Pectin lyase and polygalacturonase were produced by fermentations, as described by Silva et al. (31), using Pichia pastoris clones transformed with the pectin lyase gene AN2569.2 and the polygalacturonase gene AN4372.2, both from Aspergillus niger, as described by Bauer et al. (32). This method ensures the production of monocomponent enzymes by placing the gene under the control of a methanol promoter, allowing the enzyme to be expressed in large amounts by addition of methanol to the fermentation. Protein concentrations were determined by the bicinchoninic acid (BCA) assay with bovine serum albumin (BSA) as a standard, as described by the manufacturer (Thermo Fisher Scientific, Rockford, IL).
Pectin lyase activity was assayed by incubating pectin lyase enzyme with 1.5 g/liter citrus pectin substrate at a 0.25% (wt/wt) enzyme-to-substrate (E/S) ratio (Sigma-Aldrich, Steinheim, Germany) in McIlvaine buffer at pH 3 or pH 7 and 25°C in triplicate. The reaction was followed for 10 min in an Infinite200 microplate reader (Tecan, Salzburg, Austria) by recording the absorbance at 235 nm. Units of activity were defined as the number of μmol of unsaturated uronide released per minute using 5,500 M−1cm−1 as the extinction coefficient (31).
Polygalacturonase was assayed by incubating polygalacturonase with 3 g/liter polygalacturonic acid (Sigma-Aldrich, Steinheim, Germany) at a 0.125% E/S ratio in McIlvaine buffer at pH 3 or 7 and 37°C in triplicate. The amount of reducing ends was quantified in the reducing ends assay described by Thomassen et al. (26). In brief, after 10 min of incubation, the enzymatic reaction was stopped by extracting 20 μl into a freshly made and preheated p-hydroxybenzoic acid hydrazide solution, and the mixture was then incubated at 70°C for 10 min. The amount of reducing ends was then quantified by determination of the absorbance at 410 nm. Units of activity were defined as the number of μmol of galacturonic acid released per minute, using free galacturonic acid as a standard.
Animals.
Five suckling pigs were acquired from a commercial farm in Denmark at 26 days of age, 2 days before they would otherwise have been weaned. The animals were females of mixed breed, had been given a wheat-based creep feed since 7 days of age, and were healthy. Furthermore, they had not been given antibiotics.
The animals were euthanized at the National Veterinary Institute at the Technical University of Denmark by an overdose of pentobarbital and jugular vein puncture. All handling of animals was performed by trained personnel and veterinarians and fulfilled the regulations of the Danish Ministry of Justice.
Contents were collected from the stomach and middle jejunum, placed in sterile tubes, and snap-frozen in dry ice before storage at −80°C. Contents from the terminal ileum were collected, placed in sterile tubes, and mixed 1:1 with 50% (wt/wt) glycerol before they were snap-frozen in dry ice and stored at −80°C. The use of frozen samples rather than fresh samples has formerly been verified (33, 34), and frozen samples have previously been used in similar experiments (35, 36).
Characterization of digestive enzymes.
Stomach and jejunal contents were homogenized, followed by centrifugation at 4,000 × g for 10 min at 4°C, and the pH in the supernatant was then measured using a pH strip (pH-fix; Macherey-Nagel, Germany). Dry matter was determined by drying of the samples at 105°C for 24 h in duplicate for each sample.
The pepsin in the stomach supernatant was assayed by incubating 50 μl supernatant in 250 μl 0.06 M HCl with 20 g/liter hemoglobin in triplicate for each sample. The reaction was run at 37°C and 900 rpm and terminated after 60 min with 500 μl of 5% (wt/vol) trichloroacetic acid. After 10 min of mixing, the samples were centrifuged for 2 min at 14,000 × g, and the amount of pepsin in 100 μl of the supernatant was measured by determination of the absorbance at 280 nm. Controls were made by adding the trichloroacetic acid before addition of the gastric sample. Units of activity were defined as the number of nmol of tyrosine released per minute using an extinction coefficient of 1,250 M−1 cm−1. The pepsin concentration was calculated by comparison to a standard curve of the concentrations of commercial pepsin (catalog number P7125, batch no. SLBB6557V; Sigma-Aldrich, Steinheim, Germany).
Small intestine proteases in the jejunal supernatant were assayed with an azurine-cross-linked (AZCL) casein assay (Megazyme, Bray, Ireland) according to the manufacturer's instructions, with some modifications: 50 μl of sample diluted 40-fold was added to 250 μl McIlvaine buffer (pH 7, 20 mM) containing 5 mg AZCL-casein in triplicate for each sample. The reaction was run at 37°C and 1,400 rpm and terminated after 5 min with 500 μl of 10 g/liter trisodium phosphate. After centrifugation for 5 min at 15,000 × g, the amount of protease in 100 μl of the supernatant was measured by determination of the absorbance at 590 nm in a microplate. Units of activity were defined as the change in 1 optical density unit at a 1-cm path length per minute. The corresponding concentration of pancreatin was determined by comparison to a standard curve of the concentrations of commercial pancreatin (catalog number P7545; batch no. SLBD0640V; Sigma-Aldrich, Steinheim, Germany).
Production of IVSF.
A porcine gastrointestinal digestion process was simulated by sequential digestion under stomach conditions and then small intestinal conditions, producing in vitro-solubilized fiber (IVSF). The concentrations and conditions were based on the measurements obtained by characterization of the animal digesta. The potato pulp used in the study, FiberBind, was kindly provided by KMC (Brande, Denmark) and is the dried residue remaining when potato starch has been extracted.
Ten milliliters of purified water with 0.32 mg/ml pepsin (catalog number P7125; Sigma) was added to 300 mg potato pulp in a 50-ml Falcon tube and adjusted to pH 3 with HCl in four replicates. After they were preheated to 39°C, pectin lyase and polygalacturonase were added at E/S ratios of 0 to 0.05% each, and the mixture was incubated at 60 rpm on a rolling mixer situated in an oven at 39°C. After 60 min, 20 ml of preheated water containing 16 mg/ml pancreatin, 50 mM bicarbonate, and 2 mg/ml bile salts, which made the pH ∼7, was added. This mixture was further incubated at 60 min and 60 rpm. The tubes were placed on ice for 5 min to stop the reaction and were centrifuged at 4,000 × g for 5 min at 4°C, after which they were filtered through qualitative filter paper (no. 417; VWR, Darmstadt, Germany). Ten milliliters of the filtrate was added to 23.3 ml isopropanol to precipitate the soluble carbohydrates, and the mixture was centrifuged at 4,000 × g for 5 min. The supernatant was discarded, and the pellet was lyophilized and weighed.
Production of UFSF.
Ultrafiltered soluble fiber (UFSF) mimicking the one produced by Thomassen et al. (26) was made by adding 10 g of potato pulp to 1,000 ml 0.1 M phosphate buffer, pH 6, and preheating the mixture to 60°C. Pectin lyase enzyme and polygalacturonase substrate were added at 1% each, and the reaction was run for 1 min at 300 rpm, after which the reaction was stopped by 10 min of boiling. The solution was then centrifuged at 5,000 × g for 60 min, and the supernatant was ultrafiltered with a 100-kDa-molecular-mass-cutoff VivaFlow 200 filter (Vivascience, Hannover, Germany) with the addition of 5 liters of water until 90 ml remained in the retentate. The carbohydrate in the retentate was then precipitated by addition of isopropanol to 70% (vol/vol), and the mixture was centrifuged at 4,000 × g for 5 min. The pellet was then lyophilized.
Carbohydrate composition.
Monosaccharide composition analysis was done as described by Ravn and Meyer (37). In brief, samples were hydrolyzed by trifluoroacetic acid (soluble carbohydrate) or sulfuric acid (potato pulp), followed by quantification on a high-pH anion-exchange chromatography (HPAEC)-pulsed amperometric detection (PAD) system using a Dionex CarboPac PA1 analytical column (2 mm by 250 mm) combined with a CarboPac PA1 precolumn (2 mm by 50 mm) and 0.25 to 500 mM NaOH (37). Rhamnose, arabinose, galactose, glucose, mannose, xylose, and galacturonic acid were included as standards.
Size determinations were done with high-performance size exclusion chromatography (HPSEC) analysis by dissolving carbohydrate samples at 3 g/liter in 0.1 M sodium acetate, pH 6, with 0.02% sodium azide and filtering with a 0.22-μm-pore-size filter. Samples where then injected in a Shodex OHpak SB-806 HQ column (8.0 mm by 300 mm; Showa Denko KK, Kawasaki, Japan) and eluted with 0.1 M sodium acetate (pH 6). The injection volume was 50 μl, and the flow rate was 0.5 ml/min at 30°C on a system consisting of a P680 high-pressure liquid chromatography (HPLC) pump, an ASI-100 automated sample injector, and a Shodex RI-101 refractive index detector (Showa Denko KK). Pullulan standards of 1.3, 10, 110, 400, and 800 kDa were used.
