Abstract
AIM
To identify the presence of various bone morphogenetic proteins (BMPs) and their receptors in normal sclera of human, rat and guinea pigs, and to determine whether their expression changed with form-deprivation myopia (FDM) in guinea pig sclera.
METHODS
The expression of BMPs and BMP receptors were detected using reverse transcription polymerase chain reaction (RT-PCR) and immunofluorescence. Two-week-old guinea pigs were monocularly form-deprived with a translucent lens. After fourteen days induction of FDM, total RNA was isolated and subjected to RT-PCR to examine the changes of BMPs and BMP receptors in tissues from the posterior sclera. Western blotting analysis was used to investigate their changes in protein levels.
RESULTS
Human sclera expressed mRNAs for BMP-2, -4, -5, -7, -RIA, -RIB and BMP-RII. Conversely, rat sclera only expressed mRNA for BMP-7 and BMP-RIB, while the expression of BMPs and BMP receptors in guinea pigs were similar to that of humans. Human sclera also expresses BMP-2, -4, -5,-7 in protein level. Fourteen days after the induction of myopia, significant decreased expressions for BMP-2 and BMP-5 in the posterior sclera of FDM-affected eyes (P<0.05 vs internal control eyes).
CONCLUSION
Various BMPs were expressed in human and guinea pig sclera. In the posterior sclera, expressions of BMP-2 and BMP-5 significantly decreased in FDM eyes. This finding indicates that various BMPs as components of the scleral cytokines regulating tissue homeostasis and provide evidence that alterations in the expression of BMP-2 and BMP-5 are associated with sclera remodeling during myopia induction.
Keywords: form-deprivation myopia, bone morphogenetic protein, human sclera, guinea pig sclera
INTRODUCTION
Myopia has emerged as a major health issue in east Asia because of its high prevalence and high sight-threatening pathologies associated with high myopia[1].In Southeast Asia high myopia is closer to 20% of the general population[2]. Strong evidence from clinical and experimental studies indicates that the sclera play an important role in influencing the size of the eye and the refractive state of the eye[2],[3].
Postnatal scleral growth, like other connective tissues, is under the control of growth hormone or its downstream effectors[4]. Transforming growth factor beta (TGF-β) had been shown to play a major role in sclera remodeling as well as biomechanical changes[2],[5],[6]. Bone morphogenetic proteins (BMPs), which belong to the TGF-βsuper family had been found in a number of ocular tissues and play an important role in eye development and differentiation of ocular tissues[7]. Despite the involvement of BMPs in eye development and adult eye tissues, to our knowledge, the homeostasis of BMPs in the sclera has not been fully documented, the relationship between BMPs and axial elongation in myopia remains obscure.
It is well known that form-deprivation myopia (FDM) guinea pigs are considered as a suitable animal model to be used for the study of human myopia[8]. In this paper we examined human, rat and guinea pigs sclera for BMP-2 to BMP-7 and reported their changes during myopia induction in guinea pigs sclera.
SUBJECTS AND METHODS
Ethics Statement
All experimental procedures were conducted in conformity with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No.85-23, revised 1985). The study protocol was approved by the Review Committee for the Use of Human or Animal Subjects of Medical College of Qingdao University.
Animals
Twenty pigmented guinea pigs (approximately 15d old) were reared on a cycle of 12h illumination and 12h darkness daily during the experimental period. Water supplemented with vitamin C and food (guinea pig pellets and fresh vegetables) were freely available. The room temperature was 22 °C.
Experimental Design and Myopia Induction
The animals were randomly assigned to either of 2 groups: diffusers (n=10) and normal controls (n=10). The diffusers (white translucent hemispheric occluder with a diameter of 12 mm) were mounted on a matching plastic ring and glued to the periorbital fur of the eye for the whole two week period of this experiment. In diffusers group, the fellow eye was untreated as a matched control.
Biometric Measurements
Biometric measurements included streak retinoscopy and ultrasonography. Retinoscopy was performed in a dark room using a streak retinoscope and trial lenses approximately 15 min after the eyes had been fully cyclopleged with 2 drops of tropicamide. The refraction was recorded as the mean value of the horizontal and vertical meridians. Axial length of the eye was measured by an A-scan ultrasonograph with 11 MHz. The measurements were performed under topical anaesthesia by 0.4% Oxybuprocaine Hydrochloride (Alcon, Belgium). Ocular refraction and axial length were collected at the start and end of the experiment.
