Abstract
AIM
To investigate whether the complement system is involved in a murine model of oxygen-induced retinopathy (OIR).
METHODS
Forty C57BL/6J newborn mice were divided randomly into OIR group and control group. OIR was induced by exposing mice to 75%±2% oxygen from postnatal 7d (P7) to P12 and then recovered in room air. For the control group, the litters were raised in room air. At the postnatal 17d (P17), gene expressions of the complement components of the classical pathway (CP), the mannose-binding lectin (MBL) pathway and the alternative pathway (AP) in the retina were determined by quantitative real-time polymerase chain reaction (RT-PCR). Retinal protein expressions of the key components in the CP were examined by Western blotting.
RESULTS
Whole mounted retina in the OIR mice showed area of central hypoperfusion in both superficial and deep layers and neovascular tufts in the periphery. The expressions of C1qb and C4b genes in the OIR retina were significantly higher than those of the controls. The expression of retinal complement factor B (CFB) gene in OIR mice was significantly lower than those of the controls. However, the expressions of C3 and complement factor H (CFH) genes were higher. The protein synthesis of the key components involved in the CP (C1q, C4 and C3) were also significantly higher in OIR mouse retina. Although MBL-associated serine protease 1 (MASP1) and MASP2 were detected in both the OIR and the control groups, the expressions were weak and the difference between the two groups was not significant.
CONCLUSION
Our data suggest that the complement system CP is activated during the pathogenesis of murine model of OIR.
Keywords: oxygen-induced retinopathy, complement activation, classical pathway, retina, mouse
INTRODUCTION
Retinopathy of prematurity (ROP), a retinal vascular disease of premature infants, is a leading cause of childhood visual impairment all over the world[1]. Infants with ROP are at higher risk for developing certain other eye conditions including strabismus, amblyopia, retinal detachment, vitreous hemorrhage, and neovascular glaucoma[2]. However the underlying mechanism of ROP is poorly understood. Clinically, ROP is characterized by two stages: an early phase of vascular injury with obliteration of immature vessels and a second phase of vascular repair[3]. Oxygen-induced retinopathy (OIR) is an established model for ROP in humans. In the murine model, there are two phases in the pathological process. In the first phase, exposure of neonatal animals to high oxygen tension induces cessation of normal blood growth and regression of existing vessels, and subsequently causes obliteration of normal retinal vessels. In the second phase, along with hyperoxia induced vessel loss and subsequent relative hypoxia, overexpression of pro-angiogenic factors including vascular endothelial growth factor (VEGF), angiopoietin, insulin-like growth factor (IGF)-1 causes retinal neovascularization, which leads to alterations in the existing vasculature and pathological new capillary growth[4]. This animal model reproduces the two phases of ROP-initial vaso-obliteration, and subsequent neovascularization-along with some complications like vascular leakage[3]. A large number of studies have employed the murine OIR model to investigate of the angiogenic mechanisms underlying the ROP in vivo[5]–[7].
The complement system has been recognized as a key link in innate immunity. It consists of plentiful plasma and membrane bound proteins that play a vital role in defensing against infection and in modulating of immune and inflammatory responses[8]. There is growing evidence that the complement system is more than a mediator of innate immunity. Recent work has demonstrated that inappropriate activation of the complement system has been involved in neovascular ocular diseases including age-related macular degeneration (AMD) and diabetic retinopathy (DR)[9]–[11]. Previous study indicated the complement component complement factor H (CFH), C3a and C5a were involved in laser-induced choroidal neovascularization (CNV), a model of neovascular AMD[9],[12],[13]. On the other hand inhibition of complement has been demonstrated to be protective against laser-induced CNV[14],[15]. It was shown that complement factor H and factor B polymorphisms have been closely associated with a higher risk of developing AMD[16]–[18]. Increasing evidence from in vitro and clinical studies suggested a proangiogenic role of the complement system in the development of pathological neovascularization in DR[10],[11].