Protein determination.
The concentration of soluble protein in the extracted fibers was estimated using a micro-BCA protein assay reagent kit (Pierce, Rockford, IL) per the manufacturer's instructions with BSA as a standard.
In vitro fermentations.
A fiber product was produced in the GI tract reactor to simulate the in situ production of what would be expected to reach the terminal ileum in an animal if the animal were fed enzymes and potato pulp. This fiber was then fermented in small-scale fermentations by the method of Vigsnæs and colleagues (35, 36). Briefly, IVSF from the simulated gastrointestinal reactor was fermented for 24 h at a final concentration of 0, 2.5, 5, or 10 g/liter of fiber (the control, IVSF-2.5, IVSF-5, and IVSF-10 groups, respectively) in a glucose-free medium consisting of 2 g/liter peptone water, 1 g/liter yeast extract, 0.1 g/liter (1.71 mM) NaCl, 0.04 g/liter (0.23 mM) K2HPO4, 0.04 g/liter (0.29 mM) KH2PO4, 0.01 g/liter (0.04 mM) MgSO4·7H2O, 0.01 g/liter (0.07 mM) CaCl2·2H2O, 2 g/liter (23.81 mM) NaHCO3, 0.5 g/liter bile salts, 0.5 g/liter l-cysteine hydrochloride, 0.005 g/liter hemin, 10 μl/liter vitamin K1 (0.02 mM), 2 ml/liter Tween 80, and 0.05 ‰ (wt/vol) resazurin. Inulin (INU; OraftiHPX, Orafti, Oreye, France) and UFSF, both of which were added at 5 g/liter (INU-5 and UFSF-5, respectively), were used as positive controls. The fibers were dissolved by 15 min of agitation in a boiling water bath, which provided the added advantage of sterilizing the fibers, which were contaminated with Bacillus cereus as well as other bacteria. The samples from the terminal ileum of piglets, which had been stored in 25% glycerol, were thawed on ice, pooled, and diluted 5-fold in 10 mM degassed phosphate-buffered saline. Digesta were pooled across piglets, as in earlier studies (23, 34, 38), in order to control biological variation (39). This mixture was diluted 10-fold in the fiber-containing medium, which had been degassed in an anaerobic cabinet overnight in Nunc 14-ml round-bottom tubes. The resulting 1% solution of ileal content was then fermented for 24 h in an anaerobic cabinet at 37.5°C. All treatments were performed in 10 replicates. A fermentation mixture that was harvested immediately after inoculation and that had no added fiber served as a baseline control, and fermentation of this mixture was also performed in 10 replicates.
pH and organic acid analysis.
Following the fermentations, the tubes were centrifuged for 5 min at 5,500 × g at 4°C, and the pH was measured with a pH meter. The sample was then resuspended, and the suspension was transferred to a 2-ml Eppendorf tube, which was spun at 13,000 × g for 10 min. The supernatant was then filtered through a 0.22-μm-pore-size filter and assayed for OAs using HPLC. A Shimadzu HPLC system fitted with an RSpak KC-811 column and a refractive index detector and that used 12 mM H2SO4 at a flow rate of 0.6 ml/min at 63°C was used. The standards used were lactic acid, acetic acid, propionic acid, isobutyric acid, n-butyric acid, isovaleric acid, and n-valeric acid.
DNA extraction.
Fermentation samples were spun at 13,000 × g for 10 min, and the DNA contained in the cell pellets was purified with a Maxwell LEV blood DNA purification kit (Promega Corporation, Madison, WI, USA) as described by Ingerslev et al. (40).
16S rRNA gene PCR.
A PCR targeting the V1/V2 regions of the bacterial 16S rRNA gene from the bacteria contained within the fermentations was performed. The PCR was performed using the universal primers V1-forward (5′-AGAGTTTGATCCTGGCTCAG-3′) and V2-reverse (5′-CTGCTGCCTYCCGTA-3′) (41) (Sigma-Aldrich, Broendby, Denmark). Both primers were tagged with a 6-nucleotide bar code at the 5′ end, and each specific bar code was assigned to a specific DNA sample. The reaction was carried out in 50-μl reaction mixtures containing 5 μl of 5× Gold Taq buffer (Applied Biosystems, Branchburg, NJ, USA), 1 μl of each primer (20 μM), 2 μl of 10 mM deoxynucleoside triphosphates, 4 μl of 25 mM MgCl2, 0.5 μl of AmpliTaq Gold polymerase (Applied Biosystems), 34.5 μl of nuclease-free H2O, and 2 μl of DNA template (10 ng/μl). Reaction times and cycling conditions were 94°C for 6 min; 30 cycles of 94°C for 45 s, 57°C for 45 s, and 72°C for 90 s; and 72°C for 10 min. The resulting PCR products were then analyzed on an Agilent 2100 bioanalyzer using an Agilent DNA 1000 kit (Agilent Technologies, Waldbronn, Germany) and further pooled in equimolar ratios (50 ng per bar-coded sample). The pooled DNA was then purified of primers and detergents using a Qiagen MinElute PCR purification kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions.
Illumina MiSeq sequencing.
The DNA was submitted to the National High-Throughput DNA Sequencing Centre at the University of Copenhagen, Copenhagen, Denmark, for sequencing on an Illumina MiSeq 250PE platform. The long reads obtained were analyzed using BION-meta software (more information about BION-meta software and acquisition of the software is available from the Danish Genome Institute, Aarhus, Denmark). In brief, demultiplexing was performed according to the primer and bar code sequences. Forward and reverse sequences were joined, with no gaps, a maximum mismatch percentage of 80, and a minimum overlap length of 20 bp being allowed. Next, the sequences at both ends were cleaned by removal of bases with a quality of less than 98%, which is equivalent to a Phred score of 17. Identical sequences were further dereplicated into consensus sequences. Consensus sequences of at least 260 nucleotides in length were mapped into a table according to the individual bar codes. Finally, the consensus sequences were taxonomically classified against the sequences in the Ribosomal Database Project II (RDP-II) SSU database (RDP II; http://rdp.cme.msu.edu/index.jsp) using a word length of 8 and a match minimum of 90%. The top 1% of the sequences obtained from the RDP-II database showing similarity were used for taxonomic classification of the consensus sequences. The resulting operational taxonomic units (OTUs) in each bar-coded sample were normalized in order to enable direct statistical comparisons of relative abundance in each sample.
Statistics.
The dose-response relationship of enzyme dosage ([E/S]) versus dry matter release in the intestinal reactor was fitted to a modified Monod equation of the form percent release = ϕ1 + {ϕ2 · [E/S]/(ϕ3 + [E/S])}, where ϕ1 is the value of the control, ϕ2 is the maximum value with the control value subtracted, and ϕ3 is the enzyme concentration at which half of the maximum release is attained. Analysis of variance (ANOVA) was used to evaluate significant differences in dosage. pH and organic acid concentrations were analyzed with an ANOVA, followed by Tukey's test, with the fiber treatment being the main effect, using the lm- and HSD.test procedures in R. Two-way ANOVAs of the fiber composition and organic acid data were avoided due to uneven variances, and instead, when multiple one-way ANOVAs were performed, adjustments for multiple comparisons were made by use of the Sidak correction. Sequencing data were analyzed at a given taxonomic level by ANOVA of log-transformed data, followed by Tukey's post hoc test and correction for multiple comparisons by use of the Sidak correction (42) when multiple OTUs were compared. The microbial community was analyzed by subjecting species-level data to principal coordinates analysis (PCoA) with treatment groups as constraints and using the Bray-Curtis dissimilarity index with the capscale procedure in R, followed by analysis of similarities (anosim procedure) as well as k-means clustering (k-means procedure). Shannon indices were calculated by use of the diversity command. Principal coordinates regression, e.g., using the scores from the first axis of the PCoA as a predicting variable, was used to compare the overall bacterial composition with the presence of individual OAs.
RESULTS
Characterization of piglet digestive parameters.
The dry matter content, the pH, and the pepsin levels in the stomach digesta were determined in order to use the values to produce a realistic in vitro GI system. There was substantial variation within the piglets, especially in the activity of pepsin, which was, on average, 43.3 units/ml digesta (pooled standard deviation [SD], 3.3 units/ml digesta) but ranged from 13.4 to 98.1 units/ml digesta. The average dry matter content was 21.42% (wt/vol) and ranged from 6.78 to 30.65% (wt/vol), whereas the pH averaged 4.16 and ranged from 4.1 to 4.6.
