Abstract
AIM
To determine the role of subjective assessment using McMonnies dry eye questionnaire in diagnosing dry eye disease and its association with clinical tests.
METHODS
There were 500 patients screened for dry eye using McMonnies dry eye questionnaire between May to October 2013 at the outpatient Department of Ophthalmology of a medical college hospital. All 500 patients were subjected to clinical tests. Dry eye was defined as having one or more symptoms often or all the time. Positive signs were if one or both eyes revealed tear film breakup time (TBUT) of ≤10s, a Schirmer test score of ≤10 mm, a Rose Bengal staining score of ≥1, a Lissamine green staining score of ≥1 or existence of meibomian gland disease (≥grade 1). Statistical analysis was performed to describe the distribution of symptoms and signs, to assess the correlations between McMonnies score (MS) and variable clinical signs of dry eye, and to explore the association between dry eye symptoms and variable clinical signs. Analysis was performed using software package Epi info. A Probability (P) value using Chi-square test of <0.005 was taken as significant.
RESULTS
Dry eye prevalence with symptoms (questionnaire), Schirmer test, TBUT, Rose Bengal staining and Lissamine green staining was 25.6%, 15.20%, 20.80%, 23.60%, and 22.60% respectively. Among those with severe symptoms (MS>20), 75.86% had a low TBUT (<10s), 58.62% had a low Schirmer's I test (≤10 mm), 86.20% had Rose Bengal staining score of ≥1, 79.31% had Lissamine green staining score of ≥1. We found statistically significant associations between positive Schirmer test and arthritis (P<0.002), dryness elsewhere (P<0.001), contact lens use (P<0.002), systemic medication (P<0.0001), sleeping with eyes partly open (P<0.002), history of dry eyes treatment (P<0.0001), environmental factors (P<0.001), swimming (P<0.001).
CONCLUSION
Subjective assessment plays an important role in diagnosing dry eye disease. There is strong correlation between MS and Schirmer test, TBUT, Rose Bengal staining and Lissamine green staining in normal as well as marginal and pathological dry eye.
Keywords: McMonnies dry eye questionnaire, Schirmer test, tear film breakup time test, Rose Bengal staining, Lissamine green staining
INTRODUCTION
Dry eye causing chronic ocular irritation is extremely distressing both for patients and ophthalmologists. The pre-ocular tear film-air interface is the principle refractive surface of the eye. The maintenance of stable quality tear film is of paramount importance with regard to good vision. Tear film consists of three layers. The most superficial layer of tear film is lipid layer, 0.11 µm thick produced by the meibomian glands. It serves to retard evaporation, allows smooth movements of the lids, prevents contamination of the tear film, and thickens and stabilizes the tear film. The middle layer the widest at 7.0 µm is the aqueous layer produced by the main lacrimal gland as well as accessory lacrimal glands of Krause and Wolfring. Aqueous tear deficiency is the most common cause of dry eye. Aqueous layer constitutes over 90% of tear film. The layer closest to the cornea is the mucin layer 0.02-0.05 µm thick, produced by conjunctival goblet cells. Mucin layer lowers tear's surface tension and forms loose adsorptive coating rendering the corneal and conjunctival surfaces wettable[1]–[3]. Each layer of tear film can be affected by different diseases, each causing clinically dry eye or keratoconjunctivitis sicca.
Dry eye conditions have been classified into two major categories: 1) tear deficient dry eye (TDDE), in which there is deficiency of lacrimal component of tears; 2) evaporative dry eye (EDE), where the cause is excessive evaporation.
TDEE can further be separated into Sjogren syndrome (SS) dry, eye an autoimmune disorder affecting the lacrimal and salivary glands and non SS that encompasses the range of other causes of tear deficiency. EDE is caused by alternation or deficiency in lipid secretion by meibomian glands resulting in increased evaporation of aqueous tear from ocular surface. The leading cause is meibomian gland dysfunction[4].
At present there is no single gold standard test for developing a clinical diagnosis of dry eye. A battery of tests are employed to test different component layers of tear film in addition to patients self-reported symptoms. Clinical signs and symptoms very often lack coherence with subjective symptoms and complain by the patients and vice versa. This makes it hard to grade the severity and course of the disease and also the results of clinical studies[5]. Different questionnaires have been designed to address this problem[6]–[11]. Still the correlation between subjective complains and objective symptoms is not satisfying as is the test-retest reliability. Despite the subjective nature of symptom reporting, they are more repeatable and reliable than are the commonly employed objective tests and should always be a part of dry eye evaluation. The two most commonly used dry eye questionnaires are OSDI and MacMonnies dry eye questionnaires. We have chosen MacMonnies dry eye questionnaire as this is more comprehensive for dry eye screening with inclusion of patients demographic details like grades based on age and gender combined, dry eye symptoms with their frequency, history of dry eye treatment, history of systemic diseases commonly associated with dry eye, history of systemic medications causing dry eye, history of swimming, contact lens use, sleep with eyes open, alcohol consumption and effect of all these on dry eye symptoms etc. It is reported to have moderate to excellent test-retest reliability and good validity also.
