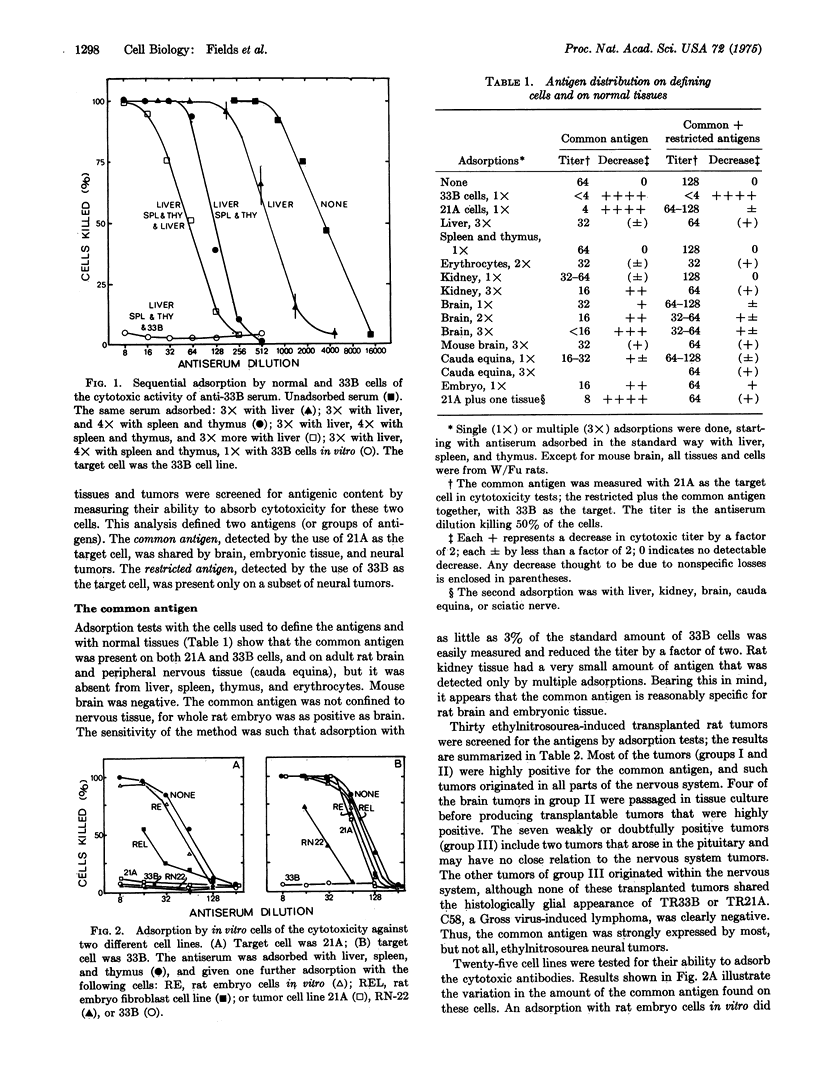
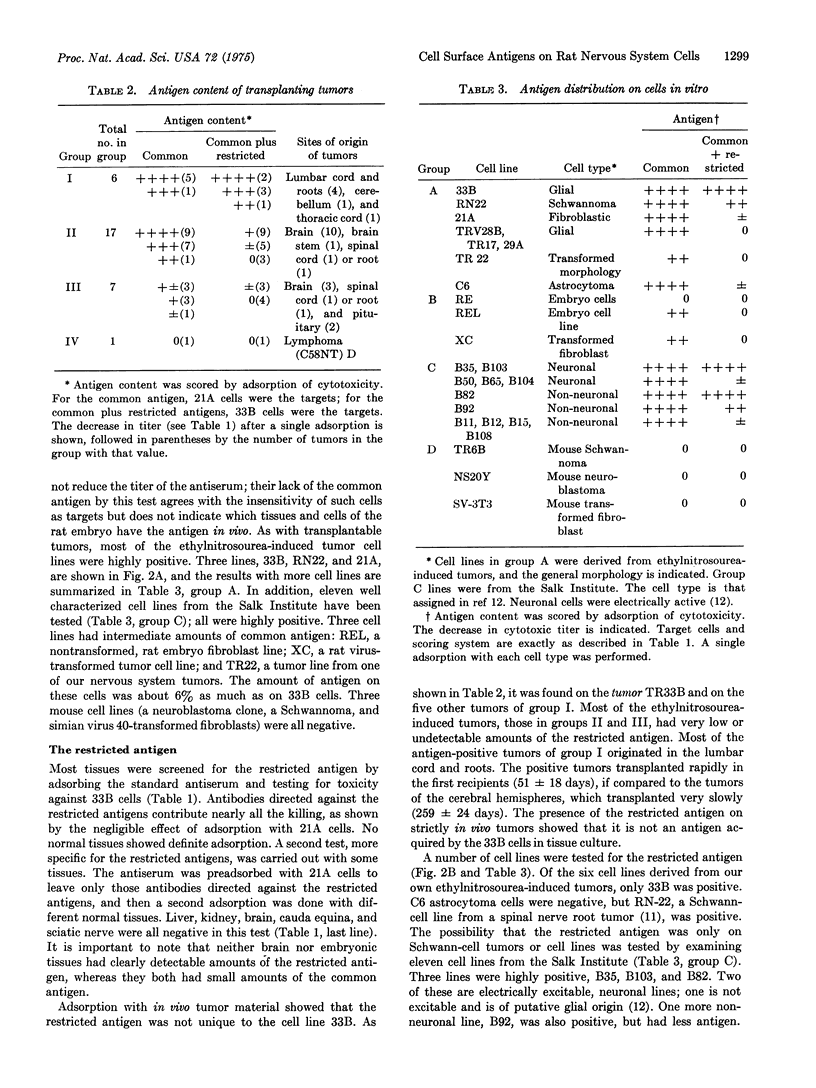
Abstract
Tumors of the central and peripheral nervous system were induced in rats with ethylnitrosourea. Many of these tumors were transplanted in syngeneic recipients, and several cell lines were derived from them. An antiserum raised against one such cell line in C3H mice defined two cell surface antigens in cytotoxicity tests. One, the common antigen, was present on rat brain and embryonic tissues and was present in large amounts on most tumors or cell lines from the nervous system. Fibroblastic cell lines had smaller amounts of this antigen, which also could be detected by immunofluorescence. The other, restricted antigen was not detected on normal or other, restricted antigen was not detected on normal or embryonic tissues. It was present on six tumors from the nervous system, on one glial cell line, and on a Schwann-cell line RN22. In addition, it was present on four out of eleven cloned cell lines isolated from rat tumors at the Salk Institute. Two of the positive clonal lines had been shown to have properties unique to neuronal cells. The restricted antigen was therefore expressed on the cell surface of some, but not all, glial, Schwann, and neuronal neoplastic cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeson R., Herschman H. R. Modulation of cell-surface antigens of a murine neuroblastoma. Proc Natl Acad Sci U S A. 1974 Jan;71(1):187–191. doi: 10.1073/pnas.71.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. W., Glaves D., Vose B. M. Embryonic antigen expression in chemically induced rat hepatomas and sarcomas. Int J Cancer. 1972 Sep 15;10(2):233–243. doi: 10.1002/ijc.2910100202. [DOI] [PubMed] [Google Scholar]
- Benda P., Someda K., Messer J., Sweet W. H. Morphological and immunochemical studies of rat glial tumors and clonal strains propagated in culture. J Neurosurg. 1971 Mar;34(3):310–323. doi: 10.3171/jns.1971.34.3.0310. [DOI] [PubMed] [Google Scholar]
- Coakham H. Surface antigen(s) common to human astrocytoma cells. Nature. 1974 Jul 26;250(464):328–330. doi: 10.1038/250328a0. [DOI] [PubMed] [Google Scholar]