The average protease content in the ileum was 39.2 units/ml digesta (pooled SD, 1.32 units/ml digesta) and ranged from 7.0 to 78.0 units/ml. The dry matter content in the ileum was, on average, 13.4% (wt/vol) (range, 7.28 to 19.30), and the average pH was 6.6 (range, 6.0 to 7.0).
In vitro GI tract reactor.
The GI tract reactor was set up on the basis of the measurements from the piglet digesta with an additional overhead. The protease content in particular was deemed to be of importance, and therefore, protease was added to an amount equal to twice the highest measured concentration for both the stomach and the small intestine steps. The lowest measured pH in the stomach was 3.5, and therefore, the pH used in this step was 3. The pH in the small intestine was set to be neutral (pH 7).
The activities of the polygalacturonase and the pectin lyase under stomach conditions (pH 3) were 22.70 U/mg and 0.02 U/mg, respectively. Under small intestine conditions (pH 7), the activities were 18.79 U/mg and 2.38 U/mg, respectively.
Characterization of IVSF and UFSF.
The release of water-soluble fibers from potato pulp showed a clear dose dependency as a function of enzyme dosage, which could be modeled by a nonlinear 3-parameter equation, which in turn suggested that the maximal release was 26.9% of the initial dry matter. The proportion of the initial dry matter released without addition of pectinolytic enzymes was 5.56% ± 0.1%, and since less than 20% of this fraction could be accounted for by HPAEC-PAD or as protein, it presumably consists of various proteins, lignin, and salts. The compositions of all other fractions released showed that they contained large amounts of galactose, galacturonic acid, and, to a lesser degree, arabinose and rhamnose. Analysis of variance of the data revealed that there was a plateau in yield at a dosage consisting of an E/S ratio of 0.05%; for this reason, an E/S ratio of 0.03% was chosen as a cost-beneficial dosage with which to do future work with. At this dose, 24.6 mg dry matter is released from 100 mg potato pulp, corresponding to 9.5 mg galactose. The composition of the in vitro-released fiber, IVSF, and UFSF is shown in Table 1, and it is seen that IVSF contained more galacturonic acid and less galactose than UFSF, as well as slightly more protein. UFSF also contained a small amount of mannose, presumably from the pectinase formulation. HPSEC analysis of IVSF revealed a molecular size distribution with two distinct peaks, notably, a large fraction of ∼900 kDa as well as a smaller fraction of ∼10 kDa (Fig. 1). The HPSEC chromatogram of the UFSF showed that high-molecular-mass polysaccharides (i.e., polysaccharides with molecular masses of >800 kDa) were much more abundant than the low-molecular-mass polysaccharide fractions. Estimation of the amount of monosaccharide was done by comparing the area under the curve (AUC) of the monosaccharide peak (24.6 min) with the total AUC. The AUC of the monosaccharide peak constituted ∼3.8% of the total AUC in UFSF, while this number was 10.6% in IVSF, suggesting that the monosaccharide content was 2.8-fold higher in IVSF.
TABLE 1.
Composition of fibers extracted from potato pulp by simulated in vitro digestion (IVSF) and by using an established purification process (UFSF)a
| Fiber type | Column composition (% [wt/wt] released dry matter) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rha | Ara | Gal | Glc | Xyl | Man | GalA | Protein | NA | |
| IVSF | 3.4 ± 0.4* | 6.5 ± 0.8 | 36.3 ± 3.6* | 2.4 ± 0.4* | 0 ± 0 | 0 ± 0* | 20.3 ± 5.0* | 13.2 ± 0.5* | 17.9 ± 0 |
| UFSF | 2.4 ± 0.1 | 5.4 ± 0.3 | 45.6 ± 1.5 | 4.7 ± 0.4 | 0 ± 0 | 6.7 ± 0.2 | 9.3 ± 0.2 | 10.7 ± 0.4 | 15.2 ± 0 |
Total dry matter release was determined by weighing after freeze-drying, and the monosaccharide composition was determined by acid hydrolysis followed by HPAEC-PAD analysis. Protein was determined with a bicinchoninic acid assay. NA, nonaccountable (the fraction which could not be recovered by HPAEC-PAD or protein determination). Data for monosaccharides were calculated as dehydrated values (n = 4). *, significant difference (P < 0.05) within column by t test after correction for multiple comparisons.
FIG 1.
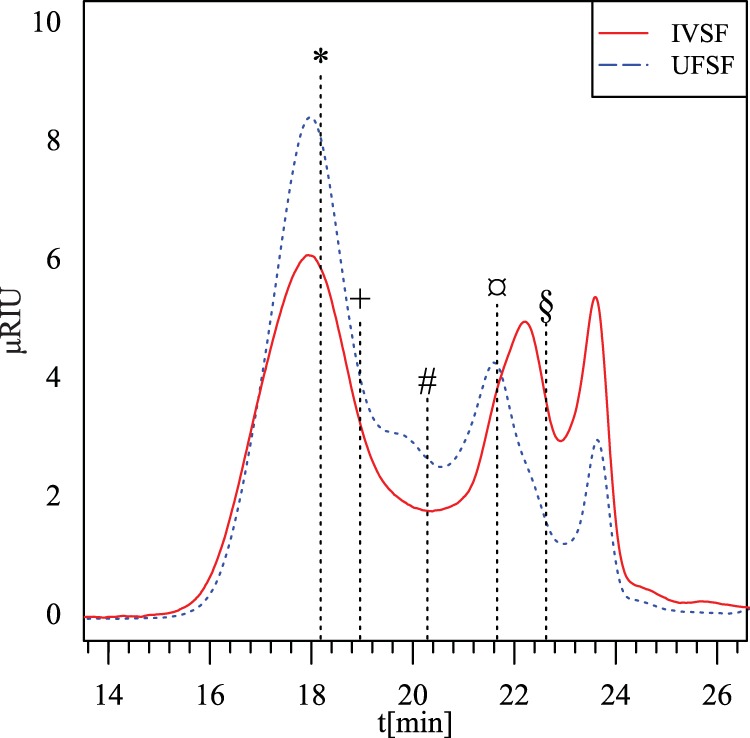
High-performance size exclusion chromatogram of the fibers extracted from potato pulp by simulated in vitro digestion (IVSF) and by using an established purification process (UFSF). *, 800 kDa; +, 400 kDa; #, 110 kDa; ¤, 12 kDa; §, 1.3 kDa. μRIU, refractive index unit; t, time.
In vitro fermentation characteristics.
IVSF, UFSF, and inulin were fermented in the terminal ileal content from piglets in an in vitro reactor for 24 h. The fermentations resulted in a fiber dose-dependent decrease in pH (P < 0.0001, general linear model), as shown in Fig. 2. The pH in the INU-5 fermentation was the same as that in the IVSF-5 fermentation (P = 1, Tukey's post hoc test), although the pH was not as low as that in the IVSF-10 fermentation. The UFSF-5 treatment induced a pH similar to that in the INU-5 and IVSF-5 treatments but a pH higher than that in the IVSF-10 treatment.
FIG 2.
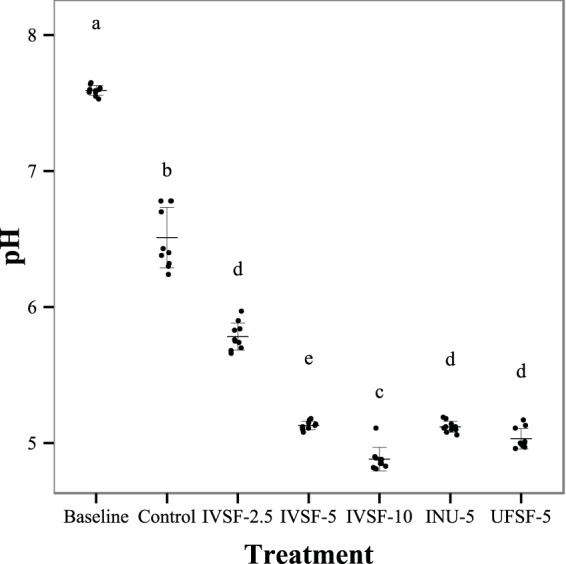
pH as a function of treatment after 24 h of in vitro fermentation of IVSF at 2.5 to 10 mg/ml, INU-5, or UFSF-5. Data are for 10 fermentations. Nonsimilar letters indicate significant differences.
Organic acids.
