Abstract
AIM
To assess the efficacy of topical Semaphorin-3A (SEMA3A) in the treatment of allergic conjunctivitis.
METHODS
Experimental allergic conjunctivitis (EAC) mice model induced by short ragweed pollen (SRW) in 4-week-old of BALB/c mice, mice were evaluated using haematoxylin and eosin (H&E) staining, immunofluorescence and light microscope photographs. Early phase took the samples in 24h after instillation and late phase took the samples between 4 to 14d after the start of treatment. The study use of topical SEMA3A (10 U, 100 U, 1000 U) eye drops and subconjunctival injection of SEMA3A with same concentration. For comparison, five types of allergy eyedrops were quantified using clinical characteristics.
RESULTS
Clinical score of composite ocular symptoms of the mice treated with SEMA3A were significantly decreased both in the immediate phase and the late phase compared to those treated with commercial ophthalmic formulations and non-treatment mice. SEMA3A treatment attenuates infiltration of eosinophils entering into conjunctiva in EAC mice. The score of eosinophil infiltration in the conjunctiva of SEMA3A 1000 U-treated group were significantly lower than low-concentration of SEMA3A treated groups and non-treated group. SEMA3A treatment also suppressed T-cell proliferation in vitro and decreased serum total IgE levels in EAC mice. Moreover, Treatment of SEMA3A suppressed Th2-related cytokines (IL-5, IL-13 and IL-4) and pro-inflammatory cytokines (IFN-γ, IL-17 and TNF-α) release, but increased regulatory cytokine IL-10 concentration in the conjunctiva of EAC mice.
CONCLUSIONS
SEMA3A as a biological agent, showed the beneficial activity in ocular allergic processes with the less damage to the intraocular tissue. It is expected that SEMA3A may be contributed in patients with a more severe spectrum of refractory ocular allergic diseases including allergic conjunctivitis in the near future.
Keywords: Semaphorin 3A, experimental allergic conjunctivitis, eosinophil infiltration, serum total IgE, cytokines, instillation, subconjunctival administration
INTRODUCTION
Allergy has been called “the 21st century disease”. In Japan the prevalence of allergic conjunctival diseases is estimated to be as high as 15%-20% of the population and is on the rise [1].
Allergens such as pollen, effects of air pollution may cause dilatation of blood vessels in the conjunctiva, the membrane covering the eye. The resulting reddening of the eyes is called allergic conjunctivitis.
The most common cause is an allergy to pollen in the hay fever season. In eye allergy conjunctivitis, seasonal allergic conjunctivitis (SAC) is the most common form, the most important seasonal triggers are ragweed pollens in Japan [2]. Characterized by itching, eyelid swelling, conjunctival swelling (chemosis), and mucus deposition, conjunctiva inflammatory process is an accumulation of eosinophils. Severe ocular symptom of irritation adversely affect the quality of life (QOL) of patients [1]. Treating patients with allergic conjunctivitis may improve their everyday “QOL. ”
Currently, therapies for ocular allergy have evolved to a point where there are now highly efficacious treatments for most of patients. Despite this, those with the most severe, chronic allergic conjunctivitis still experience substantial disease symptomatology. These patients represent up to 30% of all allergic conjunctivitis sufferers, and thus constitute a significant unmet need [3].
Semaphorin-3A (SEMA3A) is a representative secreted-type semaphorin and the first semaphorin to be identified in vertebrates. And it is well documented that SEMA3A functions as an axon guidance molecule[4], [5]. Currently, not only nerve, SEMA3A is expressed by activated T cells and downmodulates T cell activation in vitro[6]. Neuronal functions, involved in vascular biology and suppression of tumor, it has also been found that SEMA3A can be a key regulator in inflammatory diseases such as rheumatoid arthritis and lopus nephritis[7],[8]. Organogenesis, angiogenesis, and invasion of malignant tumors, academic activity in various regions has been such obviously[9]. Recently, SEMA3A been shown to have important functions in the immune system, and is widely accepted by the concept of “immune semaphorin” [10].
In the cornea, SEMA3A released from surrounding corneal fibroblasts triggers, also play a significant role as a corneal epithelial junction protein[11]. The CD4+NP-1+ T cells are a T cell subset involved in the control of the immune responses.
In this study, we investigated the therapeutic potential of SEMA3A in EAC mice model. We show that topical administration of SEMA3A inhibits allergy clinical symptoms and infiltration of eosinophils in mouse conjunctive tissue, simultaneously, in reducing ocular surface inflammation, might help to restore conjunctival goblet cells, which secrete mucins that prevent the formation of dry spots associated with ocular allergy.
MATERIALS AND METHODS
Experimental Allergic Conjunctivitis Mice Model
BALB/c mice purchased from SLC (Shizuoka, Japan) were housed in the standard mouse facility. All surgical procedures were performed under general anesthesia. The experimental procedures have been approved by ethical committee of Yokohama City University. Each experimental group contained at least six mice. Female 4-week-old BALB/c mice were injected with xylazine hydrochloride (5 mg/kg) and ketamine hydrochloride (35 mg/kg). Throughout the experimental procedures, all efforts were made to minimize the number of animals used and their suffering.
BALB/c mice were intraperitoneally injected with short ragweed pollen (SRW) (50 µg of SRW and 2 mg of alum (St. Louis, MO) on immun-day 0, 5 and immun-day 10 by active immunity. After immunization (day 0), in ophthalmic group: the eyes were challenged with SRW in phosphate-buffered saline (PBS) (2 µg in 10 mL per eye) via eye drops and 24h later their conjunctivas, spleens, were harvested for analyses. For examination of the effects of the EAC during the late induction (or effector) phase, actively immunized mice were treated with the SEMA3A just before or at the same time as the challenge.
Recombinant SEMA3A was prepared as Takahashi et al[12], Conditioned medium containing SEMA3A was collected 3-5d after transfection and was applied to Talon (Clontech, Palo Alto, CA, USA) column according to the manufacturer's instructions. Purified SEMA3A was diluted with PBS.
