Abstract
AIM
To evaluate the early expression of mannose-binding lectin 2 (MBL2) in human corneal epithelial cells (HCECs) infected by Aspergillus fumigatus (AF).
METHODS
HCECs cultured in vitro with AF antigens and sampled at 0, 0.5, 1, 2, 4, 6 and 8h. The expression of MBL2 mRNA was evaluated by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR). The expression of MBL2 protein in supernatant fluid was shown by enzyme linked immunosorbent assay (ELISA). MBL2 protein in HCECs was detected by immunocytochemistry at 0 and 24h.
RESULTS
MBL2 mRNA and protein are expressed in normal HCECs. The expression of MBL2 mRNA and protein in supernatant fluid begin to increase after being stimulated with AF antigens. The most significantly peak of MBL2 mRNA is in 2h. The protein of MBL2 in supernatant fluid decrease gradually after 0.5h. The protein in HCECs expression increase after stimulation of 24h.
CONCLUSION
MBL2 receptor expressed in normal HCECs in vitro. The stimulation by AF antigens can increase the early expression of it.
Keywords: mannose-binding lectin, human corneal epithelial cells, Aspergillus fumigatus
INTRODUCTION
In recent years, the importance of innate immune increase in the study of immune mechanisms of fungal infection[1]–[5]. The innate immune system which mainly through pattern recognition receptors (PRRs) recognize highly conserved structure of pathogenic microorganism called pathogen associated molecular pattern (PAMPs) is the first line to resist fungal antigens for cornea[6]–[9]. Mannose-binding lectin 2 (MBL2) is a kind of plasma protein synthesized by the liver and secreted into the serum. It is a family members of the collectin which belongs to C-type lectin superfamily members[10]. As soluble mediator, MBL2 make an important contribution to innate immune protection and work along with epithelial barriers, cellular defenses such as phagocytosis, and pattern-recognition receptors that trigger pro-inflammatory signaling cascades[11]. But up to now the expression of MBL2 in human corneal epithelial cells (HCECs) is still unknown. We also try to get the roles of MBL2 when the HCECs recognize the pathogenic fungi. Based on these, we investigated the early expression of MBL2 in HCECs infected by Aspergillus fumigatus (AF) antigens in order to evaluate the relationship between MBL2 and antifungal function in HCECs.
SUBJECTS AND METHODS
Subjects
AF strains (No.3.0772) was bought from China General Microbiological Culture Collection Center. High glucose medium, newborn calf serum and trypsin were from American HyClone products. Sabouroud culture was purchased from American Sigma company. Trizol Reagent was from American Invitrogen products. Polymerase chain reaction (PCR) primers and marker were from Dalian Takara products. MBL2 enzyme linked immunosorbent assay (ELISA) kits was from American R&D products. Immune cell chemistry SP kit and MBL2 antibodies were from Beijing Biosynthesis company products.
Aspergillus Fumigatus Antigens
AF grew in Sabouroud medium, 28°C for 5d; Physiological saline flushed the fungi surface; Collected the fluid; 3000 r/min centrifugal 5min; Inactivated 30min in 70% alcohol; Washed three times by phosphate buffered saline (PBS)[12]. The above antigenic stimulation liquid was saved in -20°C and should be used in 2wk.
Human Corneal Epithelial Cells Culture
HCECs were cultured in high glucose medium, 37°C, 5%CO2. Near 80% confluence, the cells were cultured in serum free Dulbecco's modified Eagle's medium (DMEM) for 24h. Cells were used for semiquantitative reverse transcription- PCR (RT-PCR) and immunocytochemistry. The supernatant fluid was used for ELISA.
Stimulation of Aspergillus Fumigatus Antigens
HCECs were cultured with AF antigenic stimulation liquid after discarding the medium. The expression of MBL2 mRNA in HCECs were detected by RT-PCR at the stimulation of 0, 0.5, 1, 2, 4, 6 and 8h. The expression of MBL2 protein in supernatant fluid was shown by ELISA at same time points. MBL2 protein in HCECs was detected by immunocytochemistry at 0 and 24h.
