Abstract
AIM
To assess the effects of eye rubbing on corneal thickness (CT) and intraocular pressure (IOP) measurements obtained 0-30min after habitual eye rubbing in symptomatic patients.
METHODS
Measurements of IOP and CT were obtained at five locations (central, temporal, superior, nasal and inferior) before, and every 5min for 30min interval after 30s of eye rubbing, for 25 randomly selected eyes of 14 subjects with ocular allergy and 11 age-matched normals. Differences in measurements were calculated in each group [Baseline measurements minus measurements recorded at each time interval after eye rubbing (for IOP), and for each corneal location (for CT)] and comparison were then made between groups (allergic versus control) for differences in any observed effects.
RESULTS
Within groups, baseline mean IOPs in the allergic patient-group (14.2±3.0 mm Hg) and in the control group (13.1±1.9 mm Hg) were similar at all times, after eye rubbing (P >0.05, for all). The maximum reduction in IOP was 0.8 mm Hg in the control subjects and the maximum increase was also 0.8 mm Hg in the allergic subjects. Between groups (allergic versus control), the changes in IOP remained under 1 mm Hg at all times (P=0.2) after 30min of eye rubbing. Between 0 and 30min of CT measurements after eye rubbing, the mean central CT (CCT), inferior CT (ICT), superior CT (SCT), temporal CT (TCT) and nasal CT (NCT) did not vary significantly from baseline values in the control and allergic-subject groups (P>0.05, for both). Between both groups, changes in CT were similar at all locations (P>0.05) except for the TC which was minimally thinner by about 4.4 µm (P=0.001) in the allergic subjects than in the control subjects, 30min following 30s of eye rubbing.
CONCLUSION
IOP measured in allergic subjects after 30s of habitual eye rubbing was comparable with that obtained in normal subjects at all times between 0 and 30min. Although, CT in the allergic subjects were similar to those of the control subjects at all times, it varied between +10 and -7.5 µm following eye rubbing, with the temporal cornea showing consistent reductions in thickness in the subjects with allergy. However, this reduction was minimal and was considered to not be clinically relevant.
Keywords: allergy, corneal thickness, intraocular pressure, eye rubbing, tonometry, pachymetry
INTRODUCTION
Eye rubbing is a common activity that occurs sporadically when awakening, before sleep, and throughout the day in response to, fatique, emotional stress, or ocular irritation. Dry eyes and symptoms of allergy are known to provoke eye rubbing[1], [2], and frequent eye rubbing could result in very long episodes of vigorously forceful knuckle rubbing, often seen in some cases of keratoconus (KC)[2]–[5]. All three conditions (dry eyes, allergy and KC) are multifactorial in etiology and are prevalent in our region due to the influence of hot and sunny weather and the high rate of consanguineous marriages[1],[2],[6],[7].
It has been demonstrated that intraocular pressure (IOP) is affected significantly by any contact that applanates or indents the ocular surface and displaces intraocular fluid and was shown to increase by approximately 100% and 300% when the distal end of the index finger was used to apply a light and firm force respectively to the temporal sclera, through the adnexal skin in an open normal eye with IOP of 15 mm Hg[8],[9]. Studies[10]–[13] have also shown that an increase in the compressive rubbing forces exerted in eye rubbing at the corneal surface increases the level of IOP and that repeated episodes of eye rubbing was necessary to observe a significant change in IOPg (a Goldman equivalent IOP)[13]. Similarly, chronic eye rubbing has long been implicated in the development and progression of KC[14] and recent studies[1], [3], [4], [15], [16] have also shown similar associations.
Eye rubbing was shown to have considerable effect on corneal topography by increasing the surface irregularity index and also inducing a 0.5 diopter of astigmatism after 60s of experimental eye rubbing[10]. Slight rubbing for 10s using one finger and in a smooth circular movement, repeated 30 times over a 30-minute period was shown to significantly reduce the keratocyte density in human corneas, and also leads to a greater concentration of inflammatory mediators in the tears[17]. The use of light to moderate force applied on closed eyelid by the finger pad of an index finger for 15min was shown to reduce central and midperipheral human corneal epithelial thickness by 18.4% and recovery to baseline was observed 15-30min centrally and 30-45min mid-peripherally, after eye rubbing [5]. In contrast, immediately after 30s of circular eye rubbing (mild to moderate force over closed eye lids) using the index finger in 10 subjects, the changes in total corneal thickness (CT), epithelial thickness and Bowman's membrane thickness were not significant[18]. Kalogeropoulos et al[19] also found no changes in epithelial thickness profile of their subjects after 10min of eye rubbing. While, the existing studies[5],[10]–[13], [17]–[19] have disagreed in their results, they have utilized different instruments in the study of the effects of eye rubbing on CT and IOP. The subjects used have been asymptomatic patients and thus the results obtained may not be a direct representation of the effects of eye rubbing in patients who actually experience regular symptoms of ocular itching. Again, the pattern/frequency/force utilized in the eye rubbing (circular[5],[18] and horizontal[10]) and who performed the eye rubbing (the examiner[5] or the subject[10], [13]) have differed and most importantly, some studies[10]–[13] did not take into consideration the consistent reported IOP reducing effects of topical anesthetics used prior to obtaining their measurements, reaching up to 8 mmHg in one study[20]–[23]. This makes their results difficult to assess if, the observed changes they reported were due to the anesthetics used or a result of the experimental eye rubbing itself. Eye rubbing as a behavior in symptomatic patients such as patients with ocular allergy is of great concern to eye care practitioners during history taking of patients owing to the frequency of occurrence of these symptoms in patients during routine clinical practice[24]. It is also a concern because about 15% of the worldwide population was reported to be affected by ocular allergies and this percentage increases in industrialized nations[25].