Different concentrations of SEMA3A (10 U, 100 U, 1000 U) and PBS were as a therapeutic agent or as control. Ophthalmic group were challenged (10 µL per eye) via eye drops with twice a day for 4wk. Subconjunctival injection group were administered by injection (2 µL per eye) with once in 2d for 4wk.
Experimental Design
All experiments presented have been repeated at least two times, and consistent results were obtained. Data are depicted as means±SE (or SD). Statistical significance was determined by Student's t-test. P<0.05 was considered statistically significant.
Allergic Conjunctivitis Clinical Scoring
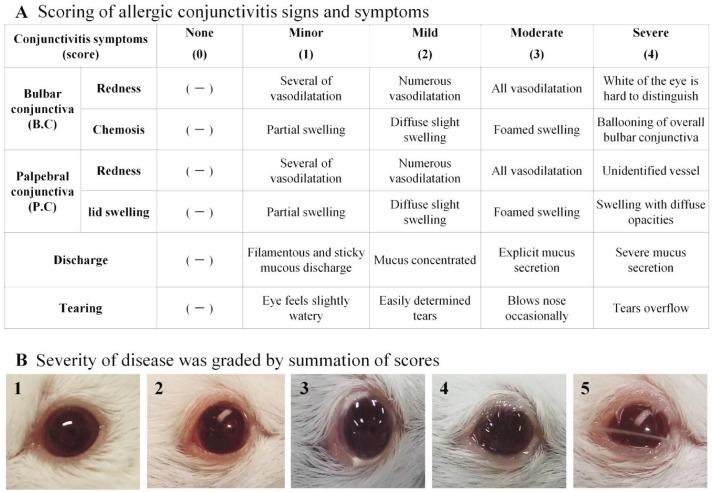
Allergic conjunctivitis (AC) clinical scoring was performed here in a masked fashion by two independent observers according to a previously validated scale[13]. The eyes were examined under microscope, scoring was performed 15-30min post challenge and done once daily from day 1 to at least day 14 (From 3wk to 4 wk had no significant changes). As shown in Figure 1, mice were examined biomicroscopically based on four independent parameters, which include redness, chemosis, discharge and tearing. Each parameter was ascribed 0 (i.e. none) to 4+ points (i.e. severe) and was summed to yield a maximum score of 24+. Changes in the symptoms scores were calculated and graphed.
Figure 1. Score for the symptoms of allergic conjunctivitis, was a total statistics of five items.
A: All mice were scored biomicroscopically 15-30min post challenge for clinical signs [Redness, Chemosis, Discharge and Tearing]. Difference in clinical symptom suppression was confirmed in the observation period for maximum 4wk (From 3wk to 4wk had no significant changes); B: Classification of allergic conjunctivitis induced by short ragweed. After the evaluation of clinical symptoms in the eye, the severity of disease was graded by summation of scores: none, 0 points (1); minimal, 1-3 points (2); mild, > 4 points or 2 grade 2 symptoms (3); moderate, 3 or 4 grade 2 symptoms (4); severe, 5 grade 2 symptoms (5).
Compare the Efficacy of SEMA3A Eye Drops with Commercial Ophthalmic Formulations of Allergic Conjunctivitis in Experimental Allergic Conjunctivitis Mice Model
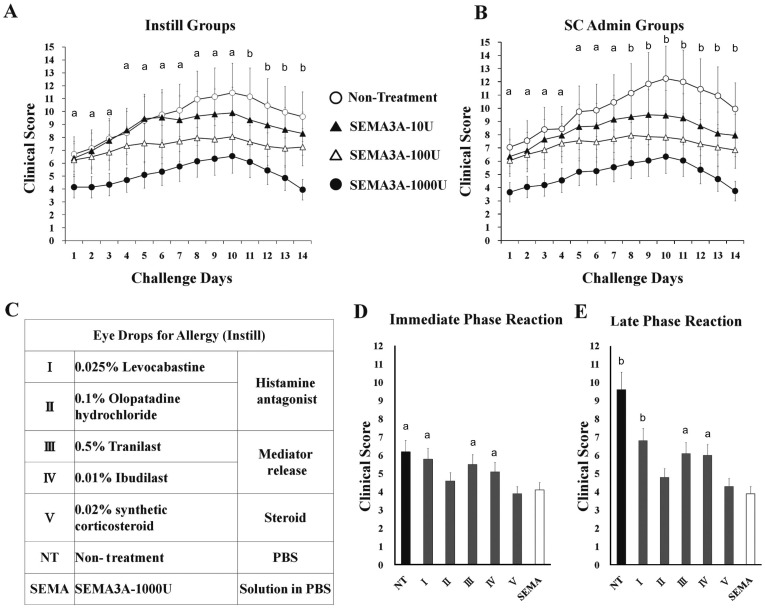
Five types conventional therapy eye drops, PBS (Non-treatment) (Figure 2D)and SEMA3A (1000 U/10 µL in PBS) solution were used in EAC mice by twice a day for maximum 4wk with topical administration. All mice were scored biomicroscopically 15-30min post challenge for clinical signs. Conjunctivitis inhibitory effect as an index was calculated and graphed the changes in the symptoms scores.
Figur 2. In instillation groups, allergy inhibitory effects of treatment with SEMA3A 1000 U has been evaluated and compared to commercial ophthalmic formulations.