Tissue Preparation
Human sclera tissues were excised from healthy adult human donor eyes (n=3; age of 18 and 19 years old) obtained from the Eye Bank of the Affiliated Hospital of Qingdao Medical College, Qingdao University, with the approval of Ethics Committee of Qingdao University (Qingdao, China) and complied with the tenets of the Declaration of Helsinki for biomedical research involving human subjects. Human sclera was embedded with optimum cutting temperature compound (Sigma, St. Louis, MO, USA), and cut into 8 µm sections at -20 °C. Sections were subsequently tiled onto slides (Corning Ltd, Tokyo, Japan), fixed with cool acetone for 15min, air-dried, and kept frozen at -20 °C until use. Rat and guinea pigs were killed by overdoses of Chloral Hydrate. The eyes were removed and cut into halves about 1 mm posterior to the ora serrata on the ice plate. The anterior segment of the eye was discarded. The posterior sclera was excised by using a 6 mm-diameter trephine around the head of the optic nerve. The head of the optic nerve was discarded. The sclera were stored at -80°C.
Immunofluorescence
The slides were washed three times with phosphate buffered saline (PBS), nonspecific binding was blocked by incubation in 10% (v/v) goat serum in PBS for 30min at room temperature. The slides were incubated at 4 °C overnight with primary antibodies BMP-2 to BMP-7 (Polyclonal rabbit antibodies, Boster Biological Technology) diluted in PBS. The antibody-treated and negative control slides were washed with PBS and exposed to fluorescein isothiocyanate-conjugated (FITC) goat anti-rabbit IgG antibodies (Boster Biological Technology) diluted in PBS and incubated at room temperature for 1h. Fluorescent signals were detected using a fluorescent microscope (TS-100, Nikon).
Nuclear Acid Extraction, Reverse Transcription and Polymerase Chain Reaction
Sclera was grinded in liquid nitrogen. Total RNA were extracted from sclera [normal human sclera n=3; normal rat sclera n=3; normal guinea pigs sclera n=3; form deprived guinea pig sclera n=5; contralateral control sclera n=5 with a kit (NcleoSpin RNA II; Macherey-nagel; Germany)] according to the manufacturer's instructions. The total RNA was quantified by measuring OD260 and OD280 (optical density 260/280 higher than 1.9). cDNA was synthesized with 5 µg total RNA, 2 µL random 6 mers, 0.5 µL Oligo dT Primer, 2 µL PrimeScript™ Buffer, and 0.5 µL PrimeScript™ RT Enzyme Mix I (TaKaRa, Dalian, China) at 37°C for 15min. The reaction was suspended at 85°C for 5s. Conditions for PCR were 95°C for 30s, 35 cycles of 95°C for 5s, 55°C (56°C for BMP-3, BMPR-lB respectively) for 30s and 72°C for 30s. The final extension step was at 72°C for 7min. The PCR products were then electrophoresed on a 2% agarose gel containing 10 × Gelred reagents in parallel with 50 bp DNA markers. The nucleotide sequences of the primers used in the experiments are summarized in Table 1. β-actin was used as an internal control. Images were captured and relative band intensities were quantitated and compared using the AlphaImager-3400 Computerized Densitometry System (Biozym, Oldendorf, Germany). Because guinea pigs nucleotide sequences haven't been reported, we designed probes to cover regions where the human and rat BMP sequences show high homology. It is therefore reasonable to expect that our primers specifically recognize the examined BMPs. To prove the identity, all the experiments were performed at least 3 times and the products were sequenced in TaKaRa, Dalian, China.
Table 1. Sequences, annealing temperatures (Tan.) and predicted product sizes of the primers used.