All the fibers were highly fermentable. Fermentation treatment had a significant effect (ANOVA, P < 0.001) on all organic acids (Table 2). Total OA levels markedly increased with the concentration of IVSF, and INU-5 and UFSF-5 treatments resulted in OA levels between the OA levels from IVSF-2.5 and IVSF-5 treatments. The level of lactic acid was especially markedly increased by the experimental fiber (20 times from the baseline level) and was significantly higher than that in the INU-5 treatment group. Lactate was also the most affected OA in relative terms, constituting 3% of total OAs in the control treatment and 17% in the IVSF-10 treatment. The propionate level increased with dosage, although the levels plateaued beyond IVSF concentrations of 5 g/liter. Both butyrate and valerate levels increased from the baseline levels with all treatments, although the only treatment that had results statistically significantly different from those for the other treatments was the IVSF-2.5 treatment. The acetate level increased with the IVSF concentration, as well as in the INU-5 and UFSF-5 treatments. As expected, a highly significant correlation (r2 = 0.94, P < 0.00001) existed between the total OA concentration and pH.
TABLE 2.
Concentrations of OAs as a function of treatment after 24 h of in vitro fermentation of IVSF at 2.5 to 10 mg/ml, INU-5, or UFSF-5
| Group | Concna (mmol/liter) |
|||||
|---|---|---|---|---|---|---|
| Lactate | Propionate | Acetate | Butyrate | Valerate | Total | |
| Baseline | 0.41 ± 0.04D | 0.89 ± 0.02E | 2.53 ± 0.05E | 0.11 ± 0.02B | 0 ± 0A | 3.95 ± 0.09F |
| Control | 0.84 ± 0.09D | 5.87 ± 0.18D | 19.73 ± 3.17D | 0.96 ± 0.50AB | 0.29 ± 0.25A | 27.69 ± 4.15E |
| IVSF-2.5 | 0.95 ± 0.07CD | 8.09 ± 1.69C | 22.25 ± 1.92CD | 3.65 ± 2.85AB | 1.40 ± 1.21A | 36.34 ± 1.51D |
| IVSF-5 | 4.23 ± 0.69BC | 11.79 ± 0.63A | 28.36 ± 1.34AB | 1.86 ± 0.77AB | 0.50 ± 0.38A | 46.73 ± 1.22B |
| IVSF-10 | 9.11 ± 3.85A | 10.77 ± 1.51AB | 30.50 ± 5.09A | 4.49 ± 4.29A | 1.52 ± 1.92A | 56.39 ± 3.68A |
| INU-5 | 2.21 ± 0.86CD | 10.30 ± 0.63AB | 26.08 ± 1.86BC | 2.72 ± 1.49AB | 0.94 ± 0.69A | 42.25 ± 1.96C |
| UFSF-5 | 6.19 ± 3.60AB | 9.56 ± 0.59BC | 23.57 ± 1.75CD | 3.22 ± 2.23AB | 0.95 ± 0.89A | 43.49 ± 1.84BC |
Values are for 10 piglets for each group. Nonsimilar letters denote significant differences (P < 0.05) for each column by one-way ANOVA after correction for multiple comparisons.
Microbiota composition.
After MiSeq sequencing of the 16S rRNA gene PCR products followed by pattern demultiplexing, sequence cleaning, uniquification, and chimera filtering by the BION-meta software, 1,279,205 sequences were available for taxonomic classification.
At the phylum level, the samples were generally heavily dominated by the phylum Firmicutes. The native microbiota, e.g., the microbiota in the baseline samples, consisted of more than 97% Firmicutes, with reads for members of the classes Bacilli, Clostridia, and Erysipelotrichia accounting for 47, 33, and 17% of the total reads, respectively. Fermentation for 24 h without added fiber resulted in increasing numbers of organisms of the class Clostridia, even in the no-fiber control group, whereas the presence of the in vitro-solubilized fibers was associated with a rise in the numbers of organisms of the class Negativicutes. Incubation with INU-5 resulted in a decrease in the numbers of organisms of the class Clostridia and an increase in the numbers of organisms of the class Negativicutes, whereas the UFSF-5 incubation eliminated organisms in the class Clostridia entirely, while it increased the numbers of organisms of the class Bacilli and the class Negativicutes.
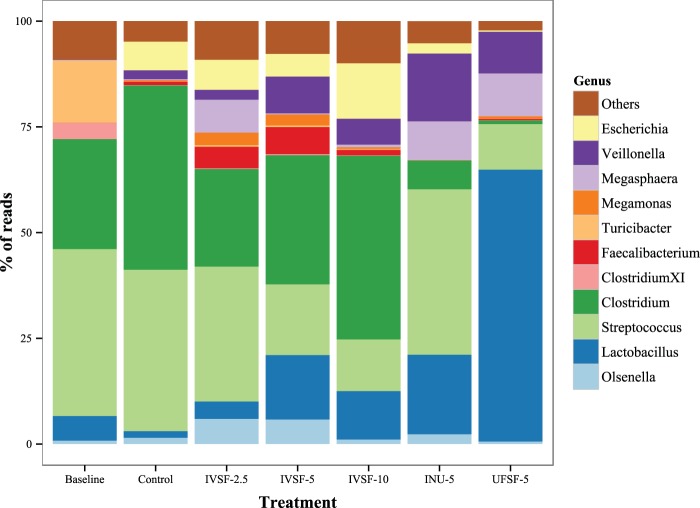
At the genus level (Fig. 3), the fibers made in vitro caused a significant decrease in the numbers of organisms of the genus Streptococcus and a significant increase in the numbers of organisms of the genus Lactobacillus. The amount of Clostridium bacteria did not change significantly from that in the control group treated with IVSF, although it was decreased significantly in the INU-5 and UFSF-5 treatment groups. Clostridium perfringens was found in all groups, but its levels were not changed by the addition of IVSF and the numbers of Clostridium perfringens organisms were significantly depressed by the INU-5 and UFSF-5 treatments. No Clostridium difficile organisms were found in any of the groups. Only about 90 reads for Clostridium cluster XIVa of more than a million total reads were found. The numbers of organisms of the genus Olsenella were elevated only in the IVSF-2.5 and IVSF-5 treatment groups. The ratio of the number of organisms of the genus Clostridium to the number of organisms of the genus Lactobacillus was markedly heightened with the control treatment but was not different between the other treatments. Exclusion of data for the control group from the analysis to retain homoscedasticity suggested that the IVSF-10 group had a higher ratio of these two genera than the UFSF-5 group, mainly since in this study the amount of Clostridium bacteria was very low. The numbers of organisms of the genus Megasphaera were increased significantly by all fiber treatments except the IVSF-2.5 treatment. Members of the family Enterobacteriaceae were present in all baseline samples, and their levels increased from those at the baseline in all fermentations, including the control fermentation, although the numbers in the INU-5 and UFSF-5 groups were slightly lower than those in the control group. Bifidobacteria were consistently observed only in the INU-5 treatments and constituted only less than ∼0.1% of the microbiota.
FIG 3.
Genus-level composition of the microbial community after sequencing. DNA from 24-h in vitro fermentations of IVSF at 2.5 to 10 mg/ml, INU-5, or UFSF-5 was sequenced. Data are for 9 to 10 fermentations in each group.
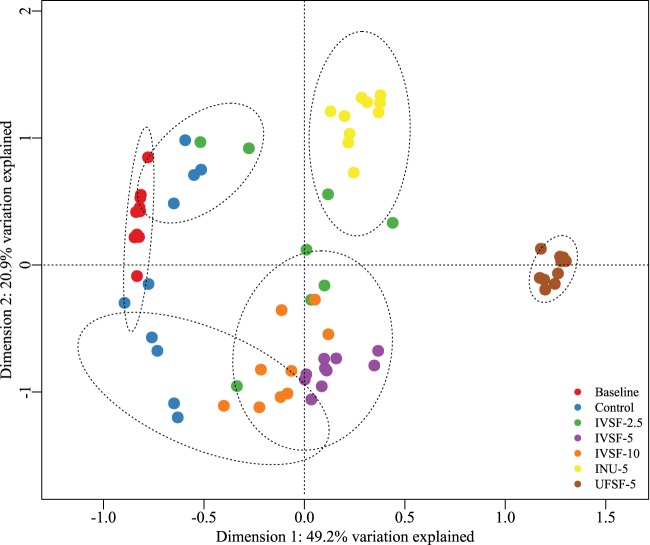
At the genus level, the Shannon diversity index after treatment was significantly lower than that at the baseline for the control and UFSF-5 groups; the IVSF-2.5, IVSF-5, IVSF-10, and INU-5 groups had significantly higher indices than the control and UFSF-5 groups. A constrained analysis of principal coordinates (CAP) on species-level data by use ofthe treatment groups as constraints resulted in 71.1% of the variation being explained on the first two dimensions, as seen in Fig. 4. On the first axis, Lactobacillus spp. as well as Megasphaera spp. had positive coefficients, whereas species of Clostridium, Streptococcus hyointestinalis, and Erysipelotrichaceae turicibacter had negative coefficients. On the second axis, Streptococcus hyointestinalis, Lactobacillus delbrueckii, and an unclassified Turicibacter species had positive coefficients, whereas various species of Clostridium and unclassified Lactobacillus species had negative coefficients. Clustering by use of the k-means algorithm showed distinct clusters, namely, one containing the UFSF-5 treatment, a second cluster containing the INU-5 treatment, a third cluster containing the IVSF-5 and IVSF-10 treatments, and a fourth cluster containing the control group. In contrast, the IVSF-2.5 group was not clearly clustered, and although the control samples were clearly separated on the first axis, there was no separation on the second axis. The clustering was supported by analysis of similarities (R = 0.811, P < 0.001).