The current study has used noncontact devices in obtaining IOP measurements and CT measurements at five different locations, and has assessed the effects of habitual eye rubbing on normal subjects in comparison to symptomatic patients in order to directly evaluate the differences in the effects if any. Noncontact devices were used to obtain both measurements so as to avoid the influence of topical anesthetics[20]–[23] (which is often used prior to obtaining measurements with instruments requiring contact with the cornea) on measured values.
Therefore, the aims were to determine: 1) whether eye rubbing result in any significant effect in IOP and/or CT measured at any of the five locations, at any time between 0 to 30min in normal and symptomatic patients; 1), whether the effects, (if any) on IOP and CT are significantly different from one CT location to another in both patient groups; and 1), the duration of any observed effect. We also tested the hypothesis that 30s of habitual eye rubbing will affect the IOP and CT in both groups similarly. The importance of this study lies in further understanding the effects of eye rubbing on IOP and CT, and determines whether it is necessary for clinicians to monitor patients with ocular allergy for eye rubbing, and as such counsel them appropriately or take necessary precautions during procedures such as, pachymetry, ocular topography and tonometry.
SUBJECTS AND METHODS
Subjects
The study adhered to the tenets of the Declaration of Helsinkiand was approved by the Research Ethics Committee of the University; all participants gave informed consent after fully understanding the nature of the study. The participants consisted of 14 subjects aged between 22 and 24y who have been previously diagnosed of ocular allergy by the consulting ophthalmologist, and 11 oculo-visually normal subjects (control) aged between 20 and 24y. Study was conducted between March and August 2013 when complaints of allergic conjunctivitis are most common in the university clinic. The Allergic-patient group was recruited from patients visiting the optometry clinic for complaints of ocular itching and who following diagnosis had seasonal allergic conjunctivitis (SAC) or perennial allergic conjunctivitis (PAC) which are localized type 1 hypersensitivity reactions with a hallmark presentation of itching. Patients with SAC and PAC were chosen among other allergies because they form the bulk of most allergies treated by eye care specialists with other forms like chronic vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC), and giant papillary conjunctivitis (GPC), comprising a much smaller percentage, and unlike other forms of ocular allergy, papillae is often absent[24]. The control subjects were normal patients randomly recruited from students of the optometry department. No patient had worn contact lenses previously, had had refractive surgery or had any ocular sign of papillae. The exclusion criteria were a family history or presence of Keratoconus, previous use of hard contact lenses, a positive family history of glaucoma, or current use of any medication known to have possible effects on corneal health and IOP.
Methods
Experimental procedures
At baseline, IOP measurements were obtained from both eyes of each subject with a CT-80 noncontact tonometer (Topcon Corp., Tokyo, Japan) by one examiner (UO). A second examiner (AA) obtained CT measurements using a noncontact specular microscope SP-3000P (Topcon Corp., Tokyo, Japan) before eye rubbing. Which instrument was to be first used at baseline and which patient eye was to be rubbed, was randomized. All randomization was done by a post-graduate student using a series of random numbers generated from a Microsoft Excel spreadsheet who also ensured that both examiners were blinded to each other's measurements. All measurements were made between 11.00 a.m. and 2.00 p.m., in order to minimize the effects of diurnal variation[26], [27].
To ensure that measurements are taken on-time and immediately after eye rubbing and also because IOP measured by nasal CT (NCT) takes lesser time than CT measured at 5 locations, no randomization was performed, instead, IOP re-measurements were first obtained followed by CT (at the five different locations) re-measurements, after eye rubbing at 0, 5, 10, 20, 30min. For each patient, only one eye was rubbed for a period lasting for 30s. The subjects were instructed to rub their eyes in a horizontal pattern using the front side of the first three fingers (palm) aligned parallel to the lid surface in a horizontal direction (moving back and forth from nasal to temporal end) as is commonly observed in itch due to ocular allergy and dry eye. In all cases, eye rubbing was performed over closed eyelids and in natural conditions by applying a force needed to ensure that discomfort experienced by the subjects was as would normally be if their eyes were itchy. To ensure uniformity the subjects were instructed to perform eye rubbing in the same manner and with consistency over 30s. The contralateral eye remained open during eye rubbing, with steady primary gaze fixation on the first letter of the visual acuity chart.