A: Instillation groups (Instill); B: Subconjunctival administration groups (SC admin). Topical SEMA3A (10 U, 100 U, 1000 U)- treatment and PBS (as non-treatment) were used in the therapy experiments. Each experimental group consisted of six mice. aP< 0.05; bP < 0.01, SEMA3A 1000 U treatment group compared with NT; C: Commercial ophthalmic formulations, which are mainly used in the allergic conjunctivitis. NT (non-treatment) used PBS as control; D: The score of the symptoms of allergic conjunctivitis in the immediate phase reaction in 24h (average value of total evaluation). Data represent means ± SD of six mice/group, a P < 0.05; bP < 0.01, SEMA3A 1000 U treatment group compared with commercial ophthalmic formulation and non-treatment groups; E: The score of the symptoms of allergic conjunctivitis in the late phase reaction on day 14 (average value of total evaluation). Data represent means±SD of six mice / group. aP < 0.05; bP < 0.01, SEMA3A 1000 U treatment group compared with commercial ophthalmic formulation and NT groups.
Evaluation of Eosinophil Infiltration
For analysis the conjunctival tissue, the hair around the perimeter about 5 mm wide was drop cut, tissue was cut off the lid around to leave about 2 mm ahead of the eyelid and all conjunctival lamina propria and the entire eyelid was fixed in buffered 10% formalin. Then, they were cut into cross sections 3 µm thick and stained with hematoxylin-eosine for detection of eosinophils, respectively. In each section, infiltrating cells in the lamina propria mucosae of the palpebral and bulbar conjunctivas were counted by two masked observers. Palpebral zone: connective tissue between the epithelium and the Meibomio gland in the centre of the lid. Bulbar conjunctiva: near the cornea in reflection zone that form the conjunctival sac. In each slide there were two non-consecutive sections and three slides per eye and per stain were counted. Number of eosinophils in conjunctival fornix was counted under microscope at 10×40 magnification. Each experimental group consisted of six mice. Data are presented as mean ± SD.
Cellular Proliferation Assay
The spleen was removed from suppression confirmation normal BALB / c mouse immune T cell proliferation. BALB/c splenocytes were cultured at 5 × 105 to 2 × 106 cells/well in anti-CD3 coated T-cell Activation Plates (96-well BioCoat™ antimurine CD3 plate, BD Biosciences, Franklin Lakes, NJ, USA). Splenocytes were administered at a concentration of 10 U, 100 U, and 1000 U Semaphorin-3A, the control group was administered PBS. Cells were cultured 45-48h at 37°C put in CO2 incubator. Cell proliferation was then measured by the optical density (OD) of 490 nm measurement wavelength 700 nm reference wavelength using a microplate spectrophotometer.
Measurement of Total IgE in Serum
Animals were sacrificed 24 hr after the challenge. Blood samples were collected by drawing blood from the heart under ether anesthesia. The concentration of total IgE was measured using a mouse IgE EIA kit (Yamasa, Tokyo, Japan), according to the manufacturer's protocol.
Measurement of the Levels of Inflammatory Markers in the Corneal Tissue
Animals were sacrificed 24h after the challenge. Corneal samples were minced and homogenized in ice-cold PBS containing a protease inhibitor (Complete; Roche Diagnostics, Mannheim, Germany) with a polytron tissue homogenizer. Precipitate was removed by centrifugation, and supernatant was collected.
Levels of cytokines in the corneal tissue were measured by the MILLIPLEX MAP Mouse Cytokine/Chemokine Panel (Millipore, USA) according to manufacturer's instructions and analyzed with the Bio-Plex System (Bio-Rad Laboratories, USA). Values are expressed as pg/mL after subtraction of baseline levels.
Immunohistochemistry
Mouse globes were dissected and frozen in OCT embedding medium (Sakura Finetec, Torrance, CA, USA) using dry-ice-cooled isopentane. Embedded frozen tissues were sectioned at 8 µm. Frozen sections were fixed with phosphate-buffered 4% formaldehyde for 10min at room temperature. Sections were washed in PBS before being blocked with 5% goat serum albumin (Sigma) in PBS for 60min at room temperature. Primary antibody (rabbit anti-SEMA3A; Sigma) was added (1:1000) and incubated overnight at 4°C. Secondary fluorophore-coupled antibody (goat anti-rabbit Alexa-Fluor 488; Invitrogen) together with Alexa-Fluor 546 phalloidin (Invitrogen) was added (1:600) for 2h at room temperature. After washing and 4′, 6′-diamidino-2-phenylindole (DAPI; 0.1 µg/mL; Sigma) counterstaining, coverslips were mounted in Prolong Gold Antifade reagent (Molecular Probes). Examined with an LSM 710 Meta confocal microscope (Carl Zeiss, Jena, Germany).
RESULTS
SEMA3A Treatment Reduced Clinical Signs of Allergic Conjunctivitis Both in the Immediate Phase Reaction and the Late Phase Reaction
Score for the symptoms of allergic conjunctivitis, was a total statistics of five items (Figure 1A). Classification of allergic conjunctivitis induced by short ragweed. After the evaluation of clinical symptoms in the eye, the severity of disease was graded by summation of scores (Figure 1B: 1-5). Difference in clinical symptom suppression was confirmed in the observation period of 4wk (from 3wk to 4wk had no significant changes).
As shown in Figure 2, SRW-sensitized mouse AC model in clinical observations revealed typical symptoms of allergic conjunctivitis. Immediate phase were detected within 30min post challenge and persisted until 24h (Figure 2D). Chemosis, lid edema, redness, and tearing scores were significantly reduced. In instillation groups, allergy inhibitory effects of treatment with SEMA3A-1000 U mice has been evaluated and compared to mice with commercial ophthalmic formulations, which are mainly used in the current clinical (scoring evaluation criterion was based on Figure 1A).
Clinical effect of five types conventional therapy eye drops (Figure 2C) and SEMA3A eye drops contrast showed that SEMA3A treatment mice had significantly reduced clinical signs of allergic conjunctivitis, and the average value of total evaluation significantly low both in the immediate phase reaction (Figure 2D, 24h) and the late phase reaction (Figure 2E, 14d).