Reverse Tanscription-polymerase Chain Reaction
For conventional RT-PCR, liver tissue samples (n=6) and HCECs were crushed in an agate mortar under liquid nitrogen, and then homogenized in 5 mL Trizol. Insoluble material was removed by centrifugation (12 000 g, 5min, 4°C). Total RNA was isolated by RNA purification. Contamination of the purified RNA by genomic DNA was prevented by performing PCR with the specific primers for MBL2 and β-actin (Table 1). The primers were synthetized by Sangon from Shanghai. After 5min of heat denaturation at 96°C, the PCR cycle was conducted at 96°C for 60s, 57°C (MBL2) for 60s each, and 72°C for 60s. Thirty-five cycles were performed with each primer pair. The final elongation cycle consisted of 72°C for 4min. Six micro liters PCR was loaded on a 2% agarose gel. After electrophoresis, the amplified products were visualized by fluorescence. Base pair (bp) values were compared with GenBank data. For verification and comparison, human liver tissues carrying the genes for the investigated protein were used as a reference. PCR products were also confirmed by sequencing. To estimate the amount of amplified PCR product, we performed β-actin PCR with specific primers for each investigated tissue. For this additional PCR, we used the conditions described.
Table 1. Primers and Productions sizes.
| Gene | Primer sequence | Product size (bp) |
| MBL2 | F: GGAGCCATTCAGAATCTCATC | 389 |
| R: TGCTTTGTTGGTGCTGTTAGT | ||
| β-actin | F: TGACGTGGACATCCGCAAAG | 205 |
| R: CTGGAAGGTGGACAGCGAGG |
Enzyme Linked Immunosorbent Assay
We detected the protein concentration of MBL2 with ELISA kit. We input the standard product spectrophotometry (A) value and concentration in Excel, forming fitting curve, then we could get the concentrations of generation.
Immunocytochemistry
Immunocytochemistry was performed by usage of the streptavidin-peroxidase method. HCECs were cultured on slides. Near 80% confluence, cells were exposed to AF antigenic stimulation liquid at 0 and 24h. Then fixed in 4% paraformaldehyde for 30min and distilled water for 3 times. After getting rid of endogenous peroxidase with 3% hydrogen peroxide for 5-10min, cells reacted with goat confining liquid at room temperature for 10-15min. After that, cells reacted with MBL2 antibody (rabbit polyclonal antibody, dilution of 1:300) at 4°C overnight, then with a biotin-conjugated anti-rabbit secondary antibody at 37°C for 10-15min, and followed by a peroxidase-conjugated streptavidin for 10-15min. Cells were developed with diaminobencidine. The developing time was controlled according to the scene under microscope. Then cells were counterstained with hematoxylin for 1min, dehydrated, and finalized with neutral balsam. Negative control cells were performed using PBS instead of primary antibodies.
Statistical Analysis
All data were presented as mean±SD. The data were analyzed using SPSS 17.0. The differences were analyzed by variance analysis or t-test. P<0.05 was considered as statistically significant.
RESULTS
Expression of Mannose-binding Lectin 2 mRNA
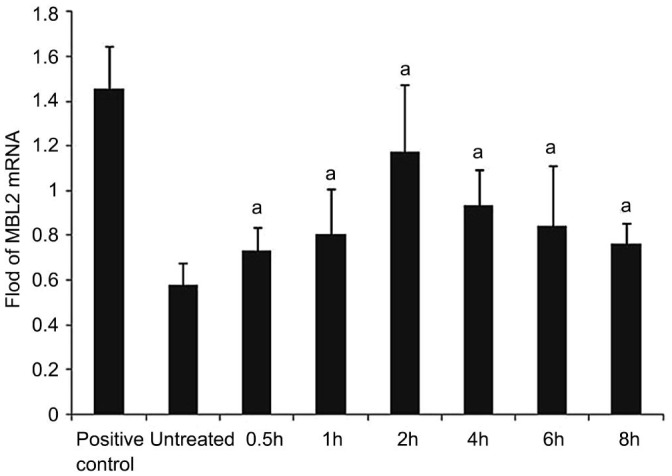
MBL2 specific cDNA amplification products (389 bp) were detected in all groups (n=6) for HCECs (Figure 1). The β-actin control PCR was positive and of similar amount for all investigated groups, as expected, and it allowed a quantitative correlation of the amplified product. Bp values were equivalent to the expected DNA products compared with GenBank data (Figure 2).
Figure 1. RT-PCR results of MBL2.