ROP is a retinal vascular disease of premature infants. The hallmark of ROP is exuberant neovascularization triggered by retina ischemia/hypoxia, which is the result of retina vessel regression[19],[20]. Although neovascularization is associated with the complement system, whether the complement system is involved in the pathogenesis of retinal vascular disease is still unclear. To document whether complement system is associated with the pathogenesis of retinal neovascularization in the animal model of OIR, we detected the expressions of the key components of the complement system in the retina.
MATERIALS AND METHODS
Animals
C57BL/6J mice were purchased from Laboratory Animal Center of Chongqing Medical University (Chongqing, China). Mice were housed and exposed to a 12h: 12h light-dark cycle. All animal procedures performed in this study complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Chongqing Medical University (ID#2011-22). Every effort was made to minimize animal discomfort.
Induction of Murine Model of Oxygen-induced Retinopathy
We made the OIR model followed a procedure described by Smith et al[4]. There were 20 mice in each group. Briefly, litters of newborn mice were randomly divided into two groups after birth. For the OIR group seven-day-old (P7) C57BL/6J mice were exposed to 75%±2% oxygen for 5d with the nursing mothers and then returned to room air for 5d. For the control group, the litters were raised in room air.
Angiography with High-molecular-weight Fluorescein-dextran
Retinal neovascularization were examined at P17. Three mice randomly selected from each group were deeply anesthetized by intraperitoneal pentobarbital sodium injection and sacrificed by intracardiac perfusion with phosphate buffer saline (PBS) containing 1 mL of 50 mg/mL FITC-dextran (molecular weight 2 000 000, Sigma-Aldrich Chemical Co. St Louis, MO, USA). The binoculus from each mouse were enucleated and fixed in 4% paraformaldehyde for 10min at room temperature. The retinas were dissected and mounted on microscope slides[21]. Images of mounted retinas were taken with 5 times magnifications with a fluorescence microscope (Leica, Bannockburn, IL, USA).
Retinal Sample Collection
All animals were euthanatized by an overdose of pentobarbital sodium followed by cervical dislocation. The binoculus from each mouse were enucleated and carefully dissected under a surgical microscope. The anterior segment of the eye including the cornea, the iris, and the lens were removed. Retinas were separated from RPE/choroid complex. Retinal samples were stored at -80°C for further RNA or protein extraction.
Real-time Quantitative Polymerase Chain Reaction Analysis
Total retinal RNA was extracted from tissues using the TRIzol (Invitrogen, Paisley, UK) following the manufacturer's instructions[22]. RNA concentrations were measured with a Nano instrument (NanoDrop Technologies, Wilmington, DE, USA). Complementary DNA (cDNA) was generated using the PrimeScript® RT reagent Kit (Takara Biotechnology, Dalian, China) following the manufacturer's instructions. Real-time quantitative polymerase chain reaction (PCR) was performed on a Bio-Rad c-1000 (Bio-Rad Laboratories, Hercules, CA, USA) using primers specific for mice C1qb: Primer ID (Mm-QRP-20447), mice C4b: Primer ID (Mm-QRP-20445), mice complement factor B (CFB): Primer ID (Mm-QRP-20442), mice CFH: Primer ID (Mm-QRP-20446), mice MBL-associated serine protease 1 (MASP1): Primer ID (Mm-QRP-20493), mice MASP2: Primer ID (Mm-QRP-20492), mice C3: Primer ID (Mm-QRP-20444) and standardized to glyceraldehyde-3-phosphatedehydrogenase (GAPDH) (forward, 5′-ATGGTGAAGGTCGGTGTGAAC-3′; reverse, 5′-TTACTCCTTGGAAG-3′) (GeneCopoeia Inc., Rockville, MD, USA). The specificity of all the primers was verified by GeneCopoeia Inc. Samples underwent 40 cycles of amplification in a volume of 20 µL using the all-in-one qPCR Mix (GeneCopoeia Inc.). The conditions were 95°C for 10min, followed by 40 cycles of 10s at 95°C, 20s at 60°C and 15s at 72°C. Fluorescence data were acquired at 72-95°C to decrease the amount of nonspecific signal and amplification of specific transcripts was confirmed by melting curve profiles at the end of each PCR. Data from each sample were obtained in triplicate. The relative amount of target mRNA was calculated from the obtained ΔCt values for target and endogenous reference gene GAPDH by using 2-ΔΔCt cycle threshold method.