SEMA3A Treatment Attenuates Infiltration of Eosinophils into Conjunctiva in Experimental Allergic Conjunctivitis Mice
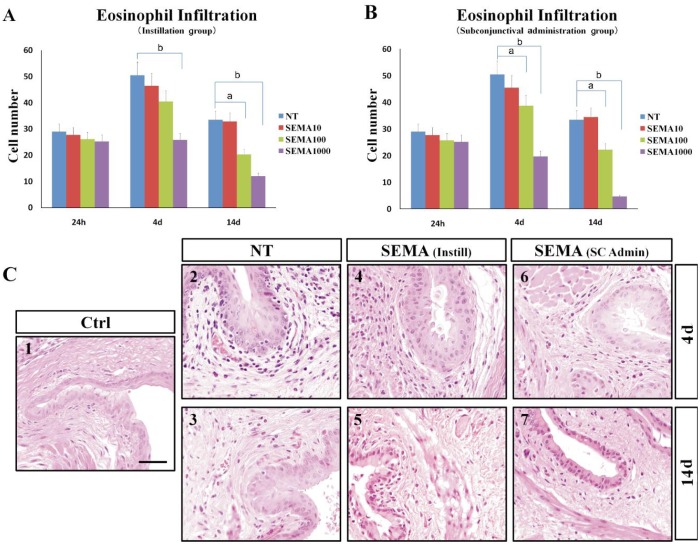
Conjunctiva as a first line of the eye has a variety of barrier function and often exposed to allergens such as viruses, bacteria and pollen. Eosinophils were the predominant inflammatory cells in the affected conjunctivae and were present in specimens collected at 24h, 4d, and 14d after topical challenge (Figure 3A, 3B). Number of eosinophils in conjunctival fornix was counted under microscope at 10×40 magnification. At a concentration difference of SEMA3A, there was a significant difference in eosinophil infiltration score (Figure 3). SEMA3A 1000 U concentration lower than those of the non-treated and the other low-concentration SEMA3A -treated mice maximum about 70 percent (Figure 3B).
Figure 3. SEMA3A administration prevents development of allergic conjunctival inflammation.
A: Instillation groups (Instill); B: Subconjunctival administration groups (SC admin). Each experimental group consisted of six mice. aP < 0.05; bP < 0.01, compared with NT; C: Light micrograph of conjunctival sections in mice from allergic untreated and SENA3A 1000 U treated groups. Eosinophil infiltration in the conjunctiva on day 4 and day 14 after short ragweed (SRW) challenge. C1 (Ctrl, control group): Sensitized but not challenged (the others groups are all sensitized and SRW challenged); C2, 3 (NT, Non-treatment group): PBS ocular instillation group; C4, 5 [SEMA (instill)]: SEMA3A 1000 U ocular instillation treatment group. C6, 7 [SEMA (SC admin)]: SEMA3A 1000 U subconjunctival injection treatment group. Scale bar: 40 um.
Furthermore, the eosinophil infiltration score showed no significant difference in subconjunctival administration group and instillation group (Figure 3A, 3B).
SEMA3A Treatment Suppressed T-cell Proliferation in Vitro Studies
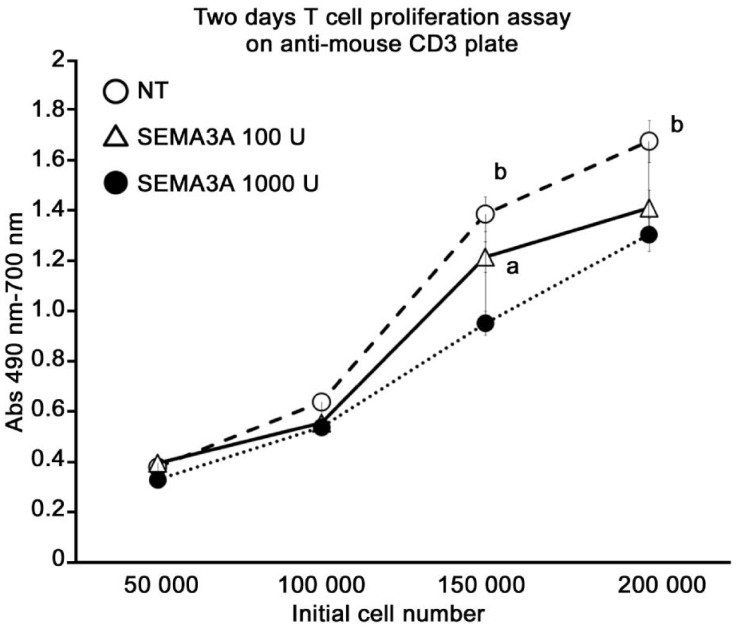
Two day proliferation assay using unsensitized mouse splenocytes on T cell activation plates. A significant reduction of proliferation in the in vitro inhibition of T cell proliferation was confirmed by SEMA3A administration of the concentration-dependent (Figure 4). SEMA3A 1000 U treatment group was maximum about 31 percent lower than non-treatment group.
Figure 4. A significant reduction of proliferation in the in vitro inhibition of T cell proliferation was confirmed by SEMA3A administration of the concentration-dependent.
In vitro, T cell proliferation inhibition of SEMA3A was confirmed on anti-Mouse CD3 plate after 48h incubation. SEMA3A 1000 U treatment group was maximum about 31 percent lower than NT group. All groups are run in triplicate. Pooled values of three repeat experiments are expressed as mean ± SE. aP < 0.05; bP < 0.01, SEMA3A 1000 U treatment group compared with the NT group or SEMA3A 100 U treatment group.
SEMA3A Showed the Tissue Penetration and the Effect of Maintaining the Barrier Function of the Conjunctival Epithelium by Administration of High Concentrations
Mice conjunctiva was analyzed by immunohistochemistry on day 4 and SEMA3A was shown to be highly expressed in the conjunctival tissue as indicated by green staining in 1000 U SEMA3A-treated mice (Figure 5).
Figure 5. SEMA3A showed tissue penetration and the effect of maintaining the barrier function of the conjunctival epithelium by administration of high concentrations on day 4.