| Gene NCBI accession number (rat and human) | Updream primer | Downdream primer | Tm (°C) | Size (bp) |
| BMP-3 | ||||
| NM_017105.1 | TTGGCTGGAGCGAATGGATTA | GCTCAGGAATCCCAGAGACGAC | 56 | 159 |
| NM_001201.2 | ||||
| BMP-4 | ||||
| NM_017105.1 | TTTGTTCAAGATTGGCTCCCAAG | AAACGACCATCAGCATTCGGTTA | 55 | 101 |
| NM_001201.2 | ||||
| BMP-5 | ||||
| NM_012827.2 | TCCACAGAACAATTTGGGCTTACA | ACCATGAACGGCTGCTTTGAC | 55 | 120 |
| BC020546.2 | ||||
| BMP-6 | ||||
| NM_013107.1 | CGCCTTCCTCAACGACCGCGG | GGAATCTGGGATAAGTTGAA | 55 | 120 |
| NM_001718.4 | ||||
| BMP-7 | ||||
| NM_001191856.1 | TCCGGTTTGATCTTTCCAAGA | CCCGGATGTAGTCCTTATAGATCCT | 55 | 81 |
| NM_001719.2 | ||||
| BMPR-IA | ||||
| S75359.1 | GCCGTTTTGAAGCTGATGTCA | TCTTTGCGAGCGTCTTCTTGA | 55 | 88 |
| NM_004329.2 | ||||
| BMPR-IB | ||||
| NM_001024259.1 | TGGCTGACATGTACAGCTTTGG | GGCACTCGTCACTGCTCCAT | 56 | 198 |
| D89675.1 | ||||
| BMPR-ll | ||||
| NM_080407.1 | ACTGCAGATGGACGCATGG | AATCTCGATGGGAAATTGCAG | 55 | 199 |
| NM_001204.6 | ||||
| β-actin | ||||
| NM_031144.2 | GGCACCACACTTTCTACAATG | GGGGTGTTGAAGGTCTCAAAC | 55 | 133 |
| NM_001101.3 |
Western Blotting Analysis
Tissues were homogenized in ice-cold extraction buffer (0.01 mol Tris-HCl at pH 7.4, 0.15 mol NaCl, 1% w/v Triton X-100, 0.1% SDS, 1% deoxycholic acid, 1 m mol EDTA) as well as protease inhibitors (1 µmol pepstatin, 1 µg/mL leupeptin, and 0.2 mmol PMSF). After homogenization, samples were placed on ice for 30 min and centrifuged at 12 000×g for 15min. The supernatant was decanted and the precipitate was discarded. Protein concentrations were determined according to the BCA method by using an Enhanced BCA Protein Assay Kit (Boster, Wuhan, China). Each protein sample (30 µg) was mixed with 5× sample buffer for SDS polyacrylamide gel electrophoresis. The mixture was boiled for 3min, electrophoresed on 10% SDS polyacrylamide gel, and transferred to nitrocellulose membranes (Pall Corporation, East Hills, NY, USA). Protein loading and transfer efficiency were monitored by staining the membranes with 1% Ponceau S. The membranes were washed three times with Tris Buffered Saline Tween (TBST pH 7.6) and soaked in a blocking solution (100 mL, Beyotime Institute of Biotechnology, Haimen, china. 5% w/v skim milk powder in 2.5 mmol Tris-HCl and 14 mmol NaCl plus 0.05% Tween-20) for 1h at room temperature. Then overnight incubation with primary antibodies to the BMP-2, BMP-4 and BMP-5 (Polyclonal goat antibodies ) at a 1:100 dilution (0.2 ml, Boster Biological Technology, Wuhan, China) at 4 °C in blocking solution was performed. The following day, the membranes were washed three times with TBST and incubated with a horseradish peroxidase-conjugated secondary antibody at a 1:1000 dilution (0.4 µg/mL; Boster, Wuhan, China) for another 1h at room temperature. After three times of washing with TBST, the reaction products were visualized with BeyoECL Plus western blotting detection reagents (eyotime Institute of Biotechnology, Haimen, China). Images were captured with Fuji Film LAS3000 imaging system and analyzed with MultiGauge software (Fuji Film, Tokyo, Japan). β-actin (Kang Chen, China) was used as a housekeeping protein to normalize the protein load.
Statistical Analysis
Ocular refraction and biometric measures data were expressed as means±SD for each group. Differences between both eyes of each animal and between the groups were statistically compared using paired sample t-test and one-way ANOVA with Bonferroni Correction. Statistical analysis was performed using the SPSS 15.0 statistical software. The results were considered statistically significant at P <0.05.