FIG 4.
Constrained analysis of a principal coordinates plot on normalized bacterial reads at the species level using Bray-Curtis dissimilarities. The k-means method was used for clustering (clusters include a 95% confidence interval). DNA from 24-h in vitro fermentations of IVSF at 2.5 to 10 mg/ml, INU-5, or UFSF-5 was sequenced. Data are for 9 to 10 fermentations in each group.
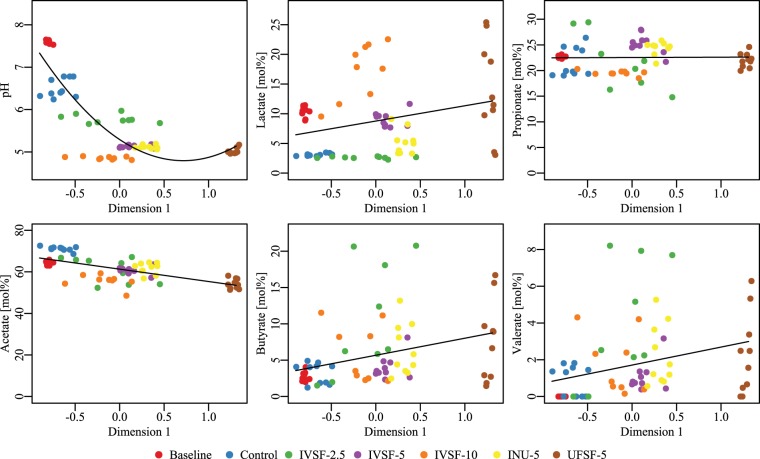
The scores obtained from the constrained analysis of principal coordinates were further used as an independent variable to test correlations of the scores with the pH and the OA composition using linear regression (Fig. 5). A significant negative association between the score on dimension 1 (CAP1) and the pH and acetic acid concentration was observed, whereas there was a positive association between the score on dimension 1 and the lactic acid, butyric acid, and valeric acid concentrations. This, in turn, suggests that a gut microbiota rich in species belonging to the genera Lactobacillus and Megasphaera and poor in organisms of the genus Clostridium is associated with a low pH and acetic acid concentration but high levels of butyric acid, valeric acid, and lactic acid.
FIG 5.
Associations between pH and fatty acids versus bacterial flora (CAP1). All associations apart from the association with propionate were significant (P < 0.05, general linear model). pH and OA levels were measured, and DNA from 24-h in vitro fermentations of IVSF at 2.5 to 10 mg/ml, INU-5, or UFSF-5 was sequenced. Data are for 9 to 10 fermentations in each group.
DISCUSSION
In the present paper, the solubilization of potato galacto-rhamnogalacturonan 1 from potato pulp was attempted in a system simulating the piglet upper gastrointestinal tract. The operating parameters of the system were chosen from direct measurements on piglets. The pHs of the stomach and the small intestine were measured to be 4.1 to 4.6 and 6.0 to 7.0, respectively, which are in agreement with those reported in the literature (43). A pH of 3.0 was chosen as the value for the stomach, as this level of acidity is realistic in piglets that have eaten little or after an overnight fast (44, 45). Proteolytic activity, which can potentially degrade the pectinolytic enzymes, was added to mimic the measured values, as the values in the literature are not directly applicable due to differences in the methods and the definition of units of activity. The release of water-soluble fiber from the potato pulp required very little enzyme, with about 30 μg of each enzyme (E/S ratio, 0.03%) releasing ∼21% of the dry matter from 300 mg potato pulp. With amounts of each enzyme above 90 μg, there was no further release. The two monocomponent enzymes were not purified beyond ultrafiltration before use, so the use of even lower doses may be feasible, as the enzyme dose was determined from the total amount of protein after Pichia fermentation. Previous reaction schemes have involved an E/S ratio of 1%, which would correspond to 3 mg of each enzyme, an approximately 33 times higher enzyme dosage (26, 27), making in situ production an attractive possibility.
The monosaccharide composition of the released fraction suggests that this was indeed galacto-rhamnogalacturonan 1, corresponding to a backbone of rhamnose and galacturonic acid that was highly substituted with galactose chains as well as smaller amounts of arabinose, likely being flanked by domains of homogalacturonan (28, 46). UFSF was different from IVSF, in that the galactose content was higher in UFSF and the galacturonic acid content was lower. According to HPSEC analysis, both fibers encompassed two separate populations of the solubilized pectin, namely, high- and low-molecular-weight fractions. UFSF, which had a higher molecular weight than IVSF as well as a larger amount of galactose, can then be hypothesized to be richer in galactan-rich RG-1 fractions and to have smaller amounts of shorter polygalacturonan chains, which would have been filtered out in the ultrafiltration step, as in the UFSF product. The content of monosaccharide was also higher in IVSF than UFSF. The existence of a dual population in enzymatically solubilized potato pectin has previously been shown (26). An additional difference was the presence of mannose in the purified fiber, which presumably came from the enzyme preparation, e.g., as cell wall material from Pichia pastoris. Mannose has been detected (3 to 5%) in similarly produced fiber (26, 47) and appears to be >10 kDa in size.
The pH of the incubations followed a dose-dependent pattern, dropping up to 2.5 pH points when incubation was with 10 mg/ml of IVSF for 24 h. Few in vitro fermentation studies have reported using porcine ileal microbiota, but the pH was reported to drop from 6.63 to ∼6.2 and ∼5.7, respectively, by fermenting 5 or 10 g/liter of predigested feed containing various oligosaccharides in pig feces (22). Another study used various human milk oligosaccharides (HMOs), inulin, and galacto-oligosaccharides at 1 mg/ml in sow-reared or formula-fed piglet colonic contents and reported pH drops from 6.5 to 5.3 after 12 h (23). Incubations of 10 g/liter inulin or transgalacto-oligosaccharides (TOSs) in the pig GI tract contents for 4 h showed a drop from pH 6.5 to 6.3 in distal small intestine content but a drop to pH 5.8 for colonic content (48). pH values reported from in vivo studies in the terminal ileum have been reported to range from 7.8 to 8.3 (47) and from 5.7 to 6.0 (49), suggesting that the low pH values observed in the in vitro fermentation studies may be lower than those that are physiologically relevant. The colonic pH has been reported to be 6.8, regardless of diet (50), suggesting that the clearance of fatty acids, which does not occur in vitro but is substantial in the colon (51), has a stabilizing effect on pH.
The fibers in this study showed excellent fermentability, evident by the increase in organic acid content (Table 2). The exact role of the individual fatty acids in piglet health is not entirely clear, but there is general consensus that butyric acid is both a fuel and a trophic factor for colonocytes, whereas propionate and acetate enter the circulation and participate in hepatic energy production (52–54). Interestingly, the control sample, with which 24 h of incubation without fiber was used, showed increases in acetate and propionate, probably reflecting the fermentation of residual material from digesta or the glycerol added at freezing. Of further interest are the relatively high levels of lactate and the apparent dose-response relationship observed in the IVSF series and the UFSF-5 group, suggesting that lactate is a fermentation product of lactic acid bacteria. The high levels of lactic acid bacteria in these treatment groups support this notion (Fig. 3) as well as the correlations of CAP1 and the lactate concentration, as seen in Fig. 5. When fermenting inulin (55) or xylooligosaccharides (21), lactate is present after 11.5 h, and its level is markedly lowered after 30 h and is undetectable after 72 h, presumably since lactate is metabolized into other organic acids, such as propionate, through the acrylate pathway (54).