Instruments
For the assessment of IOP with the Topcon CT80 non-contact tonometer, four readings were taken with the automatic mode of the instrument but, only the last three readings were averaged to get the IOP reading for an eye. This procedure was adopted to suit the principle of IOP measurement used by the Topcon CT80 noncontact tonometer. The pneumatic system of the tonometer generates a controlled pulse of room air that is fired at the cornea, while an optoelectronic monitoring device, which directs a collimated light beam at the central cornea, senses the number of rays reflected by the indented cornea. The time taken for these rays to reach the monitoring device is converted into an IOP reading in mmHg. After the first pulse is fired at the cornea, subsequent pulses are automatically adjusted to the IOP of the subject to minimize the risk of excessive air pressure.
The need to take measurements immediately after rubbing for the corneal locations meant that one measurement from each of: central CT (CCT), superior CT (SCT), inferior CT (ICT), temporal CT (TCT) and NCT, could be obtained by the automatic mode of the instrument. The SP-3000P non-contact specular microscope is a newer version of the SP-2000P with various advanced features and algorithm integrated. Aside from the improvement in quality of the captured image, the reliability and repeatability of measurements obtained in the current version has been enhanced by its ability to obtain 5 images per eye in contrast to 3 images per eye obtained by the previous version. CT readings are obtained using a reflection of light from the anterior and posterior corneal surfaces. Focusing on the endothelium, it provides a specular image and measures the focal distance, from which CT can be calculated. Strategically located, the five fixation targets of the SP-3000P allow for one central and four peripheral fixations targets (superior, temporal, inferior, and nasal corresponding to 12, 2, 6, 10 o'clock meridians). Precise focus and centering of the endothelial cell analysis and CT can be simultaneously measured in each one automatically, by the auto tracking system of the device over an area of 8 × 8-mm2.
Statistical Analysis
The Graphpad Instat software (version 3.00-Graphpad Software Inc., San Diego, CA, USA) was used for all analysis. A P value <0.05 was considered statistically significant, and with 24 eyes the study had a power of 85% as calculated using the G* Power software 3.1.3 version. Descriptive statistics was used to represent the mean values in a table before and after eye rubbing. IOP measurements were analyzed at baseline using the repeated measures analysis of variance (RM-ANOVA). First we compared all triplicate IOP measurements for each subject in each group for any significant differences. Then, the calculated mean baseline IOP were compared between eyes of same patient in each group (within group). To determine if time of measurement of IOP affects measured values after eye rubbing, we also compared the change in IOP in each group. Change was calculated as difference in IOP (before minus after rubbing) for every time point after eye rubbing using a RM-ANOVA. For between group analysis (allergic subject group versus control group), an unpaired t-test was used to compare the ages of subjects for any significant differences between them. We then compared the baseline mean IOPs to determine the differences in IOP between the allergic and control groups.
The change in measured IOP in each group (i.e. before minus after rubbing for every time point) was then compared between groups (allergic subject group versus control group) using an unpaired t-test to determine the differences in IOP changes following eye rubbing in both groups of patients.
A single factor RM-ANOVA was used to assess the difference in CT at the five locations at baseline. RM-ANOVA was conducted five times (one for each of the five locations of measured CT) for each group. For example, baseline TCT versus TCT at 0min versus TCT at 5min versus TCT at 10min versus TCT at 20min versus TCT at 30min after eye rubbing. Analysis was also done using an unpaired t-test to determine the differences in the change in CT after eye rubbing between groups at every time point. The variation was calculated as the difference between means of: baseline CT (before rubbing) in one corneal location and CT after eye rubbing on the same location; for each time point. (For example: mean SCT at baseline minus mean SCT 0min, after eye rubbing in allergic subjects versus mean SCT at baseline minus mean SCT 0min, after eye rubbing in the control subjects). A line graph of this time point variation in IOP and CCT (following eye rubbing) as a function of mean difference (allergic minus control), was then plotted. Post-hoc pair wise comparisons were also conducted, whenever significant differences were detected and P values adjusted for multiple comparisons.
RESULTS
There were no statistical significant differences between the triplicate IOP measurements obtained in allergic eyes (n=28; P>0.05) and control eyes (n=22; P>0.05) at baseline. The baseline mean IOP measurement for the right eye was, 14.8 ± 2.8 mm Hg (range: 9.7 mm Hg to 19.0 mm Hg) and 14.5±3.1 mm Hg (9.7 mm Hg to 19.0 mm Hg; P>0.05), for the left eye in the allergic group. For the control group it was, 13.6±1.9 mm Hg (range: 10.0 mm Hg to 16.0 mm Hg) and 13.2±2.3 µm (8.0 mm Hg to 16.0 mm Hg; P>0.05), right and left eye respectively. Table 1 is value of baseline (mean±SD) IOP before, 0-30min after eye rubbing in the control and allergic groups respectively.