RESULTS
Expression mRNA of BMPs and BMPs' Receptors in Human, Guinea Pig and Rat Sclera
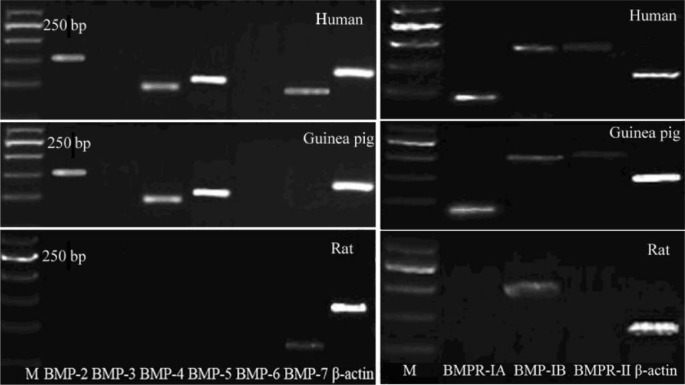
Distinct bands specific for BMP-2, BMP-4, BMP-5, BMP-7, BMPR-IA, BMPR-IB and BMPR-II were observed after reverse transcription PCR using total RNA extracted from normal human sclera (Figure 1). mRNA coding for BMP-5 was found to have the strongest expression, followed by BMP-2, BMP-4and BMP-7, whereas expression of BMP-3 and BMP-6 was not observed after 35 PCR cycles (n =3). Expression levels of mRNA coding for BMPR-IA and BMPR-IB were higher than those of BMPR-II. However, only a distinct band specific for BMP-7 was found in normal rat sclera (Figure 1). The expressions of BMPs and BMPRs in guinea pig sclera were similar to those of human. Immunofluorescence further confirmed the expression of BMPs in guinea pig sclera (Figure 2).
Figure 1. Semi-quantitative RT-PCR analysis of total RNA from 3 normal human sclera, 3 normal guinea pig sclera and 3 normal rat sclera using specific primers for BMP-2, BMP-3, BMP-4, BMP-5, BMP-6, BMP-7, BMPR I A, BMPR I B, BMPR II normalized to β-actin at 35 cycles (M: Molecular size marker).
Figure 2. Distribution of BMPs in guinea pig sclera by indirect immunofluorescence.
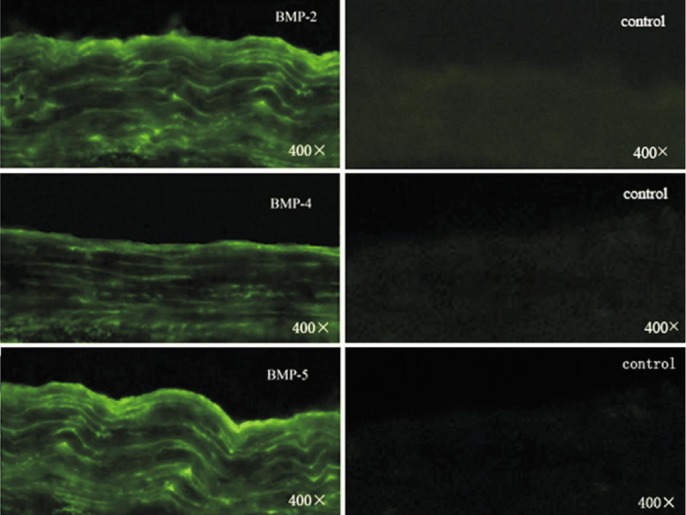
FITC marked the secondary antibody.
Refractive Error and Axial Length
Prior to the diffuser wear, there was no significant difference between the two eyes of the animals in refraction and axial length within each group (P >0.05, paired sample-t-test). The differences between the eyes of each animal in axial length were also not significant between the 2 groups at the beginning of the experiment (P >0.05, one-way ANOVA with Bonferroni correction).
At 14d, monocularly deprived eyes had myopia of -0.48±0.51 D and an axial length of 8.30±0.05 mm (P <0.001 versus normal and internal control eyes, ANOVA and Tukey post-hoc test; Figure 3. Refractive error and axial length between the eyes of the two groups were presented in Table 2.
Figure 3. Differences in refractive error and axial length after induction of myopia.
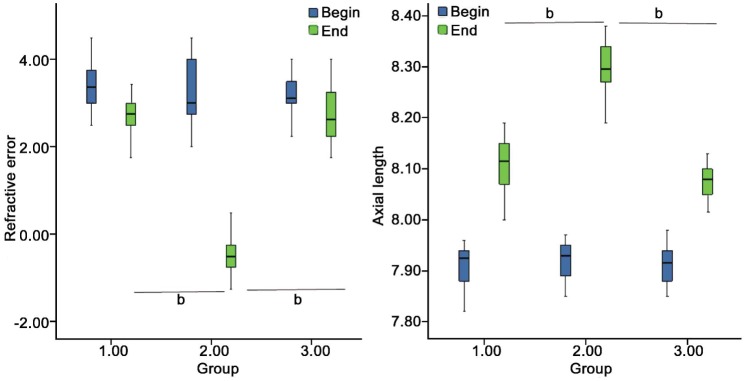
Differences in refractive error (A) and axial length (B) among FDM (group2), internal control (group1) and normal (group 3) eyes of guinea pigs is illustrated. At 14d, monocularly deprivation produced relative myopia, and axial lengths of FDM eyes were significantly greater than the others. The asterisk ‘a’ denotes bP <0.001.