Our first objective was to study the prevalence of dry eye using a validated MacMonnies dry eye questionnaire and to compare the results with those of objective tests. Our second objective was to study the effect of environmental and personal factors on dry eye symptoms in an Industrial township in western India.
SUBJECTS AND METHODS
Study Site and Context
The study was carried out in a Medical College Teaching Hospital among outdoor patients. The college is situated in the industrial township of a city in western Maharashtra, India (Total population about 17 lacs). Due to rapid industrialization, leading to rural-urban migration, the outpatients were comprised of a large number of migrant populations from rural areas. The timeframe to complete the data collection and entry was six months. The study was conducted during May-Oct 2013. A cross-sectional study design was used. A pilot study was carried out before the main study to ascertain the number of patients who could be interviewed and examined properly in a day. Based on the findings of the pilot study the final sample size was decided. For example, it was found that in a day, three patients could be interviewed properly and then subjected to dry eye tests, so given the 160d for data collection, a sample size of 480 subjects was planned rounded off to 500.
During the data collection period, three consecutive patients having various eye problems were approached daily in the waiting room of the Outpatients Department (OPD) of Ophthalmology of the Medical College Hospital. They were explained the purpose of the study and then asked to give a written informed consent to participate in the study. These patients were screened for dry eye using a validated 12-point MacMonnies dry eye questionnaire (Figure 1) and then subjected to clinical examination.
Figure 1. McMonnies dry eye questionnaire.
Institutional Ethical Committee approval was obtained before starting the study. The study was conducted following the principles outlined in the Declaration of Helsinki on human subjects.
Exclusion Criteria
Subjects with acute eye infection, extensive corneal or conjunctival pathology, lid abnormality and subjects who had undergone any eye surgery within 6mo of screening were excluded from the study.
Inclusion Criteria
All other subjects above 15y of age were included.
Instruments Used
Validated McMonnies dry eye questionnaire, fluoresceine strips, Schirmer test strips (Whatman filter paper 41), Rose Bengal test strips, Lissamine green test strips.
Procedure
Subjects demographic details including name, age, sex, place of residence (urban or rural), occupation was recorded in the proforma. All subjects were asked to fill McMonnies questionnaire which included 12 items.
Assessing Symptoms
History of following symptoms was asked with the help of the questionnaire-dry, gritty/scratchy, or filmy feeling in the eyes, burning or itching in the eyes, redness of the eyes, Blurred vision, sensation of having foreign body in the eyes and light sensitivity. They were asked if their symptoms worsen in dry climates, in windy conditions, air conditioning, with higher temperatures, with lower humidity, with prolonged use of eyes (reading, watching TV), and towards end of the day. They were also inquired about the frequency (how often a symptom is experienced) and severity (how bad or disabling the symptom is). They were also asked if and how their eye irritation or discomfort prevented them from using a computer or reading. Those patients who wear contact lenses were asked about the length of comfortable lens wearing time. Similarly those who were using computers were asked about comfortable computer use in hours. History of thyroid abnormality, arthritis, dryness elsewhere in the body, alcohol consumption, unusual sensitivity to chlorine in swimming pool, use of eye drops or any other treatment for dry eye were recorded.
Dry eye screening was done based on these symptoms as follows: normal (<10), marginal (10-20) and pathological dry eye (>20) as per guidelines of MacMonnies dry eye questionnaire. Out of 500 patients who filled the dry eye questionnaire, only 168 patients had MacMonnies scores (MS) >10 and 332 patients had a score of <10 which is considered as normal. These age and sex matched patients with normal scores of <10 were taken as controls and all the statistical analysis and inferences are drawn on this assumption. All 500 patients were subjected to objective tests.
Clinical Examination
The clinical examination included visual acuity recording, Schirmer test, slit lamp examination of anterior segment, examination of cornea after fluorescein staining, tear film breakup time test (TBUT), Lissamine green and Rose Bengal staining. The sequence of examination was as follows:
1) Visual acuity assessment by Snellen chart.
2) Schirmer test (for tear quantity). Test was performed by using sterile Schirmer (filter paper) strips which are 5×35-mm2. The test results were considered positive if length of wetting obtained was less than 10 mm or less in 5min.