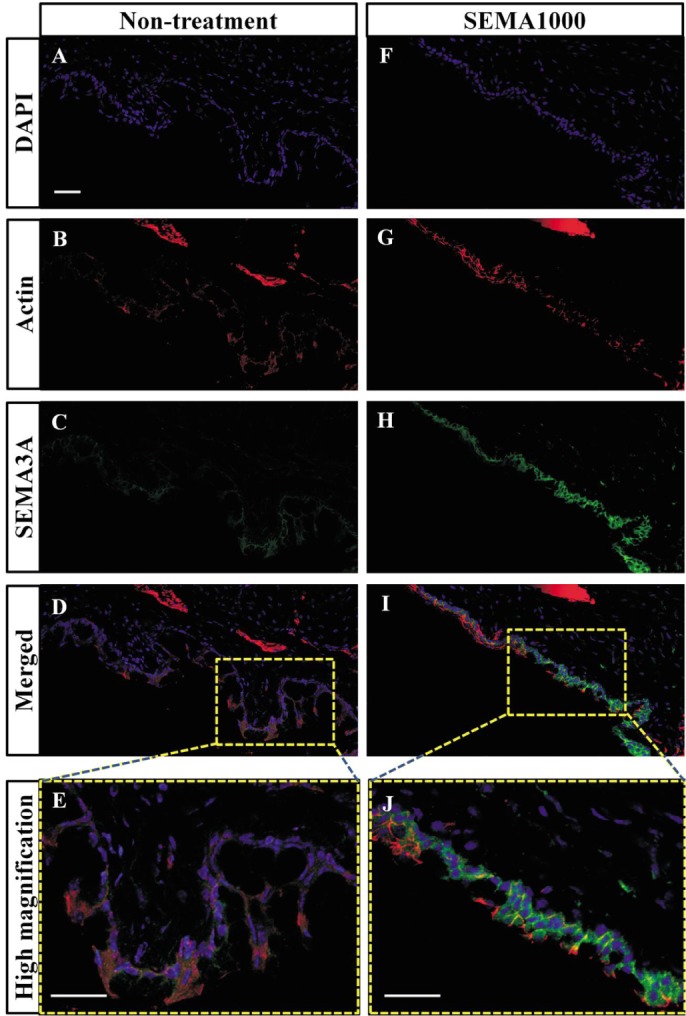
Sections were stained with antibodies against SMA3A (C,H), fluorescent Alexa Fluor 546 phalloidin (B,G) for actin, and 4′ 6′-diamidino-2-phenylondole dihydrochloride (DAPI) (A,F) was used to label cell nuclei. SEMA3A 1000 U instillation mice (F-J) compared with the non-treatment mice (A-E). Scale bar: 50um.
SEMA3A showed the inhibitory effect of allergic cellular reaction, such as conjunctival epithelial cell arrangement disturbance and dropout; goblet cell expanding and increasing, compared with the non-treatment group.
This study deals with the concept of a key role for SEMA3A in immunoregulation and provides experimental data aimed at validating the therapeutic potential of SEMA3A in allergic conjunctivitis.
SEMA3A Treatment Decreased Serum Total IgE Levels and Prevents the Development of Allergic Responses. Interestingly, Levels of the Regulatory Cytokine IL-10 were Increased by SEMA3A Treatment
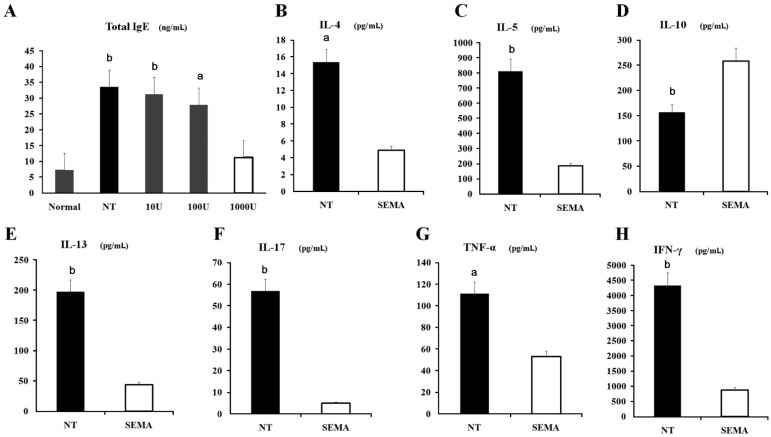
SEMA3A 1000U treated mice had significantly lower total serum IgE levels, compared with the other low-concentration SEMA3A -treated mice and non-treatment mice (Figure 6A).
Figure 6. SEMA3A treatment decreased serum total IgE levels and prevents the development of allergic responses, suppressed Th2-related cytokines (IL-5, IL-13 and IL-4) and pro-inflammatory cytokines (IFN-γ, IL-17 and TNF-α) release, but increased regulatory cytokine IL-10 concentration in the conjunctiva of EAC mice.
Serum levels of total IgE (A), in normal mice, non-treated mice, and mice treated with SEMA3A (10 U, 100 U, 1000 U) on day 14. SEMA3A 1000 U treatment group compared with NT group and the other low-concentration of SEMA3A treated groups. Levels of IL-4 (B), IL-5 (C), IL-10 (D), IL-13 (E), IL-17 (F), TNF-α (G) and IFN-γ (H) were measured by MILLIPLEX Cytokine panel. SEMA3A 1000 U treatment group (SEMA) compared with NT group. Pooled values of two repeat experiments, each with six mice per group, data represent means ± SD. aP < 0.05; bP < 0.001.
Production of both Th2-related cytokines IL-4, IL-5 and IL-13 (Figure 6B, 6C, 6E) and pro-inflammatory cytokines IFN-γ, IL-17 and TNF-α (Figure 6H, 6F, 6G) were suppressed in 1000 U SEMA3A-treated mice compared with control mice. Interestingly, levels of the regulatory cytokine IL-10 were increased by SEMA3A treatment (Figure 6D).