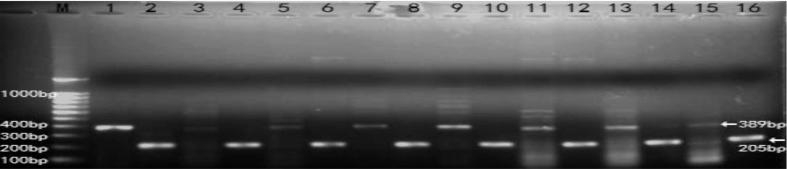
Ethidium bromide–stained agarose gels for visualization of PCR amplification products derived from the following groups (n=6 for each group): human liver tissue samples (1), untreated THCEs (3), human β-actin (2, 4, 6, 8, 10, 12, 14, 16), AF antigens stimulated 0.5h groups (5), AF antigens stimulated 1h groups (7), AF antigens stimulated 2h groups (9), AF antigens stimulated 4h groups (11), AF antigens stimulated 6h groups (13), AF antigens stimulated 8h groups (15). PCR amplification products of the human liver tissue were used for positive control. To estimate quantitative correlation, a β-actin PCR control was performed for investigated tissue. In accordance with the DNA marker (M) and the human liver tissue products, the distinct DNA bands are visible at 389 bp for MBL2.
Figure 2. MBL2 mRNA.
The cultured HCECs were incubated with AF antigens stimulation liquid for 0.5, 1, 2, 4, 6 and 8h. The mRNA expression of MBL2 in these cells was evaluated by RT-PCR, with untreated cells as control. We found that the mRNA expression of MBL2 was largely increased after treated with AF antigens. Results shown are mean±SD of six independent experiments. aP<0.05.
Enzyme Linked Immunosorbent Assay Analysis
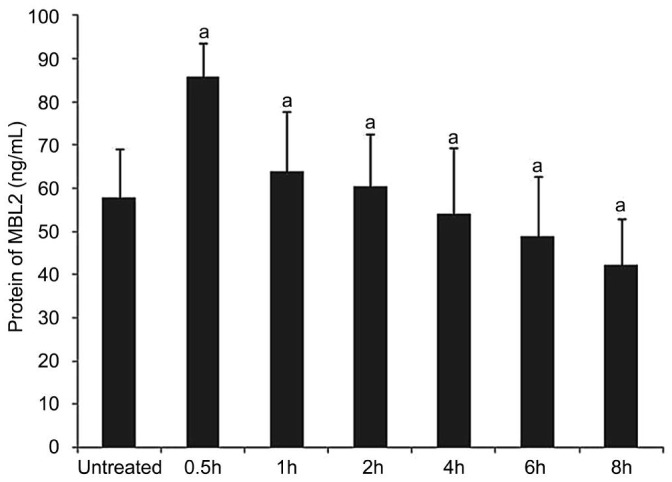
The protein of MBL2 in supernatant fluid decreased gradually after 0.5h (Figure 3).
Figure 3. MBL2 protein levels in supernatant fluid.
The expression of MBL2 protein in supernatant fluid was shown by ELISA at the stimulation of 0, 0.5, 1, 2, 4, 6 and 8h. aP<0.05.
Immunocytochemistry
HCECs were cultured on slides. Immuocytochemical staining showed that positive MBL2 expression of brown particles located in the cytoplasm (Figure 4). MBL2 protein was lowly produced by the normal HCECs. Stronger staining throughout the cytoplasm was observed in the cells exposed to AF antigens for 24h.
Figure 4. MBL2 expression in cultured HCECs evaluated by immunocytochemical staining(×200).
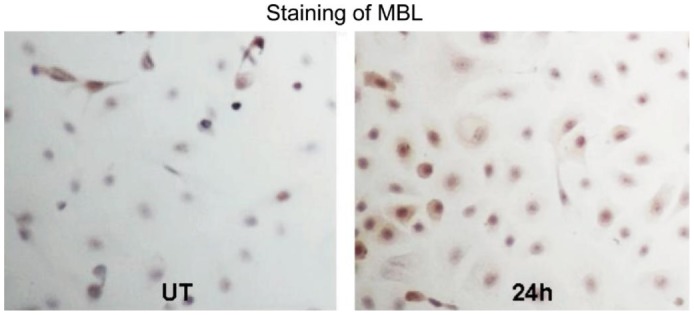
The cultured HCECs incubated with AF antigens stimulation liquid for 0 and 24h. MBL2 protein was lowly produced by the normal HCECs. Stronger staining was observed in the cells exposed to AF antigens for 24h.