- Geering G., Old L. J., Boyse E. A. Antigens of leukemias induced by naturally occurring murine leukemia virus: their relation to the antigens of gross virus and other murine leukemia viruses. J Exp Med. 1966 Oct 1;124(4):753–772. doi: 10.1084/jem.124.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. L., Searle C. E., Smith W. T. Tumours of the nervous system induced in rats by the neonatal administration of N-ethyl-N-nitrosourea. J Pathol. 1973 Feb;109(2):123–139. doi: 10.1002/path.1711090206. [DOI] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. E. Mouse brain antigen detected by rat anti-C1300 antiserum. Nature. 1974 May 3;249(452):71–73. doi: 10.1038/249071a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S. E., Herschman H. R., Lightbody J., Sato G. Synthesis by a clonal line of rat glial cells of a protein unique to the nervous system. J Cell Physiol. 1970 Jun;75(3):329–339. doi: 10.1002/jcp.1040750309. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S. E., Wechsler W. Biochemically differentiated neoplastic clone of Schwann cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2885–2889. doi: 10.1073/pnas.69.10.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Feldmann M., De Petris S. Monospecificity of bone marrow-derived lymphocytes. J Exp Med. 1973 Apr 1;137(4):1024–1030. doi: 10.1084/jem.137.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner M. NS-1 (nervous system antigen-1), a glial-cell-specific antigenic component of the surface membrane. Proc Natl Acad Sci U S A. 1974 May;71(5):1795–1799. doi: 10.1073/pnas.71.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner M. Representation of the cell surface alloantigen Thy-1 (theta) in brains of neurological mutants of the mouse. Brain Res. 1973 Jun 29;56:382–386. doi: 10.1016/0006-8993(73)90357-0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Searle C. E., Jones E. L. Tumours of the nervous system in mice treated neonatally with N-ethyl-N-nitrosourea. Nature. 1972 Dec 29;240(5383):559–560. doi: 10.1038/240559a0. [DOI] [PubMed] [Google Scholar]
- Trouillas P. Immunologie des tumeurs cérébrales: l'antigène carcino-foetal glial. Ann Inst Pasteur (Paris) 1972 Apr;122(4):819–828. [PubMed] [Google Scholar]