The microbiota was almost exclusively composed of the phylum Firmicutes, which is in contrast to the findings of other published pig metagenomic studies: in a study by Lamendella et al. (56), organisms of the Firmicutes and the Bacteroidetes phyla constituted 40 to 60% and 25 to 40% of the microbiota, respectively, depending on the sequencing platform and the classification database. These results were, however, for fecal samples from adult pigs. A more recent study using piglets weaned at 28 days and fed for 31 days showed 75% Firmicutes, 15% Bacteroidetes, and small amounts of organisms of the phylum Proteobacteria in fecal samples. In 2012, Looft et al., in an antibiotic feeding study with piglets weaned at 21 days and fed for 21 days, had results that contrasted with those of Lamendella et al. (56): 20% Firmicutes, 50% Bacteroidetes, and ∼10% Proteobacteria (57). Another study from Looft and colleagues reported ∼20 to 30% Firmicutes and 50 to 60% Bacteroidetes in animals weaned at 12 days and tested at 8 weeks (58). One explanation for the dominance of the Firmicutes in our study is the use of terminal small ileum samples rather than fecal samples, and that explanation is supported by a recent study examining the composition of the microbiota in several segments of the porcine GI tract: here, the ileal microbiota was ∼95% Firmicutes, whereas the cecal, colonic, and fecal flora was ∼50% Bacteroidetes (30).
The composition of the microbiota in the baseline group, e.g., the microbial composition in the native piglet, was dominated by the genera Streptococcus (42% ± 6%), Clostridium (27% ± 7%), Turicibacter (15% ± 2%), and Lactobacillus (5% ± 0.5%). The populations of Turicibacter, a genus previously found in the ileum of piglets (59) but isolated only from a human patient with acute appendicitis (60), were close to entirely attenuated in all 24-h fermentations, suggesting that this genus requires host interaction or has specific growth requirements. Merely incubating the bacteria for 24 h resulted in a shift toward a microbiota consisting of equal amounts of Clostridium and Streptococcus. All the fiber treatments, apart from UFSF-5, resulted in a higher bacterial diversity, as evidenced by the Shannon index. The UFSF-5 group was almost exclusively composed of species of Lactobacillus, Veillonella, Megasphaera, and Streptococcus.
Addition of UFSF resulted in a significant increase in the numbers of organisms of the genus Lactobacillus, believed to be beneficial (10), along with a significant decrease in the numbers of organisms of the genus Streptococcus and a nonsignificant increase in the numbers of organisms of the genus Clostridium. Furthermore, the amount of Clostridium bacteria was substantially and significantly depressed with both the INU-5 and UFSF-5 treatments. A point of main interest is the difference between the microbial community composition in the IVSF-5 treatment group and that in the UFSF-5 treatment group, as these treatments were supposedly chemically identical but resulted in markedly different microbiota profiles. The UFSF-5 group did, however, have 25% more galactan and half the galacturonic acid content, findings which suggest that the galactan chains of the molecule have a more pronounced effect on the genus Lactobacillus, whereas the galacturonic acid chains may be favored by bacteria belonging to the genus Clostridium. It has previously been shown that in human feces, pectin fractions with neutral sugars were efficient in increasing the amount of Bifidobacterium organisms, whereas the amount of Clostridium bacteria was increased by polygalacturonic acid, as enumerated by fluorescent in situ hybridization (FISH) (61). In contrast, another study using a pectin fraction with high levels of galacturonic acid also showed by means of FISH little growth of clostridia and increased levels of Bifidobacterium (62).
The Clostridium spp. observed in these fermentations appear to be a normal part of the porcine healthy gut microbiota, as they were present in high numbers in the baseline group and grew to high numbers in the control group, an observation consistent with the findings of other studies in healthy animals (56, 59, 63, 64). In the absence of genuine illness, the actual implications of Clostridium populations are difficult to discern, and it is also uncertain whether or not the eradication of Clostridium, as seen in the UFSF treatment group, is a positive effect. Clostridium cluster XIVa bacteria, previously found in the colon of piglets (23), were not found to any relevant extent in the samples in the present study, which could be because this study used the contents of the terminal ileum rather than the colon. Moreover, this cluster or organisms appears to preferably colonize the mucus layer (65), whereas this study used the luminal content. Clostridium perfringens appears to be a problem in the neonatal animal (66) but less of a problem in the weaning piglet, where E. coli is the main pathogenic agent (6, 7). In this study, the amount of Enterobacteriaceae in the groups treated with IVSF series was not different from that in the control group, although the amount in the INU-5 and UFSF-5 groups was decreased from that in the control group. The observation that the number of organisms of the genus Escherichia was higher in the IVSF-5 group than the UFSF-5 group can possibly be explained by the apparent 3-fold higher levels of monosaccharide in the IVSF-5 group. Escherichia is well adapted to the catabolism of monosaccharides, including galacturonic acid (67), and may have had an advantage in the early stages of the fermentation owing to the quick rate of replication of this genus.
Streptococcus spp. are also a normal component of the porcine gut microbiota, and in this study, their amount appeared to be diminished by both the fiber made in vitro and the fiber from UFSF-5. Since the abundance of Lactobacillus spp. was increased in the same groups, it is difficult to establish whether it is a direct effect of the fiber or occurs through the Lactobacillus organisms present. The consequence of this alteration is unknown.
Bifidobacterium, which is generally considered to be a health-promoting organism, is ubiquitous in the human gut microbiota, but it was not detected in notable numbers either in the baseline samples or after fermentation, and it is reported to comprise a negligible portion of the pig intestinal microbiota (23, 68, 69). The importance of Bifidobacterium in the pig remains to be illuminated.
The genus Veillonella grew well in all treatments but not at the baseline, likely owing to the fact that this genus metabolizes organic acids rather than carbohydrate (70). The amount of bacteria belonging to the genus Megasphaera, including the species Megasphaera elsdenii, suggested to protect the host against the pathogen Brachyspira hyodysenteriae (19), was also elevated by the fiber treatment, in agreement with the fact that this genus feeds on organic acids (71).
With the use of PCoA, the entire microbiota could be visualized as well as clustered at the species level. It was seen that there was marked clustering of microbiota within each treatment group but that the microbiotas from the IVSF-5 and IVSF-10 groups were closer to one another than to the microbiota from any other group. In particular, the microbiota of the UFSF-5 group clustered far from the microbiota of the IVSF-5 group, but the compositions of these two groups should be chemically similar. This could be explained by small but possibly key differences in the monosaccharide content (3.8% in UFSF versus 10.6% in IVSF). The scores that were obtained, which provide an aggregate measure of the bacterial composition, could, furthermore, be correlated with the levels of organic acids and pH, showing patterns in the production of fatty acids and microbiota, namely, that a microbiota low in acetic acid but high in butyric acid, valeric acid, and lactic acid is associated with the genera Lactobacillus and Megasphaera and but not with the genus Clostridium.
For use in animal trials, it would appear that a treatment corresponding to IVSF-5 should be used, since the treatment with a higher dose of fiber, IVSF-10, tended to produce levels of Clostridium higher than those produced by the control treatment. UFSF appeared to decrease bacterial diversity, and it is unclear if having 60% Lactobacillus has a positive impact on the host.
In conclusion, it has been established that targeted enzymatic catalysis can be used to extract the RG-1 domain of low-value potato pulp in a simulated in vitro digestion. Furthermore, the fiber that was produced was highly fermentable and capable of changing the microbial community when fermented in the contents of the terminal ileum of piglets, notably, by increasing the Lactobacillus counts. Whether the interanimal variation is too large to verify a consistent response in vivo remains to be determined. For use in animal studies, use of a fiber dosage corresponding to that in IVSF-5 may be a valid option.
ACKNOWLEDGMENTS
This work was supported by the National Veterinary Institute and the Department of Chemical Engineering, Technical University of Denmark.
We thank Sophia Rasmussen for excellent technical assistance and senior scientist Tim K. Jensen for assistance with the procurement of animal samples. Moreover, we are grateful for the fermentation medium supplied by Louise Vigsnæs.