Table 1. Fluctuations in intraocular pressure in mm Hg from baseline to 30min after eye rubbing control subjects and allergic subjects.
| Time (min) | IOP of control subjects (mm Hg) (mean age= 21.8±1.5) y | P | IOP of allergic subjects (mm Hg) (mean age=22.9±0.8) y | P |
| Baseline | 13.1±1.9 (10.0-15.7) | 14.2±3.0 (9.7-19.0) | ||
| 0 | 12.9±2.5 (7.0-15.3) | 0.68 | 14.4±2.9 (10.5-20.0) | 0.58 |
| 5 | 13.0±2.5 (6.5-15.0) | 0.93 | 13.4±2.8 (10.3-18.0) | 0.06 |
| 10 | 12.5±2.3 (7.0-15.7) | 0.24 | 13.8±3.1 (10.0-18.0) | 0.18 |
| 20 | 13.8±2.5 (8.0-18.0) | 0.08 | 14.0±3.0 (10.0-19.0) | 0.66 |
| 30 | 13.9±2.2 (9.3-17.0) | 0.11 | 14.4±3.0 (10.0-19.7) | 0.67 |
Repeated measures analysis of variance (RM-ANOVA) between baseline and each time interval (post-hoc analysis). P<0.05 is considered significant.
On analysis, there were statistical significant differences in the CT measured at the five locations (P<0.0001, for both eyes) in both groups. The order of increasing thickness was: central, inferior, temporal, nasal and superior.
Eye Rubbing-induced Variations in Intraooclar Pressure
Mean IOP measured at 0, 5, 10, 20, 30min after eye rubbing in the control (P>0.05 for all, Table 1) and allergic (P > 0.05, Table 1) patient groups did not change significantly from baseline values in all eyes. Figure 1 demonstrates the pattern of variation from baseline IOP across 30min. It can be deduced from the figure that IOP averagely decreased (not statistically significant) at all points in the control eyes but averagely increased at all points in the allergic eyes following eye rubbing. However, these variations were always below 1 mm Hg at all times. Between groups, there was no statistically significant difference in IOP changes at any time interval, after eye rubbing (P = 0.2).
Figure 1. Changes in IOP in mm Hg after 30s of habitual eye rubbing, over time in minutes.
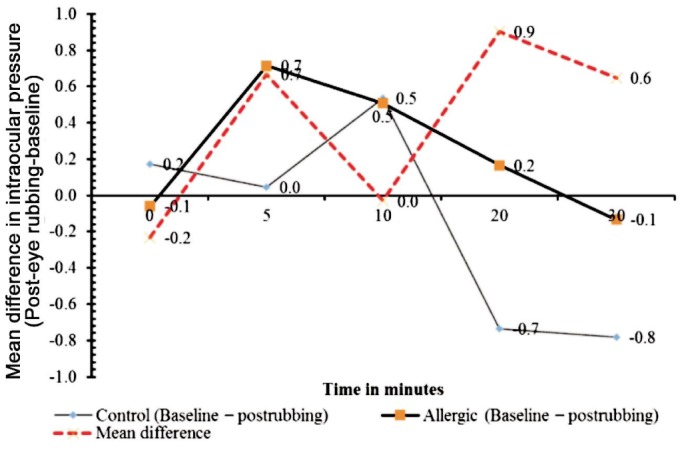
No significant difference was observed in mean IOP variations between the allergic-subject group and the control, after eye rubbing (Unpaired t-test P=0.20).
Eye Rubbing-induced Variations in Corneal Thickness at Five Locations
Tables 2 and 3 are values of baseline (mean±SD) CCT, ICT, SCT, TCT and NCT before, 0-30min after eye rubbing in control and allergic subjects, respectively. The tables show no statistical significant differences in mean CCT, ICT, SCT, TCT and NCT, after eye rubbing in the control (P>0.5, for all; Table 2) and allergic (P>0.16 for all, Table 3) patient groups, respectively. Average fluctuations over the 30min after 30s of eye rubbing were largest in the superior cornea (9.8 µm at 0min and -8.9 µm at 20min) for eyes with allergy and largest for nasal cornea (6.6 µm at 5min) in the control eyes. However, none of these fluctuations reached a statistical significant value (P>0.05).
Table 2. Fluctuations in Corneal Thickness (CT) in µm from baseline to 30min after eye rubbing in control subjects [mean age (standard deviation, SD) = 21.81(1.53)y]. Values expressed as mean (standard deviation, SD), of values.
| Time (min) | Central | Inferior | Superior | Temporal | Nasal |
| Baseline | 516.0 (26.1) | 559.0 (25.7) | 604.3 (40.7) | 565.1 (33.5) | 595.7 (29.1) |
| 0 | 515.6 (25.2) | 57.4 (26.0) | 606.8 (29.1) | 562.7 (39.1) | 593.1 (27.7) |
| 5 | 517.7 (25.2) | 559.0 (27.9) | 603.5 (33.2) | 567.6 (33.3) | 589.0 (33.2) |
| 10 | 510.3 (34.2) | 353.4 (26.3) | 604.8 (33.0) | 564.4 (37.7) | 590.3 (32.8) |
| 20 | 515.6 (23.9) | 559.3 (24.7) | 608.3 (29.6) | 562.7 (40.0) | 596.0 (30.7) |
| 30 | 515.4 (22.5) | 557.4 (27.5) | 597.3 (33.5) | 566.0 (38.0) | 596.5 (31.3) |
| P | 0.80 | 0.75 | 0.68 | 0.88 | 0.55 |
P values are results of repeated measures analysis of variance (RM-ANOVA) conducted on for example baseline TCT versus TCT at 0, 5, 10s, 20, 30min post-habitual eye rubbing. P<0.05 is considered significant.
