Abstract
Virus inactivation by chemical disinfectants is an important instrument for infection control in medical settings, but the mechanisms involved are poorly understood. In this study, we systematically investigated the effects of several antiviral treatments on hepatitis C virus (HCV) particles as model for enveloped viruses. Studies were performed with authentic cell culture-derived viruses, and the influence of chemical disinfectants, heat, and UV treatment on HCV was analyzed by the determination of infectious particles in a limiting-dilution assay, by quantitative reverse transcription-PCR, by core enzyme-linked immunosorbent assay, and by proteolytic protection assay. All different inactivation methods resulted in a loss of HCV infectivity by targeting different parts of the virus particle. Alcohols such as ethanol and 2-propanol did not affect the viral RNA genome integrity but disrupted the viral envelope membrane in a capsid protection assay. Heat and UV treatment of HCV particles resulted in direct damage of the viral genome since transfection of viral particle-associated RNA into permissive cells did not initiate RNA replication. In addition, heat incubation at 80°C disrupted the HCV envelope, rendering the viral capsid susceptible to proteolytic digest. This study demonstrated the molecular processes of viral inactivation of an enveloped virus and should facilitate the development of effective disinfection strategies in infection control not only against HCV but also against other enveloped viruses.
INTRODUCTION
Virus inactivation procedures apply numerous treatment methods, for instance, chemical inactivation, heat, or UV irradiation. Although these methods have been widely used for a long time in industrial processes and public health systems, the understanding of the viral inactivation mechanisms remains relatively low. All viruses with the exception of iridoviruses can be assigned to either enveloped or nonenveloped viruses and are composed of a protein structure protecting the viral nucleic acid genome. Therefore, inactivation methods target either the lipid envelope membrane, the viral capsid, and/or the viral genome. Hepatitis C virus (HCV) is an enveloped, positive-strand RNA virus belonging to the family of Flaviviridae. Its 9.6-kb genome is composed of the 5′-untranslated region (5′ UTR), an open reading frame encoding a large polyprotein, and the 3′ UTR (1). The polyprotein is cleaved into 10 individual proteins with the structural proteins building up the virus particle (Core, E1, and E2) and the nonstructural proteins required for RNA replication. HCV infection is considered a global health problem, with an estimated 170 million people infected worldwide (2). Once a chronic infection is established, there is a high risk for developing severe liver damage, including hepatic steatosis, fibrosis, cirrhosis, and hepatocellular carcinoma (3). In the last couple of years, treatment options have been improved, especially since the approval of direct-acting antivirals that could be used without interferon on an all oral combination therapy (4). However, there is still no protective vaccine available, rendering healthcare workers at a constant risk of acquiring HCV from occupational exposure. In addition, nosocomial transmission of HCV still accounts for a large proportion of new HCV infections each year (5–9). Together with needle stick injuries or injections with contaminated syringes, especially among intravenous drug users, which constitutes the main route of infection in developed countries (10), as well as other transmission routes involving vertical and sexual transmission (11–13), approximately three to four million people are newly infected each year (14).
Different studies have recently evaluated the environmental stability of HCV and its susceptibility to chemical biocides in quantitative suspension assays (15–19) or on dried surfaces (20, 21). However, virus inactivation mechanisms of these and other procedures and the question of which parts of the virus particles are specifically disrupted have not been addressed. Therefore, with the help of a productive HCV cell culture system, we analyzed the effect of several inactivation methods on the HCV particle and show that different disinfectant procedures target different parts of the virus. A detailed understanding of the molecular processes involved in viral inactivation will assist the development of effective disinfection strategies against HCV.
MATERIALS AND METHODS
Cell culture and reagents.
For HCV infection experiments, a human hepatoma cell line, Huh7.5, was used that is permissive for HCV infection and replication (22). The cells were grown in Dulbecco modified Eagle medium (DMEM; Invitrogen, Karlsruhe, Germany) supplemented with 2 mM l-glutamine, nonessential amino acids, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal calf serum (DMEM complete).
Plasmids, in vitro transcription, electroporation, and production of cell culture-derived HCV.
The plasmid pFK-Jc1 has been described recently and encodes the intragenotypic 2a/2a chimeric virus Jc1 (23). Infectious HCV particles were produced as described elsewhere (24). Briefly, Jc1 plasmid DNA was linearized and transcribed into RNA, followed by the electroporation into Huh7.5 cells. Virus-containing culture fluids were harvested after 48, 72, and 96 h and concentrated using centricons (Centricon Plus-70; Millipore, USA). For the determination of viral infectivity, cell-free supernatants were used to infect naive Huh7.5 target cells.