Western Blotting Analysis
Total retinal proteins extracted from tissues were homogenized and solubilized in ice-cold PBS containing protease inhibitors and detergent NP-40[22]. Total protein concentration was determined by a bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Haimen, Jiangsu Province, China). Electrophoresis was performed on 7.5% SDS-PAGE slab gel (Goodbio Techology CO., LTD, Wuhan, Hubei Province, China) and the separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The blots were blocked with 5% non-fat dry milk in TBST for 60min at room temperature. Anti-C1q (ab64632), anti-C4 (ab11863) and anti-C3 (ab11887) (Abcom Biotechnology, Cambridge, MA, USA) were used as the primary antibodies and the blots were incubated overnight at 4°C. The membrane was incubated with HRP conjugated secondary antibody for 1h at room temperature (Abcom Biotechnology, Cambridge, MA, USA). Following secondary antibody incubation, all the membranes were washed for 30min, incubated for 5min with an enhanced chemiluminescence kit (Amersham, Pittsburgh, PA, USA), and exposed to Hyperfilm enhanced chemiluminescence film (Amersham, Pittsburgh, PA, USA). Densitometric analysis was performed with the Image J software (Version1.43, Broken Symmetry Software Inc., MD, USA).
Statistical Analysis
Statistical analysis was undertaken using a SPSS software (Version 17.0, SPSS Inc., Chicago, IL, USA). Unpaired student's t-test was used to assess significance between the two groups. P<0.05 was regarded as statistically significant.
RESULTS
The OIR model is considered having two pathological stages which mimic the two phases of ROP in human. In the first stage, exposure to hyperoxia results in ceaseing growing and regression of the developing retinal capillaries. In the second stage, returning to room air causes induction of pro-angiogenic growth factors that trigger retinal neovascularization[4]. Figure 1 showed representative mouse retinal flat mounts of an OIR and a control eye at P17. The normal mouse retina presented both superficial and deep vascular layers. The vessels extended from the optic nerve to the periphery and formed a radial branching pattern in the superficial layer and a polygonal reticular pattern in the deep layer (Figure 1A). The OIR mouse retina displayed an area of central hypoperfusion in both superficial and deep layers and neovascular tufts in the periphery (Figure 1B).
Figure 1. Representative retinal flat mounts of an OIR and a control mouse eye perfused with high-molecular-weight fluorescein-dextran. The photographs were taken with a fluorescence microscope at 5 times magnification at P17.
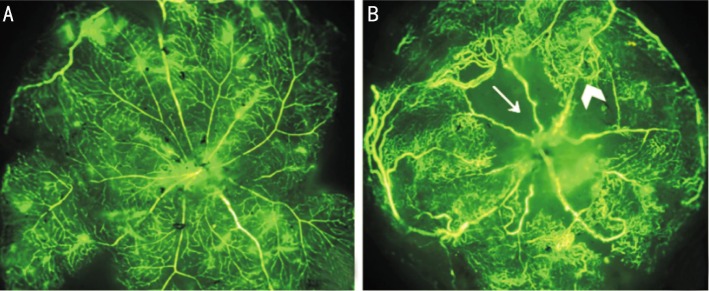
A: Room air raised mouse exhibited normal retinal vascular architecture. The vessels formed a radial branching pattern in the superficial layer and a polygonal reticular pattern in the deep layer. B: Mouse exposed to hyperoxia showed reduced vascular development, which leads to the development of neovascularization. Arrow indicates area of central hypoperfusion in both the superficial and the deep layers. Arrow head indicates peripheral neovascular tufts.