Table 3. Fluctuations in Corneal Thickness (CT) in µm from baseline to 30min after eye rubbing in allergic subjects [mean age (standard deviation, SD) = 22.9(0.8)y]. Values expressed as mean (standard deviation, SD), of values.
| Time (min) | Central | Inferior | Superior | Temporal | Nasal |
| Baseline | 527.6(37.1) | 577.9 (30.7) | 623.3(37.6) | 578.6(34.0) | 621.2(26.2) |
| 0 | 525.9(34.9) | 572.2(32.9) | 618.3(41.8) | 562.7(39.1) | 619.6 (37.9) |
| 5 | 526.8(33.5) | 573.9(32.9) | 621.1 (33.2) | 578.2(38.3) | 620.3 (31.8) |
| 10 | 529.4(32.5) | 574.8(29.4) | 613.5(45.5) | 580.8(32.9) | 620.8(34.9) |
| 20 | 526.0(44.4) | 575.3(31.4) | 632.2(32.0) | 577.5(28.9) | 614.1 (37.7) |
| 30 | 531. 9(30.3) | 579.4(31.9) | 620.4(36.7) | 580.8 (31.4) | 616.8(31.8) |
| P | 0.33 | 0.16 | 0.24 | 0.85 | 0.59 |
P values are results of Repeated Measures Analysis of Variance (RM-ANOVA) conducted on for example baseline TCT versus TCT at 0, 5, 10, 20 and 30min post-habitual eye rubbing. P<0.05 is considered significant.
Differences in CCT, ICT, SCT, NCT between group eyes (allergy versus control) showed no statistical significant differences at all times (0-30min) following 30s of eye rubbing (P>0.05, for all four locations) except at the temporal cornea where a statistically but not clinically significant reduction in thickness (4.4 µm) occurred, after eye rubbing. Figure 2 is a line graph representation of the between-group difference (allergy minus control eyes) in CT variations from baseline values at five corneal locations after eye rubbing. It can be deduced from the figure that, at all time points, TCT (the red dotted line) was consistently thinner in the allergic eyes than in the control eyes following eye rubbing.
Figure 2. Differences in CT variations in µm between subjects with allergy and normal subjects, after 30s of eye rubbing, over time in minutes.
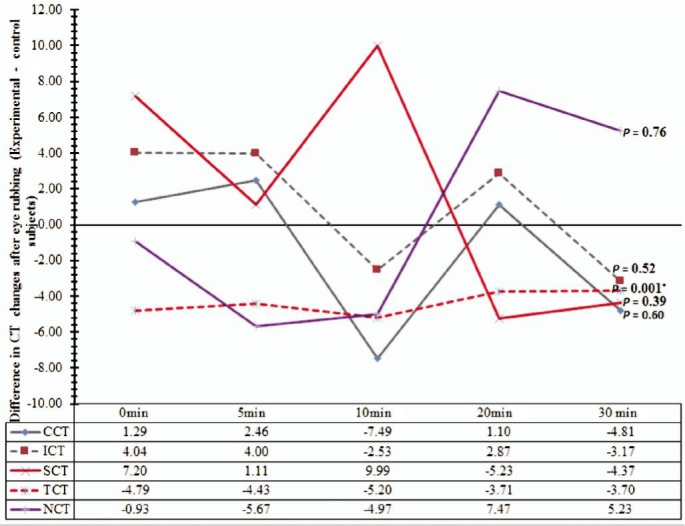
The results of unpaired t-test analysis represented by the P-values show a statistically but not clinically significant reduction in thickness at the temporal cornea after eye rubbing in the allergic-subject group. CCT: Central corneal thickness; SCT: Superior corneal thickness; ICT: Inferior corneal thickness; NCT: Nasal corneal thickness; TCT: Temporal corneal thickness.
DISCUSSION
In this study, we investigated the differences in measured IOP and CT over time after 30s of eye rubbing in normal and symptomatic patients with ocular allergy. To the best of the authors' knowledge, no study has assessed the effects of eye rubbing in patients who actually experience symptoms of ocular itching or compared the results of eye rubbing performed in normal and allergic subjects, despite the numerous reports associating eye rubbing with changes in CT.
The maximum change in IOP occurred at 30min following eye rubbing in the control eyes and at 5min (an increase of 0.8 mm Hg) post eye rubbing in the eyes with allergy (Table 1). Averagely, IOP reduced by 0. 2 mm Hg (P >0.05) and increased by 0.2 mm Hg (P>0.05) in the control and allergic eyes respectively. However, no eye had a spike in IOP that exceeded 0.9 mm Hg at any time interval. Between the normal and allergic eyes, the difference in IOP variation was similar and only varied by about 0.3 mm Hg after 30min of eye rubbing (Figure 1).