Table 2. Refractive error and axial length between the eyes of the two groups.
| Parameters | Refraction |
P | Axial length |
P | ||
| R | L | R | L | |||
| NOR | ||||||
| 0wk | 3.25±0.63 | 3.25±0.63 | 0.654 | 7.91±0.04 | 7.89±0.05 | 0.404 |
| 2wk | 2.27±0.70 | 3.02±0.54 | 0.386 | 8.06±0.06 | 8.05±0.06 | 0.595 |
| FDM | ||||||
| 0wk | 3.22±0.74 | 3.42±0.60 | 0.516 | 7.92±0.04 | 7.91±0.05 | 0.531 |
| 2wk | -0.48±0.51 | 2.72±0.61 | 0.000 | 8.30±0.05 | 8.11±0.06 | 0.000 |
Changes of the BMPs and BMPRs mRNA Expression in Guinea Pig Sclera
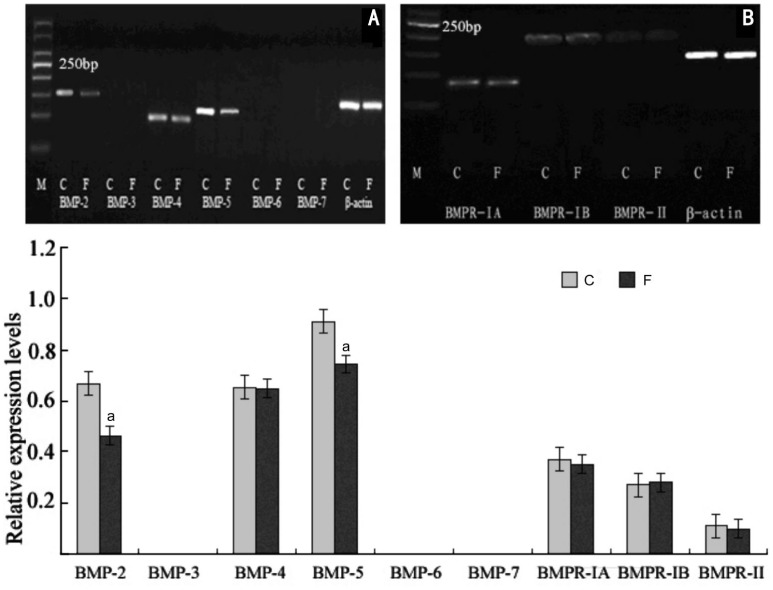
Normal guinea pig sclera express mRNA for BMP-2, BMP-4 and BMP-5. The BMPs and BMPRs mRNA expression normalized to the expression of β-actin showed that mRNA expression of FDM eyes was significantly lower than that of internal control eyes for BMP-2 (P =0.017 or P <0.05,) and BMP-5 (P =0.028 or P <0.05). However, mRNA expression for the BMP-4 was not significantly altered in FDM eyes (P =0.46; Figure 4).
Figure 4. Effect of myopia induction on the mRNA expression for BMP-2 to BMP-7 and BMPRs in guinea pigs sclera.
2wk after visual deprivation, Semi-quantitative RT-PCR showed a significant decrease in mRNA expression in BMP-2 (-26.48%, P =0.017) and BMP-5 (-18.67%, P =0.028) but not for BMP-4 compared with the internal control eyes(C) in the same animals. Bar graph shows changes in mRNA expression of BMP subtypes 2 to 7 and BMPRs in the posterior sclera during FDM (F) in guinea pigs. Values (mean±SD) were normalized for β-actin and expressed as ratios of optical density. aP<0.05.
Changes of BMP-2 and BMP-5 Protein Expression in Each Group
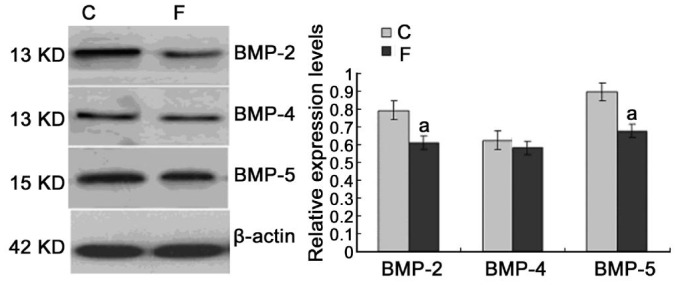
Immunoblotting for BMP-2, BMP-4 and BMP-5 showed a band at about 30 kDa. Figure 5 showed the effect of myopia induction on the protein expression for BMP-2, BMP-4 and BMP-5. After 14d of visual deprivation, significant decrease were found for BMP-2 (-21.25%, P =0. 030) and BMP-5 (-17.11%, P=0.031) compared with the internal control eyes of the same animals, while the expression for BMP-4 was not significantly changed (P =0.365; Figure 5).