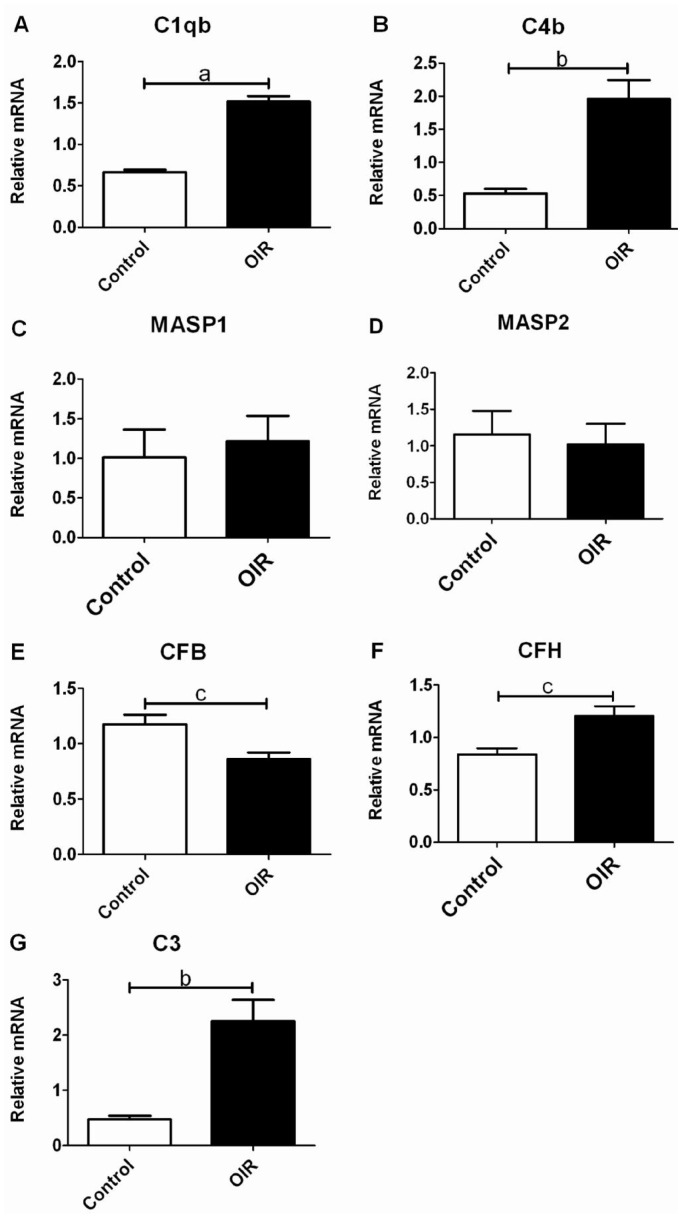
To determine whether the complement components in the OIR mouse retina were activated, we performed real-time PCR to quantitate mRNA levels of the major complement genes in the retina. We found in the OIR mouse retina the expressions of C1qb and C4b genes, two components of the classical pathway (CP), were significantly higher than those of the controls (P<0.001, P<0.01; Figure 2A, 2B). Weak expressions of MASP1 and MASP2 genes, specific components of the mannose-binding lectin (MBL) pathway, were detected in both the OIR and the control groups, and no significant was seen between the two groups (P>0.05; Figure 2C, 2D). The expression of CFB, a component in the alternative pathway (AP) was significantly lower in the OIR mouse retina (P<0.05; Figure 2E). However, the expression of CFH, another component in the AP, was significantly higher than those of the controls (P<0.05; Figure 2F). In the OIR mouse retina the expression of C3, a central component in all the three pathways, was significantly higher than those of the controls (P<0.01; Figure 2G). Real-time PCR results suggested that the CP was activated in the retina of OIR mice. While the AP and MBL pathways might play a minor role.