Disinfectants and inactivation methods.
For viral disinfection, the following disinfectants were used: 5% Triton X-100 (Carl Roth GmbH, Karlsruhe, Germany), 100% ethanol (Carl Roth), 2-propanol (Carl Roth), povidone-iodide (PVP-I) (Betaisodona; Mundipharma GmbH, Limburg an der Lahn, Germany). For heat inactivation, Jc1 virus stock was first diluted 1:10 with DMEM and then heated at 80°C for 5 min. For UV inactivation, Jc1 virus stock was diluted 1:10 with DMEM and subsequently irradiated in a six-well cell culture dish at an intensity of 0.6 J/cm2 using a UV-cross-linker CX-2000 (UVP).
Virucidal activity experiments, virus titration, and controls.
To determine the effect of inactivation procedures on viral infectivity, virucidal suspension experiments were performed. Virus was incubated with chemical disinfectants at a ratio of 1:10 for 1 min at room temperature or were treated as described above. As a control, virus was incubated with DMEM. After the incubation period, target cells were infected in a limiting dilution assay on Huh7.5 cells. The 50% tissue culture infectious dose (TCID50) was determined at 72 h postinfection as described previously (25). As an interference control, the cell culture medium of Huh7.5 cell was therefore replaced by a nontoxic dilution of the test substance, followed by incubation for 1 h at 37°C. As a corresponding negative control, cell cultures were exposed to phosphate-buffered saline (PBS) in the same manner as to the disinfectant in the nontoxic concentration and were incubated for 1 h under the same conditions. After the incubation, the disinfectant dilution or the PBS was removed from the cell cultures. Afterward, the titers of a test virus suspension were determined on these cell cultures (26). To determine the cytotoxicity of the disinfectants, one part of the PBS was mixed with nine parts of the disinfectant, followed by inoculation onto permissive cells. The cytotoxicity was determined by examining target cells by microscopy for any significant changes of the cell monolayer. The cytotoxicity was calculated in analogy to the determination of virus titer (TCID50/ml).
Quantitative detection of HCV RNA and core protein.
To measure HCV-specific RNA, the viral RNA was isolated using a High-Pure viral RNA kit (Roche, Mannheim, Germany) according to the manufacturer's recommendations. For the reverse transcription-PCR (RT-PCR), a LightCycler 480 RNA master hydrolysis probe kit (Roche, Mannheim, Germany) was used with JFH1-specific probe A-195 (TIB Molbiol, Berlin, Germany) and the primers S-147 and A-221 (MWG-Biotech) as described previously (25). Measurement was conducted at the LightCycler 480 (Roche). To quantify the HCV core protein, the samples were inactivated with 1% (vol/vol) Triton X-100 in PBS, and core protein levels were measured using a core-specific enzyme-linked immunosorbent assay (ELISA) (27).
Proteolytic digestion and proteolytic protection assay.
Samples were treated with 50 μg of proteinase K (PK; Roche)/ml for 1 h on ice. To determine the amount of protease-resistant core protein after disinfectant treatment, 50 μl of the disinfectant-virus mixture was left untreated, 50 μl was treated with 50 μg of PK/ml for 1 h on ice, and another 50 μl was lysed with 2% (vol/vol) Triton X-100 prior to PK treatment. Protease digestion was stopped by the addition of 5 mM phenylmethylsulfonyl fluoride (AppliChem, Darmstadt, Germany), heating to 95°C for 10 min, and the addition of 50 μl of 2× protease inhibitor cocktail (one pill in 5 ml of TNE; Roche). The amount of core protein was determined using a core-specific ELISA.
Statistical analysis.
A statistical analysis of all figures was performed using a one-tailed Student t test. P values were calculated, and differences are reported as significant if the P value were <0.05 (*), <0.01 (**), or <0.001 (***). Differences were considered not significant at P values of >0.05.
RESULTS
Effect of viral inactivation procedures on HCV infectivity and RNA genome stability.