DISCUSSION
MBL2 is a pattern recognition molecule of the innate immune system. It belongs to the collectin family of proteins in which lectin (carbohydrate-recognition) domains are found in association with collagenous structures[13]. MBL2 binds to a range of sugars including N-acetyl-D-glucosamine, mannose, N-acetyl-mannosamine, fucose and glucose. This permits the protein to interact with a wide selection of viruses, bacteria, yeasts, fungi and protozoa decorated with such sugars. MBL2 bound to microbial surfaces is able to activate the complement system in an antibody and C1-independent manner. MBL2 can also interact directly with cell surface receptors and thereby promote opsonophagocytosis by a complement-independent pathway. MBL2 plays an important role in the first hour/day of any primary immune response to a sugar decorated pathogen. This provides the host with a first-line of defence before the adaptive immune system becomes operative and in humans. Many studies have shown that deficiency of MBL2 increases the overall susceptibility of an individual to infectious disease[14],[15]. Increasingly, there is evidence that the association between MBL2 levels and disease is complex.
Up to now the relationship between MBL2 and fungal keratitis is still unknown. Only Wang et al[16] found with microarray that MBL2-A is among the primary responding genes during the onset of mouse fungal keratitis. Corneal epithelial cells are the first barrier of the cornea to resist the invasion of external pathogenic microorganisms[17]. Fungi can adhere to the basement membrane of the corneal epithelium with adhesin after corneal epithelial cells are damaged. Therefore, the study of the role of MBL2 in fungal keratitis innate immune stage primarily need to detect the relationship between MBL2 and corneal epithelial cells. We evaluated the early expression of MBL2 in HCECs infected by AF. The results indicate that MBL2 is normal structural protein in HCECs and exist in the cell culture supernatant fluid as exocrine PRRs. The expression of MBL2 mRNA and protein in supernatant fluid begin to increase after being stimulated with AF antigens. Then the new MBL2 protein is translated in HCECs. The results suggest that MBL2 involve in the innate immune stage of fungal keratitis as PRRs. Further, the amount of MBL2 protein in supernatant fluid increase in preliminary stage of stimulation, and gradually decline. The results suggest MBL2 protein is excreted into the extracellular space after the stimulation and is consumed gradually. It further indicate that MBL2 participate in the anti-fungal immune response as exocrine PRRs.
Our experimental results confirm although not the traditional immune cells HCECs participate in the anti-fungal immune response with MBL2. Similar to our experimental results, a growing number of studies found that MBL2 exist and play a very important role in non-traditional immune cells. Bulla et al[18] shown that MBL2 detected in cervico-vaginal lavage derives both from plasma as result of transudation and from local synthesis by vaginal epithelial cells and its level is progesterone dependent increasing in the secretive phase of the menstrual cycle. MBL2 has been detected in the cervico-vaginal cavity where it can provide a first-line defence against infectious agents colonizing the lower tract of the reproductive system. Swierzko et al[19] shown that MBL2-specific mRNA expression was detected in several normal and malignant ovarian tissues, as well as in ovarian epithelial cell lines. MBL2 is an important factor of innate immunity contributing to the clearance of microorganisms and play an antitumourigenic role. Reiber et al[20] shown that MBL2 in cerebrospinal fluid is predominantly brain-derived and all results pointed to the leptomeningeal cells as the source of the protein. This recognition of MBL2 in brain cells opens a new field of discussion about the function of the innate immune response in central nervus system in cases of acute and chronic neurological diseases.
At present, more and more research confirmed MBL2 play an important role in the immune response to fungal infection. Inactivated AF antigen used in this study can not entirely imitate the biological characteristics of viable AF, but our results show MBL2 expressed by HCECs participate in the anti-fungal immune response at least. The related functional studies will reveal the idiographic role of MBL2 in resistance to fungal infection in innate immune stage as exocrine PRRs.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81170825; No.81300730)
Conflicts of Interest: Che CY, None; Zhang JF, None; Lee JE, None; Lin J, None; Hu LT, None; Jiang N, None; Wang Q, None; Xu Q, None; Zhao GQ, None.