Figure 2. The expressions of the major complement system components mRNA in the OIR mouse retina determined by quantitative RT-PCR. Gene expressions of selected complement components of CP, MBL pathway and AP in the retina of the OIR and the control mice were shown.
A&B: mRNA levels for complement components C1qb and C4b mainly in the CP were significantly higher in the OIR mouse retina (n=3); C&D: mRNA levels for complement components MASP1 and MASP2 specifically in the MBL pathway were comparable between the two groups (n=3); E&F: mRNA levels for complement components CFB and CFH in the AP. The expression of CFB was significantly lower, while the expression of CFH was significantly higher than those of the controls (n=3); G: In the terminal pathway, the expression of C3 in the OIR mouse retina was significantly higher than those of the controls (n=3; Bars represent means±SEM; aP<0.001, bP<0.01, cP<0.05).
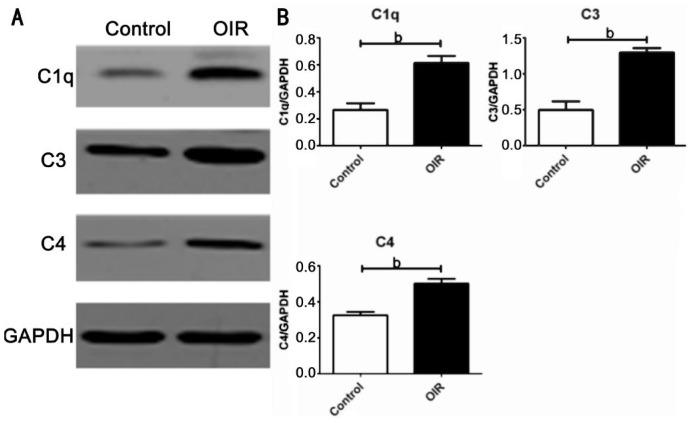
According to the results of real-time PCR quantitation of mRNA levels for complement genes, we determined the protein levels of the key components of the complement system involved in the CP (C1q, C4 and C3) by Western blotting. We confirmed the retinal C1q, C4 and C3 protein levels in the OIR mice were significantly higher (P<0.01, P<0.01 and P<0.01; Figure 3B).
Figure 3. Retinal protein expressions of the key components of complement system mainly involved in the CP in the OIR and the control groups.
A: Retinal C1q, C4 and C3 protein levels in the OIR and the control groups were determined by Western blotting (n=6). B: Densitometry analysis of the C1q, C4 and C3 protein levels in the OIR mouse retina were significantly higher than those of the control mice (Bars represent means±SEM; bP<0.01).
DISCUSSION
As a key component of innate immunity, the complement system plays a vital role in defensing against infection and in modulating the immune and inflammatory responses[8]. In recent years, increasing evidence indicated that the complement system was implicated in neovascular ocular diseases[9]–[13]. OIR is an established model for ROP in humans. We studied the expressions of several key components of the complement system in the retina of a mouse OIR model to determine if the complement system is associated with the pathogenesis of this neovasculazation condition.
Involvement of Complement System in Oxygen-induced Retinopathy
The complement system activated via distinct pathways triggers a sequence of biological reactions. The CP involving C1, C2 and C4 can be activated by immune complexes or by substances such as C-reactive protein. C1 is composed of C1q, C1r and C1s three-subunits and is the start of the CP. C1q is the main function domain[23]. We demonstrated that C1q gene was locally synthesized and increased in the OIR mouse retina, which suggests that activation of the CP may be associated with OIR.
In relation to the MBL pathway, its activation is independent of immune complex generation and involves C4, C2, MASP1 and MASP2. C4 is a component of both the CP and the MBL pathways, while MASP1 and MASP2 are the specific components of the MBL pathway[24]. We found the expression of C4b gene was significantly higher, while very low levels of MASP1 and MASP2 gene expressions were detected in the retina in both the OIR and the control groups. The different profiles between C4, MASP1 and MASP2 expressions suggest that activation of the complement MBL pathway may be less important in OIR. The higher expression of C4 may be a consequence of activation of the CP. Thus our data imply that activation of the MBL pathway may be less important in OIR, since MASP1 and MASP2 expressions were at similar low levels between the two groups.