Figure 5. Effect of myopia induction on the protein expression of BMP-2,-4,-5. Western blotting showed a significant decrease in protein expression for BMP-2 and BMP-5 but not BMP-4, compared with the internal control (C) eyes.
Values (mean±SD) were normalized for β-actin and expressed as ratios of optical density. aP<0.05.
There was no significant difference between the two eyes of the animals in refraction and axial length within each group (P >0.05, paired sample t-test). The differences between the eyes of each animal in axial length were also not significant between the 2 groups at the beginning of the experiment (P>0.05, one-way ANOVA with Bonferroni correction). At 14d, monocularly deprived eyes had myopia of -0.48±0.51D and an axial length of 8.30±0.05 mm (P <0.001 versus normal and internal control eyes).
DISCUSSION
BMPs are a large group of multifunctional growth factors which play a crucial role during embryonic development and morphogenesis, and equally essential in a variety of biological processes during the postnatal life, such as cell proliferation, differentiation, apoptosis, angiogenesis, and secretion of extracellular matrix components [10],[11]. BMPs exert their biologic effects though binding to the cell-surface serine-threonine kinase receptors BMP-RI and BMP-RII, activation of which leads to phosphorylation of intracellular signaling molecules including Smad1, Smad5, and Smad8 [11],[12].
The present study showed that human sclera expressed mRNA for BMP-2, BMP-4, BMP-5, andBMP-7, whereas BMP-3 and BMP-6 were not found. We only found BMP-7 in the rat sclera, which similar to Thomadakis et al's results[13]. The expression of BMPs mRNA in guinea pig sclera was similar to that of human, which was BMP-2, BMP-4 and BMP-5, but without BMP-7. The additional expression of BMP receptors (BMPR-IA, BMPR-IB, BMPR-II) in human sclera indicates that BMPs in fact play a role in normal sclera homeostasis.
The activation of sclera fibroblasts, predominantly mediated by the transgenic growth factor TGF-β, has been generally regarded to be responsible for sclera remodel during myopia induction [6],[14],[15]. In this study, we provided evidence for a significant down regulation of BMP-2 and BMP-5, suggesting an additional role for these members of the TGF-β superfamily in the scleral remodeling.
BMP-2 was found to promote sclera fibroblast proliferation in vitro[16],[17]. It is also expressed in human cornea and is considered a heparin-binding cytokine that can modulate corneal fibroblast apoptosis [18]. A gene microarray analysis was used to identify gene expression changes in human sclera fibroblasts in response to mechanical loads and possible mechanisms of sclera remodeling in the development of myopia and showed that mechanical stresses induced BMP-2 mRNA expression after 30min and 24h [19]. McGlinn et al [20] reported that chicks following 6 h and 3d of diffuser-wear, BMP-2 mRNA levels were significantly down-regulated in the retina and RPE. Another research showed that variations in the BMP2K gene were strongly correlated with high myopia in the Taiwanese population[21]. Most recently, BMP-2 in chick RPE showed bidirectional defocus sign-dependent changes which further suggested an important role for BMP-2 in eye growth regulation[22]. In our study, we detected BMP-2 in guinea pigs sclera, and our data showed that BMP-2 was decreased in posterior sclera of myopia eyes, which further illustrated this kind of growth factor was involved in the growth of human sclera. Further research on BMP-2 and its effects on the alteration of scleral proteoglycan and collagen synthesis will contribute to the understanding of its role on the remodeling of the sclera.
BMP-2, BMP-4 and BMP-5 were also found in human trabecular meshwork (TM), optic nerve head and may be associated with glaucoma pathogenesis [23]. Wordinger et al [24] found that BMP-4 could blocked the TGF-β2 induction of fibronectin in TM cells, the result suggests that BMPs can modulate the effects of TGF-β2 in TM cells. Further study found that in primary open angle glaucoma, elevated BMP antagonist expression by TM cells inhibits BMP-4 antagonism of TGF-β2 and this inhibition leads to increased ECM deposition and elevated IOP[25]. BMP-4 also enhanced the secretion of vascular endothelial growth factor (VEGF) by human RPE cells and may be involved in the regulation of ocular angiogenesis associated with diabetic retinopathy[26]. Mutations in BMP-4 cause ocular malformations including anophthalmia-microphthalmia (AM) and may be a candidate gene for myopia[27]. However, in our research, we did not find significant changes in BMP-4 expression in sclera during myopia induction. Further research may focus on the relationships of BMP-4 and its antagonist on the effect of TGF-β in sclera ECM remodeling.