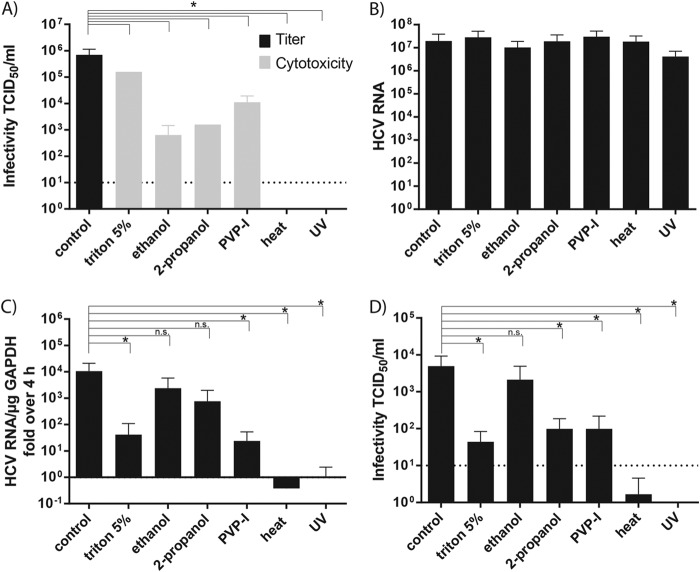
In order to systematically analyze the effect of different viral inactivation methods, we used chemical disinfectants (Triton X-100, ethanol, 2-propanol, and PVP-I) in a quantitative suspension assay as depicted in Fig. 1 (15). In these assays, nine parts of disinfectants were mixed with one part of the HCV Jc1 virus (23), and the mixture was incubated at room temperature for 1 min. In case of heat and UV inactivation, the virus was preincubated with nine parts of DMEM before the respective inactivation. After the chemical treatment or preincubation, the viral infectivity was determined in a limiting-dilution assay (Fig. 1A), and the viral particle-associated RNA was determined by quantitative RT-PCR (qRT-PCR) (Fig. 1B). To investigate RNA genome stability, the virus associated RNA was purified and subsequently retransfected into human liver cells highly permissive for HCV RNA replication. Successful RNA replication was measured by qRT-PCR (Fig. 1C) and by the release of infectious particles using inoculation of Huh7.5 cells with cell culture supernatants (Fig. 1D).
FIG 1.
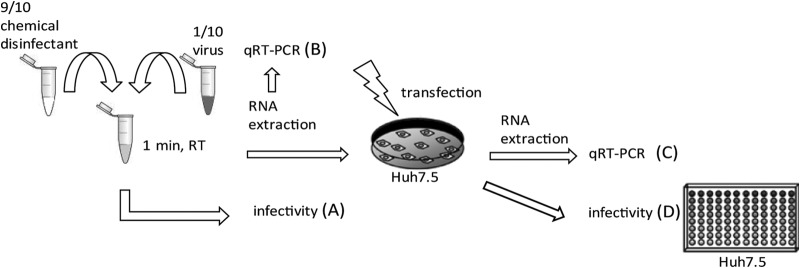
Experimental setup for studying the mode of action of HCV inactivation procedures. (A) Chemical disinfectants and virus were mixed at a ratio of 10 to 1, and the mixture was incubated at room temperature for 1 min before the infectivity was determined by TCID50 assay. In the case of heat treatment or UV irradiation, the virus were mixed at a ratio of 10 to 1 with DMEM and heated at 80°C for 5 min or UV irradiated before determination of the TCID50. (B) Virus particle-associated RNA was extracted and measured by qRT-PCR. Purified RNA was used to transfect naive Huh7.5 cells by electroporation. (C) After 72 h, Huh7.5 cells were lysed, and HCV RNA was analyzed by qRT-PCR. (D) The supernatant of the cells was harvested and used to infect naive Huh7.5 cells to determine virus titers.
All of the chemical disinfectants (Triton X-100, ethanol, and 2-propanol) significantly reduced viral infectious titers at least 2 to 3 orders of magnitude to the level of detectable cytotoxicity induced by the disinfectants (Fig. 2A). To verify that the susceptibility of the target cells for the virus infection was not influenced negatively by the treatment with the disinfectant, an interference control experiment was performed. We observed no difference in susceptibility of the target cells due to the disinfectant treatment (data not shown). For heat and UV treatment, no residual infectivity could be determined. Next, we purified the viral RNA from the differently treated samples and determined the amount of HCV RNA copies by qRT-PCR (Fig. 2B). No difference between the nontreated control and the inactivation methods was observed. To determine whether the loss of infectivity was due solely to the inability of the virus particle to penetrate into cells via the normal route of entry or whether the viral genome itself was no longer infectious, we transfected the virus particle associated RNA into highly permissive Huh7.5 cells. Successful initiation of viral RNA replication was assessed by qRT-PCR (Fig. 2C). Compared to the control-treated virus sample, no significant reduction was observed for the alcohol-treated specimen, a significant reduction for the Triton X-100- and PVP-I-treated samples, and no RNA replication was detected after treatment of the virus with UV radiation or heat. To further analyze whether infectious particles were released from the cells that still enable viral replication, the supernatants were harvested and used to inoculate naive Huh7.5 cells in a limiting-dilution assay. Productive infection of target cells similar to the control could be detected in the ethanol-treated sample, whereas treatment with Triton X-100, 2-propanol, and PVP-I resulted in a significant reduced virus production, and heat or UV treatment completely abrogated virus production (Fig. 2D). In summary, these results indicate that some inactivation procedures exert a strong influence on viral RNA stability and integrity, whereas others apparently inactivate HCV by targeting different parts of the virus particle.