Regarding CT, no statistically significant changes were observed at any location and at all-time intervals (not exceeding 30min) following eye rubbing in both the control (Table 2) and allergic eyes (Table 3), even though, CCT, NCT, TCT, IST and SCT increased after eye rubbing in the control eyes. Changes in CT were also observed in the allergic eyes. NCT, SCT, ICT increased by about 3 µm while CCT & TCT decreased by 0.3 µm and 2.9 µm, respectively. Generally, the variation in measured-CT observed in the allergic eyes after eye rubbing did not significantly differ from that which was observed in normal subjects, after eye rubbing (Figure 2). However, TCT was statistically significantly reduced by a very minimal amount (4.4 µm). This variation was within the standard deviation and as such, was considered to be clinically irrelevant. At no time was the difference in CT variation (normal versus allergic eyes) at any location, greater or lesser than 10µm.
Spikes in IOP have been demonstrated to result from: applying a digital light pressure on an open eye through the adnexal skin (IOP spiked by 50%-130%)[9]; and in strong eye closure during a blink-related compression (IOP spiked by about 2-10 mm Hg in an eye with normal IOP of 15 mm Hg), or following eye rubbing[10],[12],[13]. Thus rubbing-related spikes may be much higher because of the additive effect of spiking from eye closure and spiking resulting from rubbing compression indentation[5],[6],[12],[16]. Similarly, IOP readings obtained by a Tonopen XL after a minute of gentle horizontal eye rubbing resulted in mean values that were lower than baseline values by 2.4 mm Hg[10]. Contrary to these findings, the current results showed that fluctuations in IOP did not exceed 0.9 mmHg at any time up to 30min after 30s of eye rubbing. This result may suggest that, a cumulative effect on corneal biomechanical rigidity or a longer duration of gentle rubbing was necessary for such habitual eye rubbing to produce significant spikes in IOP, or the non-preserved anesthetic drop utilized in the previous studies during IOP measurements which has been shown to cause reduction in IOP but was not used in the current study, may have partly influenced their results[10],[13],[20]–[23]. From this result, we can therefore deduce that patients with less severe forms of conjunctivitis may not experience significant changes in IOP and CCT following habitual eye rubbing.
The thickness of the cornea was significantly decreased in the order of: SCT, NCT, TCT, ICT and CCT (P<0.0001), in the current study. This is consistent with the report that peripheral cornea is usually thicker than central cornea while the temporal and inferior corneal are thinner than the nasal and superior corneal[28]. The cohesive strength of the cornea is primarily dependent on the molecular binding strength of the proteoglycan, making the less rigid and less resistant inferocentral cornea most susceptible to ecstasies as is commonly seen in KC. Corneal epithelial thinning was shown to occur when prolonged but strong mechanical pressure was applied to the cornea such as in long term use of extended wear soft contact lens and reverse geometry lenses (orthokeratology)[29],[30]. In contrast, mild pressure induced by the use of daily wear soft lenses and overnight wear of conventional rigid gas permeable lenses was shown to cause no significant change in epithelial thickness[11],[19].
In the case of eye rubbing, mild short term eye rubbing was reported to cause significant reductions in corneal biomechanical properties and epithelial thickness whereas Parkasam and cohorts found no significant changes in total CT, epithelial thickness, and bowman's membrane thickness after 30s of mild to moderate eye rubbing. In one controlled study, no significant differences in the epithelial thickness and basal cell morphology were observed between eyes that were rubbed for 20s/min over a 10-minute period and the control eyes[5],[13],[18],[19]. The result presented in the current study is consistent with these reports[11],[18],[19] but differed from the results reported in other studies [5],[10],[13], [16]. In comparison to these studies[5],[10],[13], [16], the duration of eye rubbing was shorter in the current study (30s), no anesthesia was used because of the reported effects on CT[31], [32], and eye rubbing was performed in one episode, and patients with milder forms of allergy (SAC and PAC) were use. Compared with the direct pressure exerted by reverse geometry orthokeratology lenses (which are capable of producing rapid central corneal epithelial thinning with mid-peripheral stromal thickening) and the eye rubbing observed in KC, which involves a severe knuckle force measuring at about 4.54 kg/2.54-cm2 (about 10 times greater than the normal rubbing force in patients without KC)[2] and lasting from 10 to 180s up to 300s[33], the pattern of eye rubbing utilized in the current study may involve less risk of cell damage. Repeated eye rubbing over weeks or months with greater force may lead to potential adverse effects on corneal tissues due to the repeated rubbing episodes causing significant corneal tissue responses such as that seen in KC development and progression[34].