DISCUSSION
Allergens are proteins that have enzymatic or irritating properties that aid in penetration into the mucosa. It seems that whether it's the nose or the eye, our patients with chronic allergy are experiencing a simultaneous combination of immediate and delayed allergic responses, with both humoral and cellular aspects underlying their disease [14].
In present study, we initially developed an allergic conjunctivitis model in mice and showed the SEMA3A treatment mice had significantly reduced clinical signs of allergic conjunctivitis both in the immediate phase reaction and the late phase reaction.
Recent studies, the role of SEMA3A in the immune system has been revealed partially. 1) Enhances DC (dendritic cells) migration into lymph nodes; 2) induces apoptosis in macrophage colony-stimulating factor (M-CSF)-derived macrophages, and 3) terminates the immune response [15]–[19].
We subsequently examined the effect of SEMA3A in immune cell migration on EAC model mice. SEMA3A significantly inhibit the migration of eosinophils. Eosinophils have been studied extensively in relation to priming, and are thought to be key players in the late phase and in chronic allergic conditions[20]. Release of IL-5 and related cytokines prime the eosinophil, allowing for recruitment, accumulation in tissues and activation. These effects occur minutes after IL-5 exposure, and are therefore non-transcriptional processes, and the induced changes may persist for hours or days after exposure[21]. This eosinophilic priming translates clinically to more severe allergic responses[22].
Eosinophils can also potentiate their own chemoattraction by providing an additional source of eotaxin-1, which, when bound to its C-C chemokine receptor type 3 (CCR3), calls even more cells into tissues. Eotaxins have been extensively studied and are known to be elevated in chronic ocular allergic diseases[23]. The suppression of eosinophil migration is a key in the treatment of allergic conjunctivitis.
A role is emerging for SEMA3A in immune cell migration. SEMA3A, which is a repulsive axon guidance factor in neurons, reportedly inhibits human monocyte and T cell migration toward chemokine gradients in classical transwell assays [24],[25]. A recent imaging study has revealed that SEMA3A produced by lymphatic endothelial cells guides the entry of DCs into the afferent lymphatics due to its interaction with the Plexin-A1/Np-1 receptor complex, which is expressed on the trailing end of migrating DCs. These findings indicate that the defective T cell priming observed in Plexin-A1-deficient mice is primarily due to the inability of DCs to properly migrate into the lymph nodes. During DC migration, SEMA3A consistently acts on the rear side of DCs, where plexin-A1 is localized, and induces myosin light chain phosphorylation, resulting in increased DC velocity and transmigration by promoting actomyosin contraction [26].
The results showed SEMA3A treatment suppressed T-cell proliferation in the T cell receptor complex antibody coated plates. SEMA3A treatment also decreased serum total IgE levels and prevents the development of allergic responses in EAC mice.
These findings can be interpreted by previously published results. The pathological processes of an allergic reaction are thought to be mediated by Th2-type cells which release interleukins such as IL-4, IL-5 and IL-13[27], [28], and newly discovered Th2 cytokines with proinflammatory functions, such as IL-25, IL-31, IL-33 and IL-9[29], [30]. These cytokines are responsible for a cascade of events necessary for allergic inflammation: IgE production by Bcells, eosinophil activation and recruitment, and mast cell activation. IL-13 is a crucial mediator of hypersensivity reactions[31]–[33].
Nrp-1, which was originally identified as a cell surface glycoprotein, functions as a SEMA3A receptor[34]. Nrp-1 mediates contact between T cells and DCs through homophilic interactions that are important for initiation of an immune response [35]. In addition, Nrp-1 has been identified as a specific marker for CD4+CD25+ regulatory T cells (Tregs)[36]. Recently, one report has suggested that Nrp-1 in Tregs contributes to prolonged contact between Tregs and DCs, which inhibits T cell activation at steady state [37]. Furthermore, Nrp-1+ Tregs preferentially produce IL-10 upon SEMA3A stimulation[7].
SEMA3A does not inhibit immune synapse formation by interfering with this homotypic interaction but rather modulates T cell responses by blocking actin cytoskeleton reorganisation. This inhibition impairs the redistribution of T cell receptors (TCRs) in the DC/T cell contact zone and inhibits TCR mediated T cell activation signals [34], most likely via Plexin-A4-mediated signalling [38]. However, the secretion of SEMA3A by activated T cells during the immune response is delayed, suggesting that sema3A/Np-1 interactions participate in terminating the immune response, thus restraining overactivation. In fact, Regardless of the suppressive function that SEMA3A exerts in adaptive immune responses, this protein has also been reported to promote innate immunity through interactions with Plexin-A4 [39]. As described above and the findings in this study suggest that SEMA3A in Tregs exerts suppressive functions, presumably by mediating Treg stop signals on DCs and by producing regulatory cytokines.
In addition, SEMA3A showed the tissue penetration and the effect of maintaining the barrier function of the conjunctival epithelium. SEMA3A inhibits the inhibitory effect of allergic cellular reaction, such as conjunctival epithelial cell arrangement disturbance and dropout and goblet cell expanding and increasing. SEMA3A might help to restore conjunctival goblet cells, which secrete mucins that prevent the formation of dry spots associated with ocular allergy.
In our study, we also demonstrated therapeutic effect of commercial ophthalmic formulations concurrently in EAC mice model. Five types conventional therapy eye drops, which were mainly used in the allergic conjunctivitis, such as mediator release inhibitors, oral medicine and steroid eye drops. SEMA3A showed a clear therapeutic effect compared with commercial ophthalmic formulations.
Even now, it cannot be said that effect is to be sufficient non-steroid eye drops, the drug we need often recombination. Topical steroids are still being used in the treatment of serious allergic conjunctivitis cases because of their strong effects[40]. We produce corneal epithelial disorder refractory, a dry eye by the long-term continuous use. Increased intraocular pressure, secondary glaucoma, secondary cataract, by compromised. There is a risk to aggravate the lesions of the cornea, leading and corneal transplant, by the last surgery in steroid eye drops.