FIG 2.
Influence of treatment procedures on HCV infectivity and RNA integrity. (A) Chemical disinfectants, heat, and UV treatment were tested in a quantitative suspension assay for their efficiency in inactivating HCV by determination of the TCID50. (B) HCV RNA of the respective supernatant was isolated and quantified by RT-PCR. (C) The isolated RNA was used for re-electroporation of Huh7.5 cells. After 72 h, RNA was extracted and quantified by qRT-PCR. (D) Limiting-dilution assay was used to determine the TCID50 of the viral supernatants. Depicted are the means plus the standard deviations of three independent experiments. The background level of the assay is shown as a dotted line. Statistical analysis was performed using a one-tailed Student t test.
Effect of viral inactivation procedures on viral capsid and envelopment.
Besides the viral RNA, the viral capsid and envelope constitute possible targets for particle disruption by inactivation treatments. We analyzed whether the viral capsid was impaired due to the antiviral procedures. To this end, we measured the amount of core protein via core-specific ELISA after preincubation of virus with the respective chemical or treatment. As seen in Fig. 3A, the different inactivation methods had no effect on the total amount of viral capsid protein itself (Fig. 3A). To dissect the effect on the viral envelope, we performed a proteolytic protection assay to determine the amount of protease-resistant, enveloped core protein after treatment. In the case of an intact envelope, externally added PK was not able to cleave the viral capsid because the protease has no access to the membrane-enveloped core protein. In contrast, treatment-induced disruptions of the viral envelope permits access of the protease to the viral capsid and thus results in a digestion of core protein, which can be quantified via core-specific ELISA (19). To control that the concentration of PK used was sufficient to cleave core protein, we added a high dose of the detergent Triton X-100 as a positive control, which resolved all membranes. Only the UV-treated virus still showed protection against PK to levels comparable to those of the control-treated virus, indicating that the viral envelope was still mainly intact (Fig. 3B). The two different alcohols, ethanol and propanol, as well as heat treatment, disrupted parts of the viral envelope, resulting in ca. 70, 40, and 30% PK protection, respectively. On the other hand, heat and Triton X-100 completely destroyed viral envelopment, whereas the PVP-I-treated samples were not detectable in this assay setup (Fig. 3B). Taken together, UV light inactivation had no influence on the virus particle membrane, while chemical disinfectants and heat treatment destroyed the viral envelope rendering HCV noninfectious.
FIG 3.