In this study, patients were asked to rub their eyes the way they usually would if their eyes were itchy. This is in contrast to the method of experimental eye rubbing utilized in previous studies[5],[13],[18],[19]. Such guided eye rubbing is often limited by the fact that the patients may not be able to replicate the force with which they often rub their eyes when symptoms of ocular itching occur or maintain this force throughout the eye rubbing episode. Although, this can be argued for the allergic subjects (in whom eye rubbing is often a habit), the control subjects may find it difficult to replicate similar pattern of eye rubbing since eye itching may not be a common symptom. However, guidance was provided on the rubbing pattern expected of the subjects to ensure uniformity, and subjects were supervised all through the rubbing episode. Subjects were also encouraged to consistently apply uniform pressure over closed eyes for 30s. Despite these precautionary measures, it is still difficult to control the inter-subject variability in the amount of applied force during such habitual eye rubbing which may have influenced the results presented here. However, the contact area of the front side of the first three fingers (palm) used in this study measures about 12×16 mm which is more than the size of the index finger pad (the finger print) used in a previous study[5] and represented an area loading greater than the 3-mm diameter of the central cornea and even the 8×8 mm corneal area assessed by the SP-3000P. Whereas, the force impacted on the cornea during the eye rubbing may have been well distributed across the cornea, the study further highlights the need to: compare self-eye rubbing with eye rubbing performed by examiner to understand the significance in differences; compare the effects of eye rubbing on symptomatic patients with milder forms of ocular allergy with those with more severe ocular allergies or keratoconus suspect. It may also be necessary to acquire direct in vivo measurement of applied pressure on individual subjects to better explain exactly how much force is needed to impact on the corneal surface.
In conclusion, measurements of IOP and CT obtained at the central, nasal, inferior, superior and temporal by non contact devices, immediately and up to 30min, after 30s of eye rubbing, showed no significant changes in both normal and allergic subjects. The difference in IOP variation between the normal and allergic subjects was not significant and did not exceed 0.8 mm Hg at any time point. Regarding CCT, the difference in variation was also not significant between normal and allergic subjects and did not exceed 10µm at any time point, after eye rubbing. Although there was a consistent reduction in the TCT of allergic subjects in relation to the normal subjects, this difference was minimal and clinically irrelevant.
Acknowledgments
The authors extend their appreciation to the Research Centre, College of Applied Medical Sciences and the Deanship of Scientific Research at King Saud University for funding this research. We also appreciate the efforts of Shbear Abdulrahman Ali during data collection.
Conflicts of Interest: Osuagwu UL, None; Alanazi SA, None.
REFERENCES
- 1.Gordon-Shaag A, Millodot M, Essa M, Garth J, Ghara M, Shneor E. Is consanguinity a risk factor for keratoconus? Optom Vis Sci. 2013;90(5):448–454. doi: 10.1097/OPX.0b013e31828da95c. [DOI] [PubMed] [Google Scholar]
- 2.Korb DR, Leahy CD, Greiner JV. Prevalence and characteristics of eye-rubbing for keratoconic and non-keratoconic subjects. Invest Ophthalmol Vis Sci. 1991;32:1057. [Google Scholar]
- 3.McMonnies CW, Boneham GC. Keratoconus, allergy, itch, eye-rubbing and hand-dominance. Clin Exp Optom. 2003;86(6):376–384. doi: 10.1111/j.1444-0938.2003.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 4.Jafri B, Lichter H, Stulting RD. Asymmetric keratoconus attributed to eye rubbing. Cornea. 2004;23(6):560–564. doi: 10.1097/01.ico.0000121711.58571.8d. [DOI] [PubMed] [Google Scholar]
- 5.McMonnies CW, Alharbi A, Boneham GC. Epithelial responses to rubbing-related mechanical forces. Cornea. 2010;29(11):1223–1231. doi: 10.1097/ICO.0b013e3181d3d660. [DOI] [PubMed] [Google Scholar]
- 6.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 7.Assiri AA, Yousuf BI, Quantock AJ, Murphy PJ. Incidence and severity of keratoconus in Asir province, Saudi Arabia. Br J Ophthalmol. 2005;89(11):1403–1406. doi: 10.1136/bjo.2005.074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gloster J. Tonometry and tonography. Int Ophthalmol Clin. 1965;5(4):911–1133. [PubMed] [Google Scholar]
- 9.McMonnies CW. Abnormal rubbing and keratectasia. Eye Contact Lens. 2007;33(6):265–271. doi: 10.1097/ICL.0b013e31814fb64b. [DOI] [PubMed] [Google Scholar]