REFERENCES
- 1.Lyutskanov M. 2011. Epidemiological characteristics of post-weaning diarrhoea associated with toxin-producing Escherichia coli in large intensive pig farms. Trakia J Sci 9:68–73. [Google Scholar]
- 2.Ho WS, Tan LK, Ooi PT, Yeo CC, Thong KL. 2013. Prevalence and characterization of verotoxigenic-Escherichia coli isolates from pigs in Malaysia. BMC Vet Res 9:109. doi: 10.1186/1746-6148-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Beers-Schreurs HM, Vellenga L, Wensing T, Breukink HJ. 1992. The pathogenesis of the post-weaning syndrome in weaned piglets: a review. Vet Q 14:29–34. doi: 10.1080/01652176.1992.9694322. [DOI] [PubMed] [Google Scholar]
- 4.Vondruskova H, Slamova R, Trckova M, Zraly Z, Pavlik I. 2010. Alternatives to antibiotic growth promoters in prevention of diarrhoea in weaned piglets: a review. Vet Med 55:199–224. [Google Scholar]
- 5.De Lange CFM, Pluske J, Gong J, Nyachoti CM. 2010. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livestock Sci 134:124–134. doi: 10.1016/j.livsci.2010.06.117. [DOI] [Google Scholar]
- 6.Kim JC, Hansen CF, Mullan BP, Pluske JR. 2012. Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol 173:3–16. doi: 10.1016/j.anifeedsci.2011.12.022. [DOI] [Google Scholar]
- 7.Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM. 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr (Berl) 97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 8.Cromwell GL. 2002. Why and how antibiotics are used in swine production. Anim Biotechnol 13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- 9.Dibner JJ, Richards JD. 2005. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci 84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- 10.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco M-J, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. 2010. Prebiotic effects: metabolic and health benefits. Br J Nutr 104(Suppl):S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 11.Servin AL. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Liévin-Le Moal V, Servin AL. 2014. Anti-infective activities of Lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin Microbiol Rev 27:167–199. doi: 10.1128/CMR.00080-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkelsen LL, Jensen BB. 2004. Effect of fructo-oligosaccharides and transgalacto-oligosaccharides on microbial populations and microbial activity in the gastrointestinal tract of piglets post-weaning. Anim Feed Sci Technol 117:107–119. doi: 10.1016/j.anifeedsci.2004.07.015. [DOI] [Google Scholar]
- 14.Mikkelsen LL, Jakobsen M, Jensen BB. 2003. Effects of dietary oligosaccharides on microbial diversity and fructo-oligosaccharide degrading bacteria in faeces of piglets post-weaning. Anim Feed Sci Technol 109:133–150. doi: 10.1016/S0377-8401(03)00172-X. [DOI] [Google Scholar]
- 15.Mountzouris KC, Balaskas C, Fava F, Tuohy KM, Gibson GR, Fegeros K. 2006. Profiling of composition and metabolic activities of the colonic microflora of growing pigs fed diets supplemented with prebiotic oligosaccharides. Anaerobe 12:178–185. doi: 10.1016/j.anaerobe.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Shim SB, Williams IH, Verstegen MWA. 2005. Effects of dietary fructo-oligosaccharide on villous height and disaccharidase activity of the small intestine, pH, VFA and ammonia concentrations in the large intestine of weaned pigs. Acta Agric Scand Sect A 55:91–97. doi: 10.1080/09064700500307201. [DOI] [Google Scholar]
- 17.Houdijk JGM, Hartemink R, Verstegen MWA, Bosch MW. 2002. Effects of dietary non-digestible oligosaccharides on microbial characteristics of ileal chyme and faeces in weaner pigs. Arch Anim Nutr 56:297–307. doi: 10.1080/00039420214346. [DOI] [PubMed] [Google Scholar]
- 18.Jensen AN, Mejer H, Mølbak L, Langkjær M, Jensen TK, Angen Ø, Martinussen T, Klitgaard K, Baggesen DL, Thamsborg SM, Roepstorff A. 2011. The effect of a diet with fructan-rich chicory roots on intestinal helminths and microbiota with special focus on Bifidobacteria and Campylobacter in piglets around weaning. Animal 5:851–860. doi: 10.1017/S175173111000251X. [DOI] [PubMed] [Google Scholar]
- 19.Mølbak L, Thomsen LE, Jensen TK, Bach Knudsen KE, Boye M. 2007. Increased amount of Bifidobacterium thermacidophilum and Megasphaera elsdenii in the colonic microbiota of pigs fed a swine dysentery preventive diet containing chicory roots and sweet lupine. J Appl Microbiol 103:1853–1867. doi: 10.1111/j.1365-2672.2007.03430.x. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen LE, Knudsen KEB, Jensen TK, Christensen AS, Møller K, Roepstorff A. 2007. The effect of fermentable carbohydrates on experimental swine dysentery and whip worm infections in pigs. Vet Microbiol 119:152–163. doi: 10.1016/j.vetmic.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Moura P, Cabanas S, Lourenço P, Gírio F, Loureiro-Dias MC, Esteves MP. 2008. In vitro fermentation of selected xylo-oligosaccharides by piglet intestinal microbiota. LWT Food Sci Technol 41:1952–1961. doi: 10.1016/j.lwt.2007.11.007. [DOI] [Google Scholar]
- 22.Kim D-W, Chae S-J, Cho S-B, Hwang O-H, Lee H-J, Chung W-T, Park J-C, Kim I-C, Kim I-H. 2010. Effect of prebiotics on intestinal microflora and fermentation products in pig in vitro model. J Anim Sci Technol 52:199–204. doi: 10.5187/JAST.2010.52.3.199. [DOI] [Google Scholar]
- 23.Li M, Bauer LL, Chen X, Wang M, Kuhlenschmidt TB, Kuhlenschmidt MS, Fahey GC Jr, Donovan SM. 2012. Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J Nutr 142:681–689. doi: 10.3945/jn.111.154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Zhu Y, Li D. 2004. In vitro fermentation of various fiber and starch sources by pig fecal inocula. J Anim Sci 82:2615–2622. [DOI] [PubMed] [Google Scholar]
- 25.Meyer AS, Dam BP, Lærke HN. 2009. Enzymatic solubilization of a pectinaceous dietary fiber fraction from potato pulp: optimization of the fiber extraction process. Biochem Eng J 43:106–112. doi: 10.1016/j.bej.2008.09.006. [DOI] [Google Scholar]
- 26.Thomassen LV, Vigsnæs LK, Licht TR, Mikkelsen JD, Meyer AS. 2011. Maximal release of highly bifidogenic soluble dietary fibers from industrial potato pulp by minimal enzymatic treatment. Appl Microbiol Biotechnol 90:873–884. doi: 10.1007/s00253-011-3092-y. [DOI] [PubMed] [Google Scholar]
- 27.Thomassen LV, Larsen DM, Mikkelsen JD, Meyer AS. 2011. Definition and characterization of enzymes for maximal biocatalytic solubilization of prebiotic polysaccharides from potato pulp. Enzyme Microb Technol 49:289–297. doi: 10.1016/j.enzmictec.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Mohnen D. 2008. Pectin structure and biosynthesis. Curr Opin Plant Biol 11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Harholt J, Suttangkakul A, Vibe Scheller H, Scheller HV. 2010. Biosynthesis of pectin. Plant Physiol 153:384–395. doi: 10.1104/pp.110.156588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Looft T, Allen HK, Cantarel BL, Levine UY, Bayles DO, Alt DP, Henrissat B, Stanton TB. 2014. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J 8:1566–1576. doi: 10.1038/ismej.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva IR, Larsen DM, Meyer AS, Mikkelsen JD. 2011. Identification, expression, and characterization of a novel bacterial RGI lyase enzyme for the production of bio-functional fibers. Enzyme Microb Technol 49:160–166. doi: 10.1016/j.enzmictec.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Bauer S, Vasu P, Persson S, Mort AJ, Somerville CR. 2006. Development and application of a suite of polysaccharide-degrading enzymes for analyzing plant cell walls. Proc Natl Acad Sci U S A 103:11417–11422. doi: 10.1073/pnas.0604632103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. 2012. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol 107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 34.Rose DJ, Venema K, Keshavarzian A, Hamaker BR. 2010. Starch-entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacteria during in vitro fermentation in faecal microbiota obtained from patients with inflammatory bowel disease. Br J Nutr 103:1514–1524. doi: 10.1017/S0007114509993515. [DOI] [PubMed] [Google Scholar]
- 35.Vigsnæs LK, Holck J, Meyer AS, Licht TR. 2011. In vitro fermentation of sugar beet arabino-oligosaccharides by fecal microbiota obtained from patients with ulcerative colitis to selectively stimulate the growth of Bifidobacterium spp. and Lactobacillus spp. Appl Environ Microbiol 77:8336–8344. doi: 10.1128/AEM.05895-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holck J, Lorentzen A, Vigsnæs LK, Licht TR, Mikkelsen JD, Meyer AS. 2011. Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of Bifidobacterium spp. in human fecal in vitro fermentations. J Agric Food Chem 59:6511–6519. doi: 10.1021/jf200996h. [DOI] [PubMed] [Google Scholar]