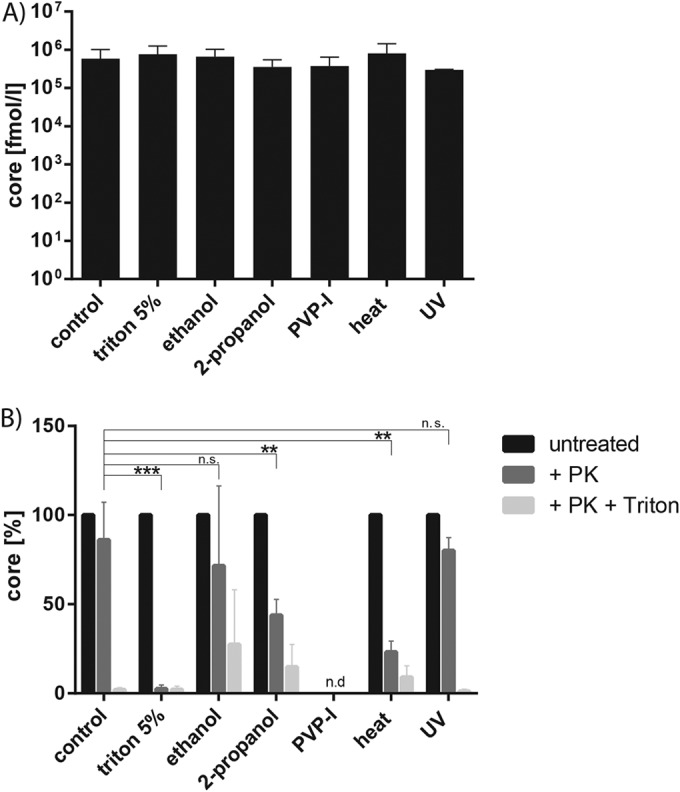
Influence of inactivation methods on viral capsid and envelope. (A) Chemical disinfectants and virus were mixed at a ratio of 10 to 1, and the mixture was incubated at room temperature for 1 min before the amount of HCV core protein was determined via core-specific ELISA. In the case of heat treatment or UV irradiation, the viruses were mixed at a ratio of 10 to 1 with DMEM and heated at 80°C for 5 min or UV irradiated before core-specific ELISA. (B) Proteolytic digestion protection assay to determine PK-resistant core protein. Therefore, one part was left untreated, one part was treated with 50 μg of PK/ml for 1 h at 4°C, and another part was lysed in 2% Triton X-100 prior to PK treatment. The amount of protease-resistant core protein was quantified with a core-specific ELISA. Depicted are the means plus the standard deviations for at least three independent experiments. n.d., not detected. Statistical analysis was performed using a one-tailed Student t test.
DISCUSSION
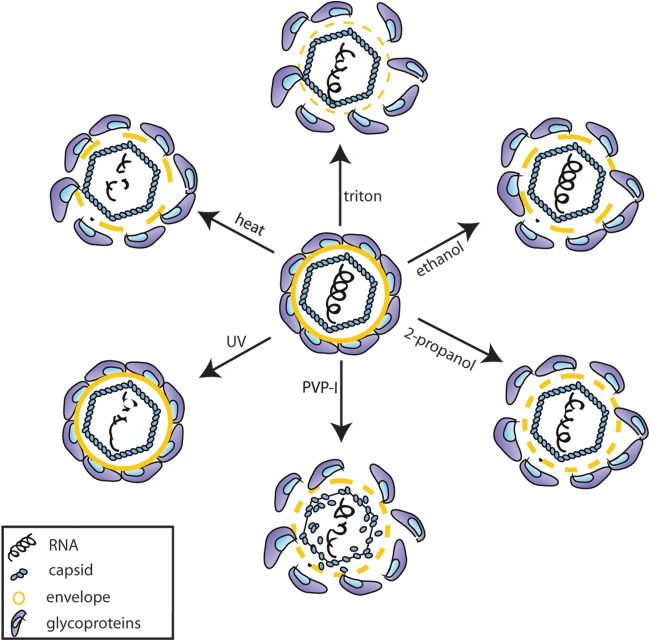
The use of viral inactivation methods is an essential part of infection control practices and plays an important role in the prevention of nosocomial infections. However, the exact antiviral mechanisms of these inactivation treatments are largely not well characterized (28). For approaches allowing the interruption of infectious virus and sterilizing strategies, knowledge of the specific mode of action should improve the application of inactivation procedure and disinfection strategies. In the present study, we could show that different inactivation methods against HCV comprising treatment with Triton X-100 and use of the alcohols ethanol and 2-propanol, PVP-I, and heat, as well as UV irradiation, resulted in a loss of infectivity for the HCV particle. Further analyses revealed that each disinfectant method targeted different parts of the viral particle (Fig. 4). Heat and UV treatment resulted in irreparable damage of the RNA and therefore in a loss of viral RNA replication. Heat treatment at 80°C, but not UV irradiation, further disrupted the viral envelope, rendering the viral capsid susceptible to proteolytic digestion. Even though we did not see an influence of either treatment method on the viral capsid, we cannot exclude that the viral capsid itself might also be damaged since the core ELISA is based on the detection of only a small part of the capsid (27). It has been shown for other viruses that heat inactivation induces structural changes in viral proteins, which might cause the loss of infectivity (29, 30) and degrades the viral RNA (31, 32). Whether heat inactivation influences only the viral proteins or also the RNA might depend on the applied temperature, as well as on the duration of heat administration. The same holds true for UV irradiation. UV irradiation, typically at a wavelength of 254 nm, is known to target nucleic acids, while leaving proteins largely preserved (29, 33). However, both viral genome and protein damage have been reported previously due to UV irradiation (30, 34, 35). Viral inactivation by alcohols is thought to be due to membrane damage and rapid protein denaturation (36) and, indeed, HCV RNA integrity was not compromised after treatment of the virus with either ethanol or 2-propanol. However, the viral envelope was damaged and resulted in a reduced protection of the capsid from externally added PK supporting the assumption that alcohols target the viral envelope. Both the actions of Triton X-100 and of PVP-I are thought to occur by targeting of the viral envelope. Triton X-100 is a nonionic surfactant commonly used as a detergent in laboratories that solubilizes proteins of the cell membrane (37), whereas PVP-I is a complex of iodine and a solubilizing