- 10.Mansour AM, Haddad RS. Corneal topography after ocular rubbing. Cornea. 2002;21(8):756–775. doi: 10.1097/00003226-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ren DH, Yamamoto K, Ladage PM, Molai M, Li L, Petroll WM, Jester JV, Cavanagh HD. Adaptive effects of 30-night wear of hyper-O(2) transmissible contact lenses on bacterial binding and corneal epithelium: a 1-year clinical trial. Ophthalmology. 2002;109(1):27–39. doi: 10.1016/s0161-6420(01)00867-3. [DOI] [PubMed] [Google Scholar]
- 12.Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82(5):637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- 13.Liu WC, Lee SM, Graham AD, Lin MC. Effects of eye rubbing and breath holding on corneal biomechanical properties and intraocular pressure. Cornea. 2011;30(8):855–860. doi: 10.1097/ICO.0b013e3182032b21. [DOI] [PubMed] [Google Scholar]
- 14.Ridley F. Contact lenses in treatment of keratoconus. Br J Ophthalmol. 1956;40(5):295–304. doi: 10.1136/bjo.40.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84(8):834–836. doi: 10.1136/bjo.84.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMonnies CW. Mechanisms of rubbing-related corneal trauma in keratoconus. Cornea. 2009;28(6):607–615. doi: 10.1097/ICO.0b013e318198384f. [DOI] [PubMed] [Google Scholar]
- 17.Kallinikos P, Efron N. On the etiology of keratocyte loss during contact lens wear. Invest Ophthalmol Vis Sci. 2004;45(9):3011–3020. doi: 10.1167/iovs.04-0129. [DOI] [PubMed] [Google Scholar]
- 18.Prakasam RK, Schwiede M, Hütz WW, Guthoff RF, Stachs O. Corneal responses to eye rubbing with spectral domain optical coherence tomography. Curr Eye Res. 2012;37(1):25–32. doi: 10.3109/02713683.2011.622850. [DOI] [PubMed] [Google Scholar]
- 19.Kalogeropoulos G, Chang S, Bolton T, Jalbert I. The effects of short-term lens wear and eye rubbing on the corneal epithelium. Eye Contact Lens. 2009;35(5):255–259. doi: 10.1097/ICL.0b013e3181b4ec39. [DOI] [PubMed] [Google Scholar]
- 20.Carel RS, Korczyn AD, Boyman R. Amethocaine and intraocular pressure. Ophthalmic Res. 1979;11:212–215. [Google Scholar]
- 21.Baudouin C, Gastaud P. Influence of topical anesthesia on tonometric values of intraocular pressure. Ophthalmologica. 1994;208(6):309–313. doi: 10.1159/000310527. [DOI] [PubMed] [Google Scholar]
- 22.Almubrad TM, Ogbuehi KC. Clinical investigation of the effect of topical anesthesia on intraocular pressure. Clin Ophthalmol. 2007;1(3):305–309. [PMC free article] [PubMed] [Google Scholar]
- 23.Ogbuehi KC. Corneal biomechanical parameters and intraocular pressure: the effect of topical anesthesia. Clin Ophthalmol. 2012;6:871–878. doi: 10.2147/OPTH.S32322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Toit G. Clinical allergy images-allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2005;18(3):148–150. [Google Scholar]
- 25.Bielory L. Allergic and immunologic disorders of the eye. Part II: ocular allergy. J Allergy Clin Immunol. 2000;106(6):1019–1032. doi: 10.1067/mai.2000.111238. [DOI] [PubMed] [Google Scholar]
- 26.Liu JH, Kripke DF, Twa MD, Hoffman RE, Mansberger SL, Rex KM, Girkin CA, Weinreb RN. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40(12):2912–2917. [PubMed] [Google Scholar]
- 27.Lattimore MR, Jr, Kaupp S, Schallhorn S, Lewis R., 4th Orbscan pachymetry: implications of a repeated measures and diurnal variation analysis. Ophthalmology. 1999;106(5):977–981. doi: 10.1016/S0161-6420(99)00519-9. [DOI] [PubMed] [Google Scholar]
- 28.Hashemi H, Asgari S, Mehravaran S, Emamian MH, Shariati M, Fotouhi A. The distribution of corneal thickness in a 40- to 64-year-old population of Shahroud, Iran. Cornea. 2011;30(12):1409–1413. doi: 10.1097/ICO.0b013e31822018dd. [DOI] [PubMed] [Google Scholar]
- 29.Jalbert I, Sweeney DF, Stapleton F. The effect of long-term wear of soft lenses of low and high oxygen transmissibility on the corneal epithelium. Eye (Lond) 2009;23(6):1282–1287. doi: 10.1038/eye.2008.307. [DOI] [PubMed] [Google Scholar]
- 30.Gifford P, Alharbi A, Swarbrick HA. Corneal thickness changes in hyperopic orthokeratology measured by optical pachometry. Invest Ophthalmol Vis Sci. 2011;52(6):3648–3653. doi: 10.1167/iovs.10-6323. [DOI] [PubMed] [Google Scholar]
- 31.Herse P, Siu A. Short-term effects of proparacaine on human corneal thickness. Acta Ophthalmol Scand. 1992;70(6):740–744. doi: 10.1111/j.1755-3768.1992.tb04879.x. [DOI] [PubMed] [Google Scholar]
- 32.Asensio I, Rahhal SM, Alonso L, Palanca-Sanfrancisco JM, Sanchis-Gimeno JA. Corneal thickness values before and after oxybuprocaine 0.4% eye drops. Cornea. 2003;22(6):527–532. doi: 10.1097/00003226-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Carlson AN. Expanding Our Understanding of Eye Rubbing and Keratoconus. Cornea. 2010;29(2):245. doi: 10.1097/ICO.0b013e3181bdefbc. [DOI] [PubMed] [Google Scholar]
- 34.McMonnies CW. Management of chronic habits of abnormal rubbing. Cont Lens Anterior Eye. 2008;31(2):95–102. doi: 10.1016/j.clae.2007.07.008. [DOI] [PubMed] [Google Scholar]