The AP provides a rapid, antibody-independent route of complement activation and amplification and involves C3, Factor B, Factor D and Factor H[24]. Factor B is the key component of the AP. As an inhibitory complement regulatory protein of the AP, CFH could inhibit C3 activation by binding to C3b and also has decay-accelerating activity for the AP C3 convertase, C3bBb. It also competes with factor B for surface-bound C3b[8],[24]. And the AP is initiated by the slow hydrolysis of circulating C3. Meanwhile C3 is also central to the function of all three complement activation pathways[23]. We found the expression of C3 gene was significantly higher in the OIR mouse retina, while the expression of CFB inhibited by elevated CFH was significantly lower. The different profiles between C3 and CFB expressions suggest that activation of complement AP may not be involved in OIR. The higher expression of C3 may be the downstream reaction of the activated CP.
At protein level, we confirmed the retinal C1q, C4 and C3 in the OIR mice were significantly higher, which is in agreement with our suggestion that the CP is involved in the pathogenesis in the animal model of OIR.
Role of Complement System in Oxygen-induced Retinopathy
The complement system has been involved in various neovascular ocular diseases. However it has been suggested that complement could play an opposite role in the pathological neovascularization in the process of CNV and OIR[25]. Firstly, activation of the complement system could mediate induction of the proangiogenic VEGF in retina pigment epithelial cells and play a proangiogenic actions in models of CNV[13], [14]. On the other hand, a recent study showed that C3-deficient and C5aR-deficient mice showed increased pathological retinal angiogenesis in OIR. The complement system may play a negative regulator role in pathological neovascularization in OIR[26]. It is interesting that in our study, the CP of the complement system in OIR model is activatied. It has been reported that activation of the complement system could polarized macrophages to a M1 antiangiogenic phenotype in vitro by inducing secretion of sVEGFR1, an established angiogenesis inhibitor[27]. Given the antiangiogenic roles of the complement system in pathological neovascularization in OIR, elevation of the complement conponents in the OIR mouse retina might represent a mechanism of compensation, which inhibits pathological retina angiogenesis[26]. While the function of activation of the complement system as a counterplayer of pathological retina angiogenesis might be overridden by dorminant proangiogenic factors in the pathologenesis process of OIR. Nevertheless, the details of the underlying mechanisms deserve further study.
A more recent study showed that CFB-deficient mice resulted in increased pathological retinal angiogenesis[28]. The data implicated the alternative complement pathway in facilitating neovessel clearance, which appears inconsistent with our results. It should be noticed that knock-out mice were used in this study to reveal the role of the complement system in OIR, which is an ideal status. However, considering the complexity of the complement system, we believe our data collected from otherwise normal mice are more reasonable to represent the disease condition. On the other hand, our data don't completely deny the possible role of the alternative complement pathway in the pathophysiology of OIR.
In conclusion, our findings suggest that activation of the CP may have implications in the pathophysiology of the murine model of OIR. Activation of the complement system might play a compensatory role in OIR. However, the detailed mechanisms remain unclear.
Acknowledgments
This work was supported by Chongqing Key Laboratory of Ophthalmology (CSTC), and National Key Clinical Specialties Construction Program of China.
Foundations: Supported partially by National Natural Science Foundation of China (No.81271033, 81470621)
Conflicts of Interest: Tao XY, None; Zheng SJ, None; Lei B, None.