carrier, which acts as a reservoir of “free” active iodine (38). With both inactivation methods, we observed a mild reduction in the ability of the RNA to replicate after the transfection of virus-associated genomes, indicating that both treatments have an influence on the viral RNA. As expected, Triton X-100 treatment resulted in complete destruction of the viral envelope, rendering the core protein susceptible to PK digestion. However, the effect of PVP-I could not be completely determined since the disinfectant targeted the core protein even in the absence of PK in the untreated control within this assay setup. It could be observed that longer incubation of HCV with PVP-I resulted in decreased amounts of core protein (data not shown), suggesting that this disinfectant has a direct effect on the viral capsid and therefore simultaneously on the viral envelope. The antimicrobial mechanism of PVP-I has been described as a direct delivery to the bacterial cell membrane, where it rapidly penetrates into the microorganism and targets key groups of proteins, nucleotides, and fatty acids in the cytoplasm and cytoplasmic membrane (38). The antiviral action against viruses has not been extensively studied, but it is likely that iodine attacks the surface proteins of enveloped viruses; it could also destabilize membrane fatty acids by reacting with unsaturated carbon bonds (39). Furthermore, lipid-enveloped viruses are generally more sensitive to chemical inactivation methods than non-lipid-enveloped viruses (36), which would support our assumption that the viral envelope constitutes a target for PVP-I. Interestingly, recent evidence suggests that some nonenveloped viruses such as hepatitis A virus and hepatitis E virus circulate in the blood of infected patient or animals enveloped in host-derived membranes but are shed as nonenveloped viruses. The two types of particle, enveloped and nonenveloped, appear to be equally infectious but are probably differently stable in the environment (40, 41).
FIG 4.
Mode of action of inactivation treatments on the HCV particle. Schematic depiction of the HCV particle, showing the glycoproteins E1 and E2 (blue), the viral envelope (yellow), and the capsid formed by the core protein (light blue), which protects the viral RNA (black). Each inactivation procedure affects the particle in a unique way, either by influencing the viral RNA or by destroying the viral envelope (solid yellow line, intact envelope; thick dashed yellow line, envelope damaged; thin dashed line, envelope strongly damaged).
In conclusion, different viral inactivation methods target specific parts of HCV particles as an example of an enveloped virus. Although heat and UV treatment mainly damage the viral genome stability, alcohol disinfectants cause a disruption of the virus particle membrane. Understanding virus inactivation on a basic mechanistic level will aid to predicting the susceptibility of nonculturable virus strains and should improve methods for combating viral transmission and inactivation.
ACKNOWLEDGMENTS
We are grateful to Takaji Wakita and Jens Bukh for the JFH1 and HCV isolates, respectively, and to Charles Rice for the Huh7.5 cells and E9E10 monoclonal antibody. Moreover, we thank all members of the Institute of Experimental Virology, Twincore, for helpful suggestions and discussions.
Twincore is a joint venture between the Medical School of Hanover and the Helmholtz Center for Infection Research, Hanover, Germany.
S.P. was supported by a stipend from the international research training group 1273 (IRTG 1273) provided by the DFG. E.S. was supported by the DFG (STE 1954/1-1) and intramural young investigator award of the Helmholtz Centre for Infection Research. T.P. was supported by a grant from the Helmholtz Association (SO-024).