In the present study, we found a very high expression of BMP-5 in normal guinea pigs sclera and there was a significantly decreased expression of BMP-5 in FDM. Other than TM and optic nerve head, BMP-5 is also expressed in adult cornea[28]. BMP-5 and its function in occular tissues were not clarified yet. The function of BMP-5 in other tissues has been wildly investigated which indicate BMP-5 is an important growth factor in maintain normal tissue homeostasis. BMP-5 expression increases during chondrocyte differentiation in vivo and in vitro and could promotes proliferation and cartilage matrix synthesis through Smad1/5/8 and p38 MAP kinase pathway[29]. In synovial tissue of patients with osteoarthritis and rheumatoid arthritis, the expression of BMP-5 decreased[30]. BMP-5 is also expressed by rat granulosa cells (GCs) and affects on the cell's proliferation and steroidogenesis in an autocrine manner [31]. Most recently, it was reported that BMP-5 modulate adrenal cell proliferation and steroidogenesis and was down-regulated in adrenocortical carcinoma[32]. Our results of decreased expression of BMP-5 in FDM guinea pigs sclera indicate that BMP-5was involved in sclera remodeling during myopia induction.
In conclusion, human sclera express various BMPs and BMPRs. BMP-2, BMP-4 and BMP-5 are also expressed in normal guinea pigs sclera and were found to be difference expressed in posterior sclera of guinea pig during myopia induction. These results suggest that BMPs may be an important role in sclera homeostasis and are therefore potential candidates for myopia control.
Acknowledgments
The authors thank Xiang-Tian Zhou for his technical assistance in animal model and De-Quan Li for his helpful suggestions pertaining to this manuscript.
Foundations: Supported by The National Youth Science Fund Project (81300790), The Development Project of Shandong Province Medical Science and Technology (No.2013WS0262) and The Youth Innovation Team Project of Medical College Qingdao University (No.600201304).
Conflicts of Interest: Wang Q, None; Xue ML, None; Zhao GQ, None; Liu MG, None; Ma YN, None; Ma Y, None.
REFERENCES
- 1.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 2.McBrien NA. Regulation of scleral metabolism in myopia and the role of transforming growth factor-beta. Exp Eye Res. 2013;114:128–140. doi: 10.1016/j.exer.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82(2):185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 4.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22(3):307–338. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 5.McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86(1):E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- 6.Jobling AI, Gentle A, Metlapally R, McGowan BJ, McBrien NA. Regulation of scleral cell contraction by transforming growth factor-beta and stress: competing roles in myopic eye growth. J Biol Chem. 2009;284(4):2072–2079. doi: 10.1074/jbc.M807521200. [DOI] [PubMed] [Google Scholar]
- 7.Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med (Maywood) 2007;232(8):979–992. doi: 10.3181/0510-MR-345. [DOI] [PubMed] [Google Scholar]
- 8.Lu F, Zhou X, Jiang L, Fu Y, Lai X, Xie R, Qu J. Axial myopia induced by hyperopic defocus in guinea pigs: A detailed assessment on susceptibility and recovery. Exp Eye Res. 2009;89(1):101–108. doi: 10.1016/j.exer.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Reddi AH. Bone morphogenetic proteins: an unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev. 1997;8(1):11–20. doi: 10.1016/s1359-6101(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Zhao M, Mundy G. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 11.ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211(1–2):105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 12.de Caestecker M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev. 2004;15(1):1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Thomadakis G, Ramoshebi LN, Crooks J, Rueger DC, Ripamonti U. Immunolocalization of Bone Morphogenetic Protein-2 and -3 and Osteogenic Protein-1 during murine tooth root morphogenesis and in other craniofacial structures. Eur J Oral Sci. 1999;107(5):368–377. doi: 10.1046/j.0909-8836.1999.eos107508.x. [DOI] [PubMed] [Google Scholar]
- 14.Jobling AI, Nguyen M, Gentle A, McBrien NA. Isoform-specific changes in scleral transforming growth factor-beta expression and the regulation of collagen synthesis during myopia progression. J Biol Chem. 2004;279(18):18121–18126. doi: 10.1074/jbc.M400381200. [DOI] [PubMed] [Google Scholar]