- 37.Ravn HC, Meyer AS. 2014. Chelating agents improve enzymatic solubilization of pectinaceous co-processing streams. Process Biochem 49:250–257. doi: 10.1016/j.procbio.2013.11.010. [DOI] [Google Scholar]
- 38.Sakata T, Kojima T, Fujieda M, Takahashi M, Michibata T. 2003. Influences of probiotic bacteria on organic acid production by pig caecal bacteria in vitro. Proc Nutr Soc 62:73–80. doi: 10.1079/PNS2002211. [DOI] [PubMed] [Google Scholar]
- 39.McBurney MI, Thompson LU. 1989. Effect of human faecal donor on in vitro fermentation variables. Scand J Gastroenterol 24:359–367. doi: 10.3109/00365528909093060. [DOI] [PubMed] [Google Scholar]
- 40.Ingerslev H-C, von Gersdorff Jørgensen L, Lenz Strube M, Larsen N, Dalsgaard I, Boye M, Madsen L. 2014. The development of the gut microbiota in rainbow trout (Oncorhynchus mykiss) is affected by first feeding and diet type. Aquaculture 424-425:24–34. doi: 10.1016/j.aquaculture.2013.12.032. [DOI] [Google Scholar]
- 41.Claesson MJ, Wang Q, O'Sullivan O, Greene-Diniz R, Cole JR, Ross RP, O'Toole PW. 2010. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res 38:e200. doi: 10.1093/nar/gkq873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Šidák Z. 1967. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc 62:626–633. doi: 10.1080/01621459.1967.10482935. [DOI] [Google Scholar]
- 43.Strube ML, Meyer AS, Boye M. 2013. Mini review: basic physiology and factors influencing exogenous enzymes activity in the porcine gastrointestinal tract. Anim Nutr Feed Technol 13:441–459. [Google Scholar]
- 44.Potkins Z, Lawrence T, Thomlinson J. 1991. Effects of structural and non-structural polysaccharides in the diet of the growing pig on gastric emptying rate and rate of passage of digesta to the terminal ileum and through the total gastrointestinal tract. Br J Nutr 65:391–413. doi: 10.1079/BJN19910100. [DOI] [PubMed] [Google Scholar]
- 45.Ange KD, Eisemann JH, Argenzio RA, Almond GW, Blikslager AT. 2000. Effects of feed physical form and buffering solutes on water disappearance and proximal stomach pH in swine. J Anim Sci 28:2344–2352. [DOI] [PubMed] [Google Scholar]
- 46.Ridley B, O'Neill M, Mohnen D. 2001. Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967. doi: 10.1016/S0031-9422(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 47.Michalak M, Thomassen LV, Roytio H, Ouwehand AC, Meyer AS, Mikkelsen JD. 2012. Expression and characterization of an endo-1,4-β-galactanase from Emericella nidulans in Pichia pastoris for enzymatic design of potentially prebiotic oligosaccharides from potato galactans. Enzyme Microb Technol 50:121–129. doi: 10.1016/j.enzmictec.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Mikkelsen LL, Erik K, Knudsen B, Jensen BB. 2004. In vitro fermentation of fructo-oligosaccharides and transgalacto-oligosaccharides by adapted and unadapted bacterial populations from the gastrointestinal tract of piglets. Anim Feed Sci Technol 116:225–238. doi: 10.1016/j.anifeedsci.2004.07.007. [DOI] [Google Scholar]
- 49.Partanen K, Jalava T, Valaja J. 2007. Effects of a dietary organic acid mixture and of dietary fibre levels on ileal and faecal nutrient apparent digestibility, bacterial nitrogen flow, microbial metabolite concentrations and rate of passage in the digestive tract of pigs. Animal 1:389–401. doi: 10.1017/S1751731107657838. [DOI] [PubMed] [Google Scholar]
- 50.Loh G, Eberhard M, Brunner R. 2006. Inulin alters the intestinal microbiota and short-chain fatty acid concentrations in growing pigs regardless of their basal diet. J Nutr 136:1198–1202. [DOI] [PubMed] [Google Scholar]
- 51.Puertollano E, Kolida S, Yaqoob P. 2014. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr Opin Clin Nutr Metab Care 17:139–144. doi: 10.1097/MCO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 52.Hijova E, Chmelarova A. 2007. Short chain fatty acids and colonic health. Bratisl Lek Listy 108:354–358. [PubMed] [Google Scholar]
- 53.Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJ. 2006. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 54.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moura P, Simões F, Gírio F, Loureiro-Dias MC, Esteves MP. 2007. PCR monitoring of Lactobacillus and Bifidobacterium dynamics in fermentations by piglet intestinal microbiota. J Basic Microbiol 47:148–157. doi: 10.1002/jobm.200610210. [DOI] [PubMed] [Google Scholar]
- 56.Lamendella R, Domingo JWS, Ghosh S, Martinson J, Oerther DB. 2011. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol 11:103. doi: 10.1186/1471-2180-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Looft T, Johnson TA, Allen HK, Bayles DO, Alt DP, Stedtfeld RD, Sul WJ, Stedtfeld TM, Chai B, Cole JR, Hashsham SA, Tiedje JM, Stanton TB. 2012. In-feed antibiotic effects on the swine intestinal microbiome. Proc Natl Acad Sci U S A 109:1691–1696. doi: 10.1073/pnas.1120238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bearson SMD, Allen HK, Bearson BL, Looft T, Brunelle BW, Kich JD, Tuggle CK, Bayles DO, Alt D, Levine UY, Stanton TB. 2013. Profiling the gastrointestinal microbiota in response to Salmonella: low versus high Salmonella shedding in the natural porcine host. Infect Genet Evol 16:330–340. doi: 10.1016/j.meegid.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Rettedal E, Vilain S, Lindblom S, Lehnert K, Scofield C, George S, Clay S, Kaushik RS, Rosa AJM, Francis D, Brözel VS. 2009. Alteration of the ileal microbiota of weanling piglets by the growth-promoting antibiotic chlortetracycline. Appl Environ Microbiol 75:5489–5495. doi: 10.1128/AEM.02220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bosshard P, Zbinden R, Altwegg M. 2002. Turicibacter sanguinis gen. nov., sp. nov., a novel anaerobic, Gram-positive bacterium. Int J Syst Evol Microbiol 52:1263–1266. doi: 10.1099/ijs.0.02056-0. [DOI] [PubMed] [Google Scholar]
- 61.Onumpai C, Kolida S, Bonnin E, Rastall RA. 2011. Microbial utilization and selectivity of pectin fractions with various structures. Appl Environ Microbiol 77:5747–5754. doi: 10.1128/AEM.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandalari G, Nueno Palop C, Tuohy K, Gibson GR, Bennett RN, Waldron KW, Bisignano G, Narbad A, Faulds CB. 2007. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl Microbiol Biotechnol 73:1173–1179. doi: 10.1007/s00253-006-0561-9. [DOI] [PubMed] [Google Scholar]
- 63.Pedersen R, Ingerslev H-C, Sturek M, Alloosh M, Cirera S, Christoffersen BØ, Moesgaard SG, Larsen N, Boye M. 2013. Characterisation of gut microbiota in Ossabaw and Göttingen minipigs as models of obesity and metabolic syndrome. PLoS One 8:e56612. doi: 10.1371/journal.pone.0056612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buzoianu SG, Walsh MC, Rea MC, Quigley L, O'Sullivan O, Cotter PD, Ross RP, Gardiner GE, Lawlor PG. 2013. Sequence-based analysis of the intestinal microbiota of sows and their offspring fed genetically modified maize expressing a truncated form of Bacillus thuringiensis Cry1Ab protein (Bt maize). Appl Environ Microbiol 79:7735–7744. doi: 10.1128/AEM.02937-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van den Abbeele P, Belzer C, Goossens M, Kleerebezem M, De Vos WM, Thas O, De Weirdt R, Kerckhof F-M, Van de Wiele T. 2013. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J 7:949–961. doi: 10.1038/ismej.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Songer J. 1996. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev 9:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richard P, Hilditch S. 2009. d-Galacturonic acid catabolism in microorganisms and its biotechnological relevance. Appl Microbiol Biotechnol 82:597–604. doi: 10.1007/s00253-009-1870-6. [DOI] [PubMed] [Google Scholar]
- 68.Mikkelsen LL, Bendixen C, Jensen BB, Jakobsen M. 2003. Enumeration of bifidobacteria in gastrointestinal samples from piglets. Appl Environ Microbiol 69:654–658. doi: 10.1128/AEM.69.1.654-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buzoianu SG, Walsh MC, Rea MC, O'Sullivan O, Cotter PD, Ross RP, Gardiner GE, Lawlor PG. 2012. High-throughput sequence-based analysis of the intestinal microbiota of weanling pigs fed genetically modified MON810 maize expressing Bacillus thuringiensis Cry1Ab (Bt maize) for 31 days. Appl Environ Microbiol 78:4217–4224. doi: 10.1128/AEM.00307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ng SK, Hamilton IR. 1971. Lactate metabolism by Veillonella parvula. J Bacteriol 105:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marounek M, Bartos S. 1987. Interactions between rumen amylolytic and lactate-utilizing bacteria in growth on starch. J Appl Bacteriol 63:233–238. doi: 10.1111/j.1365-2672.1987.tb04941.x. [DOI] [PubMed] [Google Scholar]