REFERENCES
- 1.Bartenschlager R, Frese M, Pietschmann T. 2004. Novel insights into hepatitis C virus replication and persistence. Adv Virus Res 63:71–180. doi: 10.1016/S0065-3527(04)63002-8. [DOI] [PubMed] [Google Scholar]
- 2.Shepard CW, Finelli L, Alter MJ. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH. 1997. Hepatitis C: the clinical spectrum of disease. Hepatology 26:15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 4.Pawlotsky JM. 2014. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 146:1176–1192. doi: 10.1053/j.gastro.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Comstock RD, Mallonee S, Fox JL, Moolenaar RL, Vogt TM, Perz JF, Bell BP, Crutcher JM. 2004. A large nosocomial outbreak of hepatitis C and hepatitis B among patients receiving pain remediation treatments. Infect Control Hosp Epidemiol 25:576–583. doi: 10.1086/502442. [DOI] [PubMed] [Google Scholar]
- 6.Forns X, Martinez-Bauer E, Feliu A, Garcia-Retortillo M, Martin M, Gay E, Navasa M, Sanchez-Tapias JM, Bruguera M, Rodes J. 2005. Nosocomial transmission of HCV in the liver unit of a tertiary care center. Hepatology 41:115–122. doi: 10.1002/hep.20515. [DOI] [PubMed] [Google Scholar]
- 7.Krause G, Trepka MJ, Whisenhunt RS, Katz D, Nainan O, Wiersma ST, Hopkins RS. 2003. Nosocomial transmission of hepatitis C virus associated with the use of multidose saline vials. Infect Control Hosp Epidemiol 24:122–127. doi: 10.1086/502176. [DOI] [PubMed] [Google Scholar]
- 8.Lagging LM, Aneman C, Nenonen N, Brandberg A, Grip L, Norkrans G, Lindh M. 2002. Nosocomial transmission of HCV in a cardiology ward during the window phase of infection: an epidemiological and molecular investigation. Scand J Infect Dis 34:580–582. doi: 10.1080/00365540110080926. [DOI] [PubMed] [Google Scholar]
- 9.Silini E, Locasciulli A, Santoleri L, Gargantini L, Pinzello G, Montillo M, Foti L, Lisa A, Orfeo N, Magliano E, Nosari A, Morra E. 2002. Hepatitis C virus infection in a hematology ward: evidence for nosocomial transmission and impact on hematologic disease outcome. Haematologica 87:1200–1208. [PubMed] [Google Scholar]
- 10.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. 2011. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison SM, Kelly DA. 2008. Management strategies for hepatitis C virus infection in children. Paediatr Drugs 10:357–365. doi: 10.2165/0148581-200810060-00003. [DOI] [PubMed] [Google Scholar]
- 12.Mohan N, Gonzalez-Peralta RP, Fujisawa T, Chang MH, Heller S, Jara P, Kelly D, Mieli-Vergani G, Shah U, Murray KF. 2010. Chronic hepatitis C virus infection in children. J Pediatr Gastroenterol Nutr 50:123–131. doi: 10.1097/MPG.0b013e3181c61995. [DOI] [PubMed] [Google Scholar]
- 13.Terrault NA, Dodge JL, Murphy EL, Tavis JE, Kiss A, Levin TR, Gish RG, Busch MP, Reingold AL, Alter MJ. 2013. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology 57:881–889. doi: 10.1002/hep.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavanchy D. 2011. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 15.Ciesek S, Friesland M, Steinmann J, Becker B, Wedemeyer H, Manns MP, Steinmann J, Pietschmann T, Steinmann E. 2010. How stable is the hepatitis C virus (HCV)? Environmental stability of HCV and its susceptibility to chemical biocides. J Infect Dis 201:1859–1866. doi: 10.1086/652803. [DOI] [PubMed] [Google Scholar]
- 16.Doerrbecker J, Behrendt P, Mateu-Gelabert P, Ciesek S, Riebesehl N, Wilhelm C, Steinmann J, Pietschmann T, Steinmann E. 2013. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis 207:281–287. doi: 10.1093/infdis/jis677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doerrbecker J, Meuleman P, Kang J, Riebesehl N, Wilhelm C, Friesland M, Pfaender S, Steinmann J, Pietschmann T, Steinmann E. 2013. Thermostability of seven hepatitis C virus genotypes in vitro and in vivo. J Viral Hepat 20:478–485. doi: 10.1111/jvh.12055. [DOI] [PubMed] [Google Scholar]
- 18.Paintsil E, He H, Peters C, Lindenbach BD, Heimer R. 2010. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis 202:984–990. doi: 10.1086/656212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaender S, Heyden J, Friesland M, Ciesek S, Ejaz A, Steinmann J, Steinmann J, Malarski A, Stoiber H, Tsiavaliaris G, Bader W, Jahreis G, Pietschmann T, Steinmann E. 2013. Inactivation of hepatitis C virus infectivity by human breast milk. J Infect Dis 208:1943–1952. doi: 10.1093/infdis/jit519. [DOI] [PubMed] [Google Scholar]