REFERENCES
- 1.Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382(9902):1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sapieha P, Joyal JS, Rivera JC, Kermorvant-Duchemin E, Sennlaub F, Hardy P, Lachapelle P, Chemtob S. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest. 2010;120(9):3022–3032. doi: 10.1172/JCI42142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Lofqvist C, Hellstrom A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51(6):2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 5.Maretzky T, Blobel CP, Guaiquil VH. Characterization of oxygen-induced retinopathy in mice carrying an inactivating point mutation in the catalytic site of ADAM15. Invest Ophthalmol Vis Sci. 2014;55(10):6774–6782. doi: 10.1167/iovs.14-14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Wang G, Kunte M, Shinde V, Gorbatyuk M. Modulation of angiogenesis by genetic manipulation of ATF4 in mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2013;54(9):5995–6002. doi: 10.1167/iovs.13-12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vavilala DT, O'Bryhim BE, Ponnaluri VK, White RS, Radel J, Symons RC, Mukherji M. Honokiol inhibits pathological retinal neovascularization in oxygen-induced retinopathy mouse model. Biochem Biophys Res Commun. 2013;438(4):697–702. doi: 10.1016/j.bbrc.2013.07.118. [DOI] [PubMed] [Google Scholar]
- 8.Bora NS, Jha P, Bora PS. The role of complement in ocular pathology. Semin Immunopathol. 2008;30(2):85–95. doi: 10.1007/s00281-008-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivaprasad S, Chong NV. The complement system and age-related macular degeneration. Eye (Lond) 2006;20(8):867–872. doi: 10.1038/sj.eye.6702176. [DOI] [PubMed] [Google Scholar]
- 10.Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008;7(6):2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Yang MM, Li YB, Liu GD, Teng Y, Liu XM. Association of CFH and CFB gene polymorphisms with retinopathy in type 2 diabetic patients. Mediators Inflamm. 2013;2013:748435. doi: 10.1155/2013/748435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kijlstra A, La Heij E, Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul Immunol Inflamm. 2005;13(1):3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 13.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103(7):2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bora NS, Kaliappan S, Jha P, Xu Q, Sivasankar B, Harris CL, Morgan BP, Bora PS. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol. 2007;178(3):1783–1790. doi: 10.4049/jimmunol.178.3.1783. [DOI] [PubMed] [Google Scholar]
- 15.Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, Kaliappan S, Kaplan HJ, Bora NS. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174(1):491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- 16.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 18.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 19.Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83(3):473–483. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Tian XF, Rong JB, Liu D, Yi GG, Tan Q. Protein kinase B (akt) promotes pathological angiogenesis in murine model of oxygen-induced retinopathy. Acta Histochem Cytochem. 2011;44(2):103–111. doi: 10.1267/ahc.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin T, Qiu Y, Liu Y, Mohan R, Li Q, Lei B. Expression of adiponectin and its receptors in type 1 diabetes mellitus in human and mouse retinas. Mol Vis. 2013;19:1769–1778. [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov. 2010;9(1):43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- 24.Jha P, Bora PS, Bora NS. The role of complement system in ocular diseases including uveitis and macular degeneration. Mol Immunol. 2007;44(16):3901–3908. doi: 10.1016/j.molimm.2007.06.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanai R, Thanos A, Connor KM. Complement involvement in neovascular ocular diseases. Adv Exp Med Biol. 2012;946:161–183. doi: 10.1007/978-1-4614-0106-3_10. [DOI] [PubMed] [Google Scholar]
- 26.Langer HF, Chung KJ, Orlova VV, Choi EY, Kaul S, Kruhlak MJ, Alatsatianos M, DeAngelis RA, Roche PA, Magotti P, Li X, Economopoulou M, Rafail S, Lambris JD, Chavakis T. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116(22):4395–4403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203(9):2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweigard JH, Yanai R, Gaissert P, Saint-Geniez M, Kataoka K, Thanos A, Stahl GL, Lambris JD, Connor KM. The alternative complement pathway regulates pathological angiogenesis in the retina. FASEB J. 2014;28(7):3171–3182. doi: 10.1096/fj.14-251041. [DOI] [PMC free article] [PubMed] [Google Scholar]