- 15.Seko Y, Tanaka Y, Tokoro T. Influence of bFGF as a potent growth stimulator and TGF-beta as a growth regulator on scleral chondrocytes and scleral fibroblasts in vitro. Ophthalmic Res. 1995;27(3):144–152. doi: 10.1159/000267651. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Cui D, Yang X, Wang S, Hu S, Li C, Zeng J. Bone morphogenetic protein-2: a potential regulator in scleral remodeling. Mol Vis. 2008;14:2373–2380. [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Zhao G, Xing S, Zhang L, Yang X. Role of bone morphogenetic proteins in form-deprivation myopia sclera. Mol Vis. 2011;17:647–657. [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WJ, Mohan RR, Mohan RR, Wilson SE. Effect of PDGF, IL-1alpha, and BMP-2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest Ophthalmol Vis Sci. 1999;40(7):1364–1372. [PubMed] [Google Scholar]
- 19.Cui W, Bryant MR, Sweet PM, McDonnell PJ. Changes in gene expression in response to mechanical strain in human scleral fibroblasts. Exp Eye Res. 2004;78(2):275–284. doi: 10.1016/j.exer.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 20.McGlinn AM, Baldwin DA, Tobias JW, Budak MT, Khurana TS, Stone RA. Form-deprivation myopia in chick induces limited changes in retinal gene expression. Invest Ophthalmol Vis Sci. 2007;48(8):3430–3436. doi: 10.1167/iovs.06-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu HP, Lin YJ, Lin WY, Wan L, Sheu JJ, Lin HJ, Tsai Y, Tsai CH, Tsai FJ. Anovel genetic variant of BMP2K contributes to high myopia. J Clin Lab Anal. 2009;23(6):362–367. doi: 10.1002/jcla.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Liu Y, Wildsoet CF. Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2012;53(10):6072–6080. doi: 10.1167/iovs.12-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wordinger RJ, Agarwal R, Talati M, Fuller J, Lambert W, Clark AF. Expression of bone morphogenetic proteins (BMP), BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis. 2002;8:241–250. [PubMed] [Google Scholar]
- 24.Wordinger RJ, Fleenor D, Hellberg P, Pang I-H, Tovar T, Zode G, Fuller J, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48(3):1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- 25.Fuchshofer R, Tamm ER. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347(1):279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Zhu D, Sonoda S, He S, Spee C, Ryan SJ, Hinton DR. Over-expression of BMP4 inhibits experimental choroidal neovascularization by modulating VEGF and MMP-9. Angiogenesis. 2012;15(2):213–227. doi: 10.1007/s10456-012-9254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reis LM, Tyler RC, Schilter KF, Abdul-Rahman O, Innis JW, Kozel BA, Schneider AS, Bardakjian TM, Lose EJ, Martin DM, Broeckel U, Semina EV. BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum Genet. 2011;130(4):495–504. doi: 10.1007/s00439-011-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You L, Kruse FE, Pohl J, Völcker HE. Bone morphogenetic proteins and growth and differentiation factors in the human cornea. Invest Ophthalmol Vis Sci. 1999;40(2):296–311. [PubMed] [Google Scholar]
- 29.Mailhot G, Yang M, Mason-Savas A, Mackay CA, Leav I, Odgren PR. BMP-5expression increases during chondrocyte differentiation in vivo and in vitro and promotes proliferation and cartilage matrix synthesis in primary chondrocyte cultures. J Cell Physiol. 2008;214(1):56–64. doi: 10.1002/jcp.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bramlage CP, Häupl T, Kaps C, Ungethüm U, Krenn V, Pruss A, Müller GA, Strutz F, Burmester GR. Decrease in expression of bone morphogenetic proteins 4 and 5 insynovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2006;8(3):R58. doi: 10.1186/ar1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierre A, Pisselet C, Dupont J, Bontoux M, Monget P. Bone morphogenetic protein 5 expression in the rat ovary: biological effects on granulosa cell proliferation and steroidogenesis. Biol Reprod. 2005;73(6):1102–1108. doi: 10.1095/biolreprod.105.043091. [DOI] [PubMed] [Google Scholar]
- 32.Johnsen IK, Kappler R, Auernhammer CJ, Beuschlein F. Bone morphogenetic proteins 2 and 5 are down-regulated in adrenocortical carcinoma and modulate adrenal cell proliferation and steroidogenesis. Cancer Res. 2009;69(14):5784–5792. doi: 10.1158/0008-5472.CAN-08-4428. [DOI] [PubMed] [Google Scholar]