- 20.Doerrbecker J, Friesland M, Ciesek S, Erichsen TJ, Mateu-Gelabert P, Steinmann J, Steinmann J, Pietschmann T, Steinmann E. 2011. Inactivation and survival of hepatitis C virus on inanimate surfaces. J Infect Dis 204:1830–1838. doi: 10.1093/infdis/jir535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paintsil E, Binka M, Patel A, Lindenbach BD, Heimer R. 2014. Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J Infect Dis 209:1205–1211. doi: 10.1093/infdis/jit648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blight KJ, McKeating JA, Rice CM. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol 76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset FL, Bartenschlager R. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A 103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinmann E, Penin F, Kallis S, Patel AH, Bartenschlager R, Pietschmann T. 2007. Hepatitis C virus p7 protein is crucial for assembly and release of infectious virions. PLoS Pathog 3:e103. doi: 10.1371/journal.ppat.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinmann E, Brohm C, Kallis S, Bartenschlager R, Pietschmann T. 2008. Efficient trans-encapsidation of hepatitis C virus RNAs into infectious virus-like particles. J Virol 82:7034–7046. doi: 10.1128/JVI.00118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattar SA, Springthorpe VS, Adegbunrin O, Zafer AA, Busa M. 2003. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J Virol Methods 112:3–12. doi: 10.1016/S0166-0934(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 27.Mederacke I, Wedemeyer H, Ciesek S, Steinmann E, Raupach R, Wursthorn K, Manns MP, Tillmann HL. 2009. Performance and clinical utility of a novel fully automated quantitative HCV-core antigen assay. J Clin Virol 46:210–215. doi: 10.1016/j.jcv.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Wigginton KR, Kohn T. 2012. Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr Opin Virol 2:84–89. doi: 10.1016/j.coviro.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuanualsuwan S, Cliver DO. 2003. Infectivity of RNA from inactivated poliovirus. Appl Environ Microbiol 69:1629–1632. doi: 10.1128/AEM.69.3.1629-1632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wigginton KR, Pecson BM, Sigstam T, Bosshard F, Kohn T. 2012. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ Sci Technol 46:12069–12078. doi: 10.1021/es3029473. [DOI] [PubMed] [Google Scholar]
- 31.Baert L, Wobus CE, Van Coillie E, Thackray LB, Debevere J, Uyttendaele M. 2008. Detection of murine norovirus 1 by using plaque assay, transfection assay, and real-time reverse transcription-PCR before and after heat exposure. Appl Environ Microbiol 74:543–546. doi: 10.1128/AEM.01039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woese C. 1960. Thermal inactivation of animal viruses. Ann N Y Acad Sci 83:741–751. [DOI] [PubMed] [Google Scholar]
- 33.Steinmann E, Gravemann U, Friesland M, Doerrbecker J, Muller TH, Pietschmann T, Seltsam A. 2013. Two pathogen reduction technologies—methylene blue plus light and shortwave ultraviolet light—effectively inactivate hepatitis C virus in blood products. Transfusion 53:1010–1018. doi: 10.1111/j.1537-2995.2012.03858.x. [DOI] [PubMed] [Google Scholar]
- 34.Eischeid AC, Linden KG. 2011. Molecular indications of protein damage in adenoviruses after UV disinfection. Appl Environ Microbiol 77:1145–1147. doi: 10.1128/AEM.00403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sirikanchana K, Shisler JL, Marinas BJ. 2008. Effect of exposure to UV-C irradiation and monochloramine on adenovirus serotype 2 early protein expression and DNA replication. Appl Environ Microbiol 74:3774–3782. doi: 10.1128/AEM.02049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnaitman CA. 1971. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol 108:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durani P, Leaper D. 2008. Povidone-iodine: use in hand disinfection, skin preparation and antiseptic irrigation. Int Wound J 5:376–387. doi: 10.1111/j.1742-481X.2007.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sattar SA, Springthorpe VS, Karim Y, Loro P. 1989. Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiol Infect 102:493–505. doi: 10.1017/S0950268800030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. 2013. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi M, Tanaka T, Takahashi H, Hoshino Y, Nagashima S, Jirintai, Mizuo H, Yazaki Y, Takagi T, Azuma M, Kusano E, Isoda N, Sugano K, Okamoto H. 2010. Hepatitis E Virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J Clin Microbiol 48:1112–1125. doi: 10.1128/JCM.02002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]