REFERENCES
- 1.Che CY, Li XJ, Jia WY, Li N, Xu Q, Lin J, Wang Q, Jiang N, Hu LT, Zhao GQ. Early expression of surfactant proteins D in Fusarium solani infected rat cornea. Int J Ophthalmol. 2012;5(3):297–300. doi: 10.3980/j.issn.2222-3959.2012.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biegańska MJ. Two fundamentals of mammalian defense in fungal infections: endothermy and innate antifungal immunity. Pol J Vet Sci. 2014;17(3):555–567. doi: 10.2478/pjvs-2014-0084. [DOI] [PubMed] [Google Scholar]
- 4.Che CY, Li C, Gao A, Lin J, Zhang LL, Xu Q, Wang Q, Zhao GQ. Dectin-1 expression at early period of Aspergillus fumigatus infection in rat's corneal epithelium. Int J Ophthalmol. 2013;6(1):30–33. doi: 10.3980/j.issn.2222-3959.2013.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leal SM, Jr, Vareechon C, Cowden S, Cobb BA, Latgé JP, Momany M, Pearlman E. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest. 2012;122(7):2482–2498. doi: 10.1172/JCI63239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Zhao GQ. Mechanism of the immune response to keratomycosis. Zhonghua Yan Ke Za Zhi. 2011;47(4):378–381. [PubMed] [Google Scholar]
- 7.Leal SM, Jr, Pearlman E. The role of cytokines and pathogen recognition molecules in fungal keratitis - Insights from human disease and animal models. Cytokine. 2012;58(1):107–111. doi: 10.1016/j.cyto.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaloye J, Calandra T. Host innate immune responses to microbial pathogens. Curr Vasc Pharmacol. 2013;11(2):123–132. [PubMed] [Google Scholar]
- 9.Carrion Sde J, Leal SM, Jr, Ghannoum MA, Aimanianda V, Latgé JP, Pearlman E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J Immunol. 2013;191(5):2581–2588. doi: 10.4049/jimmunol.1300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang WC, White MR, Moyo P, McClear S, Thiel S, Hartshorn KL, Takahashi K. Lack of the pattern recognition molecule mannose-binding lectin increases susceptibility to influenza A virus infection. BMC Immunol. 2010;11:64. doi: 10.1186/1471-2172-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230(1):9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 12.Che CY, Jia WY, Xu Q, Li N, Hu LT, Jiang N, Lin J, Wang Q, Zhao GQ. The roles of surfactant protein D during Aspergillus fumigatus infection in human corneal epithelial cells. Int J Ophthalmol. 2012;5(1):13–17. doi: 10.3980/j.issn.2222-3959.2012.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner MW. The role of mannose-blinding lectin in heath and disease. Mol Immunol. 2003;40(7):423–429. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 14.Fidler KJ, Hilliard TN, Bush A, Johnson M, Geddes DM, Turner MW, Alton EW, Klein NJ, Davies JC. Mannose-binding lectin is present in the infected airway: a possible pulmonary defence mechanism. Thorax. 2009;64(2):150–155. doi: 10.1136/thx.2008.100073. [DOI] [PubMed] [Google Scholar]
- 15.Ampel NM, Dionne SO, Giblin A, Podany AB, Galgiani J. Mannose-binding lectin serum levels are low in persons with clinically active coccidioidomycosis. Mycopathologia. 2009;167(4):173–180. doi: 10.1007/s11046-008-9172-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Liu T, Gong H, Zhou Q, Wang Y, Sun S, Xie L. Gene profiling in murine corneas challenged with Aspergillus fumigatus. Mol Vis. 2007;13:1226–1233. [PubMed] [Google Scholar]
- 17.Dong X, Shi W, Zeng Q, Xie L. Roles of adherence and matrix metalloproteinases in growth patterns of fungal pathogens in cornea. Curr Eye Res. 2005;30(8):613–620. doi: 10.1080/02713680590968196. [DOI] [PubMed] [Google Scholar]
- 18.Bulla R, De Seta F, Radillo O, Agostinis C, Durigutto P, Pellis V, De Santo D, Crovella S, Tedesco F. Mannose-binding lectin is produced by vaginal epithelial cells and its level in the vaginal fluid is influenced by progesterone. Mol Immunol. 2010;48(1–3):281–286. doi: 10.1016/j.molimm.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Swierzko AS, Florczak K, Cedzyński M, Szemraj J, Wydra D, Bak-Romaniszyn L, Emerich J, Sułowska Z. Mannan-binding lectin (MBL) in women with tumors of the reproductive system. Cancer Immunol Immunother. 2007;56(7):959–971. doi: 10.1007/s00262-006-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiber H, Padilla-Docal B, Jensenius JC, Dorta-Contreras AJ. Mannan-binding lectin in cerebrospinal fluid: a leptomeningeal protein. Fluids Barriers CNS. 2012;9(1):17. doi: 10.1186/2045-8118-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]