In chronic disease to repeat the inflammation, for merger (nose, trachea) and other allergic diseases is large, and spend a lot of time and expense also, more than 90% of the highest efficiency among the symptoms of allergic diseases, The consternation major obstacle in life, QOL of patients is reduced.
As the prevalence of allergic disease increases around the world, resistance to antibiotics/antivirals/or antimicotic drugs grows, and virulence of microorganisms improves its capacity of infection, it is apparent that more effective therapies and disease-modifying agents are needed[41].
It's clear that every allergic reaction in the eye, whether induced by natural or artificial challenge, not only evokes an allergic inflammatory response, but also changes the response to future exposures. The communication between mast cells, dendritic cells and T cells is intimate and constant [42]–[44], and thus our goal in developing treatments can be thought of as a balancing act: keeping the protective functions of the immune system intact while responding to the acute and chronic perturbations of ocular allergy.
In conclusion, to overcome the risks described above, for the first time our study demonstrated the therapeutic potential of SEMA3A for AC. SEMA3A as a biological agent, showed the beneficial activity in ocular allergic processes with less damage to the intraocular tissues. SEMA3A provides a new intervention strategy and potential therapy for ocular allergy; and could reduce or eliminate the usage of topical steroid. SEMA3A may be valuable presence as a therapeutic agent for allergic diseases.
Acknowledgments
Foundation: A grant-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Conflicts of Interest: Tanaka J, None; Tanaka H, None; Mizuki N, None; Nomura E, None; Ito N, None; Nomura N, None; Yamane M, None; Hida T, None; Goshima Y, None; Hatano H, None; Nakagawa H, None.
REFERENCES
- 1.Uchio E. Treatment of allergic conjunctivitis with olopatadine hydrochloride eye drops. Clin Ophthalmol. 2008;2(3):525–531. doi: 10.2147/opth.s3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takamura E, Uchio E, Ebihara N, Ohno S, Ohashi Y, Okamoto S, Kumagai N, Satake Y, Shoji J, Nakagawa Y, Namba K, Fukagawa K, Fukushima A, Fujishima H, Japanese Society of Allergology Japanese guideline for allergic conjunctival diseases. Allergol Int. 2011;60(2):191–203. doi: 10.2332/allergolint.11-RAI-0335. [DOI] [PubMed] [Google Scholar]
- 3.Mimura T, Yamagami S, Amano S, Funatsu H, Arimoto A, Usui T, Ono K, Araie M, Okamoto S. Allergens in Japanese patients with allergic conjunctivitis in autumn. Eye (Lond) 2005;19(9):995–999. doi: 10.1038/sj.eye.6701701. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75(2):217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 5.Rohm B, Ottemeyer A, Lohrum M, Püschel AW. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev. 2000;93(1–2):95–104. doi: 10.1016/s0925-4773(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 6.Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A, Hermine O. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006;36(7):1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- 7.Catalano A. The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J Immunol. 2010;15;185(10):6373–6383. doi: 10.4049/jimmunol.0903527. [DOI] [PubMed] [Google Scholar]
- 8.Vadasz Z, Ben-Izhak O, Bejar J, Sabo E, Kessel A, Storch S, Toubi E. The involvement of immune semaphorins and neuropilin-1 in lupus nephritis. Lupus. 2011;20(14):1466–1473. doi: 10.1177/0961203311417034. [DOI] [PubMed] [Google Scholar]
- 9.Morishige N, Ko JA, Liu Y, Chikama T, Nishida T. Localization of semaphorin 3A in the rat cornea. Exp Eye Res. 2008;86(4):669–674. doi: 10.1016/j.exer.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003;3(2):159–167. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- 11.Ko JA, Akamatsu Y, Yanai R, Nishida T. Effects of semaphorin 3A overexpression in corneal fibroblasts on the expression of adherens-junction protein in corneal epithelial cells. Biochem Biophys Res Commun. 2010;396(4):781–786. doi: 10.1016/j.bbrc.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Nakamura F, Strittmatter SM. Neuronal and non-neuronal collapsin-1 binding sites in developing chick are distinct from other semaphorin binding sites. J Neurosci. 1997;17(23):9183–9193. doi: 10.1523/JNEUROSCI.17-23-09183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takamura E, Nomura K, Fujishima H, Fukagawa K, Satake Y, Fukada Y, Sawa M, Uchida E. Efficacy of levocabastine hydrochloride ophthalmic suspension in the conjunctival allergen challenge test in Japanese subjects with seasonal allergic conjunctivitis. Allergol Int. 2006;55(2):157–165. doi: 10.2332/allergolint.55.157. [DOI] [PubMed] [Google Scholar]
- 14.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteosome system. J Biosci. 2006;31(1):137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 15.Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by Neuropilin-1/ Semaphorin-3A- mediated interactions. Proc Natl Acad Sci USA. 2007;104(13):5545–5550. doi: 10.1073/pnas.0700705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, Tamagnone L, Catalano A. Semaphorin3A signaling controls Fas (CD95)- mediated apoptosis by promoting Fas translocation into lipid rafts. Blood. 2008;111(4):2290–2299. doi: 10.1182/blood-2007-06-096529. [DOI] [PubMed] [Google Scholar]
- 17.Ji JD, Park-Min KH, Ivashkiv LB. Expression and function of semaphorin 3A and its receptors in human monocyte-derived macrophages. Hum Immunol. 2009;70(4):211–217. doi: 10.1016/j.humimm.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlahsa L, Zhang H, Battermann A, Verboom M, Immenschuh S, Eiz-Vesper B, Stripecke R, Engelmann K, Blasczyk R, Figueiredo C. Semaphorin 3A alters endothelial cell immunogenicity by regulating Class II transactivator activity circuits. Transfusion. 2014;54(8):1961–1970. doi: 10.1111/trf.12631. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi J, Nakamura F, Aihara M, Yamashita N, Usui H, Hida T, Takei K, Nagashima Y, Ikezawa Z, Goshima Y. Semaphorin3A alleviates skin lesions and scratching behavior in NC/Nga mice, an atopic dermatitis model. J Invest Dermatol. 2008;128(12):2842–2849. doi: 10.1038/jid.2008.150. [DOI] [PubMed] [Google Scholar]
- 20.Godthelp T, Holm AF, Fokkens WJ, Doornenbal P, Mulder PG, Hoefsmit EC, Kleinjan A, Prens EP, Rijntjes E. Dynamics of nasal eosinophils in response to a nonnatural allergen challenge in patients with allergic rhinitis and control subjects: A biopsy and brush study. J Allergy Clin Immunol. 1996;97(3):800–811. doi: 10.1016/s0091-6749(96)80158-8. [DOI] [PubMed] [Google Scholar]
- 21.Håkansson L, Heinrich C, Rak S, Venge P. Priming of eosinophil adhesion in patients with birch pollen allergy during pollen season: effect of immunotherapy. J Allergy Clin Immunol. 1997;99(4):551–562. doi: 10.1016/s0091-6749(97)70084-8. [DOI] [PubMed] [Google Scholar]
- 22.Yuki A, Terada T, Ichihara T, Fujii K, Hyo S, Kawata R, Takenaka H. Evaluating the effects of testing period on pollinosis symptoms using an allergen challenge chamber. Allergol Int. 2011;60(4):533–539. doi: 10.2332/allergolint.10-OA-0264. [DOI] [PubMed] [Google Scholar]
- 23.Leonardi A, Jose PJ, Zhan H, Calder VL. Tear and mucus eotaxin-1 and eotaxin-2 in allergic keratoconjunctivitis. Ophthalmology. 2003;110(3):487–492. doi: 10.1016/S0161-6420(02)01767-0. [DOI] [PubMed] [Google Scholar]
- 24.van Gils JM, Ramkhelawon B, Fernandes L, Stewart MC, Guo L, Seibert T, Menezes GB, Cara DC, Chow C, Kinane TB, Fisher EA, Balcells M, Alvarez-Leite J, Lacy-Hulbert A, Moore KJ. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler Thromb Vasc Biol. 2013;33(5):911–999. doi: 10.1161/ATVBAHA.112.301155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delaire S, Billard C, Tordjman R, Chédotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J. Immunol. 2001;166(7):4348–4354. doi: 10.4049/jimmunol.166.7.4348. [DOI] [PubMed] [Google Scholar]
- 26.Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier-Lavigne M, Yoshida Y, Okuno T, Mizui M, Kang S, Nojima S, Tsujimura T, Nakatsuji Y, Katayama I, Toyofuku T, Kikutani H, Kumanogoh A. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol. 2010;11(7):594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130(4):829–842. doi: 10.1016/j.jaci.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 28.Miyahara S, Miyahara N, Matsubara S, Takeda K, Koya T, Gelfand EW. IL-13 is essential to the late-phase response in allergic rhinitis. J Allergy Clin Immunol. 2006;118(5):1110–1116. doi: 10.1016/j.jaci.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, Storey H, LeCiel C, Harder B, Gross JA. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117(2):418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 30.Liew FY, Pitman NI, Mcinnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 31.Mannon P, Reinisch W. Interleukin 13 and its role in gut defense and inflammation. Gut. 2012;61(12):1765–1773. doi: 10.1136/gutjnl-2012-303461. [DOI] [PubMed] [Google Scholar]
- 32.Oh MH, Oh SY, Yu J, Myers AC, Leonard WJ, Liu YJ, Zhu Z, Zheng T. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186(12):7232–7242. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129(3):742–751. doi: 10.1038/jid.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90(4):753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 35.Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, Roméo PH. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002;3(5):477–482. doi: 10.1038/ni789. [DOI] [PubMed] [Google Scholar]
- 36.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34(3):623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 37.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28(3):402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto M, Suzuki K, Okuno T, Ogata T, Takegahara N, Takamatsu H, Mizui M, Taniguchi M, Chédotal A, Suto F, Fujisawa H, Kumanogoh A, Kikutani H. Plexin-A4 negatively regulates T lymphocyte responses. Int Immunol. 2008;20(3):413–420. doi: 10.1093/intimm/dxn006. [DOI] [PubMed] [Google Scholar]
- 39.Wen H, Lei Y, Eun SY, Ting JP. Plexin-A4-semaphorin 3A signaling is required for Toll-like receptor- and sepsis-induced cytokine storm. J Exp Med. 2010;207(13):2943–2957. doi: 10.1084/jem.20101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherif Z, Pleyer U. Corticosteroids in ophthalmology: past-present-future. Ophthalmologica. 2002;216(5):305–315. doi: 10.1159/000066189. [DOI] [PubMed] [Google Scholar]
- 41.Galletti J, Cañones C, Morande P, Borge M, Oppezzo P, Geffner J, Bezares R, Gamberale R, Giordano M. Chronic lymphocytic leukemia cells bind and present the erythrocyte protein band 3: possible role as initiators of autoimmune hemolytic anemia. J Immunol. 2008;181(5):3674–3683. doi: 10.4049/jimmunol.181.5.3674. [DOI] [PubMed] [Google Scholar]
- 42.De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40(3):619–630. [PubMed] [Google Scholar]
- 43.Epstein SP, Chen D, Asbell PA. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25(5):415–424. doi: 10.1089/jop.2008.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galletti JG, Gabelloni ML, Morande PE, Sabbione F, Vermeulen ME, Trevani AS, Giordano MN. Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol. 2013;6(1):24–34. doi: 10.1038/mi.2012.44. [DOI] [PubMed] [Google Scholar]