3) Slit lamp examination of anterior segment. To see the presence of conjunctival congestion, conjunctival discharge, mucus filament, meibomian gland dysfunction and dryness of bulbar conjunctiva.
4) TBUT test (TBUT for quality of tear film). It was recorded after fluorescein staining. The test was considered positive if average TBUT was less than 10s.
5) Ocular surface (diagnostic dye evaluation): Rose Bengal 1% and Lissamine green 1.5 mg/mL staining. It was performed by using sterile Rose Bengal paper strips and Lissamine green paper strips applied for 2min in lower outer conjunctional cul-de-sac. Both these stains highlighted epithelial surfaces that had been deprived of mucin protein protection which had exposed epithelial cell membranes. The Oxford grading scheme was used for grading ocular surface damage[10]. The grading chart is made up of five panels, each of which represents typical gradations of stain on either cornea or conjunctiva. Grading is done as 0, I, II, III, IV and V depending on number of dots per panel. Minimum being grade 0 and maximum score is V[12].
Diagnosis and Statistical Analysis
Dry eye was defined as having one or more symptoms often or all the time with MS>10. Positive signs were if one or both eyes revealed TBUT of ≤10s, a Schirmer test score of ≤10 mm, a Rose Bengal staining score of ≥1, a Lissamine green staining score of ≥1 or the existence of meibomian gland disease which was diagnosed when telangiectasia at the lid margin or plugging of the gland orifices was present (≥grade 1). Statistical analysis was performed to describe the distribution of symptoms and signs, to assess the correlations between MS [Normal (<10), marginal (10-20) and pathological dry eye (>20) as per guidelines of MacMonnies dry eye questionnaire] and variable clinical signs of dry eye, and to explore the association between dry eye symptoms and variable clinical signs. All patients with MS<10 were treated as control. Analysis was performed using software package Epi info. A probability (P) value using Chi-square test of <0.05 was taken as significant.
RESULTS
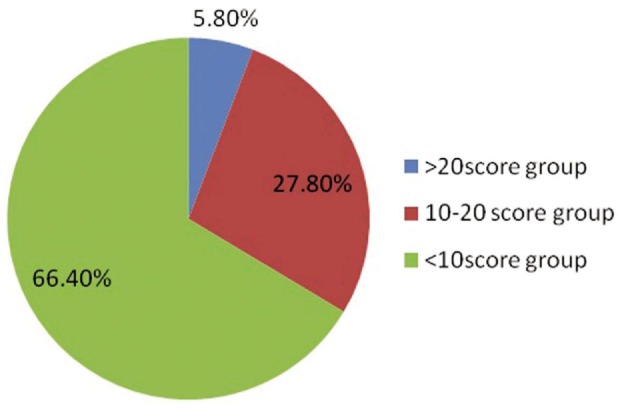
A total of 500 patients were screened for dry eye using McMonnies dry eye questionnaire between May and October 2013 at the outpatient Department of Ophthalmology of a medical college hospital. Five hundred eyes of 250 patients who complained of 1 or more symptoms were subjected to Schirmer test, TBUT, Rose Bengal staining and Lissamine green staining. Dry eye prevalence with symptoms (questionnaire), Schirmer test, TBUT Rose Bengal staining and Lissamine green staining was 25.6%, 15.20%, 20.80%, 23.60%, and 22.60% respectively. These patients were divided in three age groups. There were 114 (22.8%) patients in <25y age group, 226 (45.2%) in 25-45y, and 160 (32%) in >45y age group. Males were 248 (49.6%) while females were 252 (50.4%). Rural migrants were 48% and urban were 52%. McMonnies index was used to group the patients as <10 (normal), 10-20 (moderate dry eye) and >20 (severe dry eye) score groups. Out of a total of 500 patients 332 (66.40%) had a score of <10, 139 (27.80%) had 10-20 while 29 (5.80%) had a score of >20 (Figure 2).
Figure 2. Score groups based on McMonnies questionnaire.
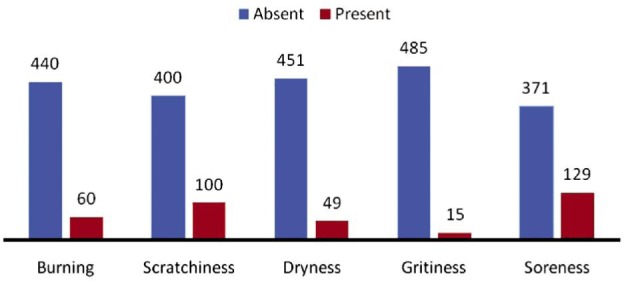
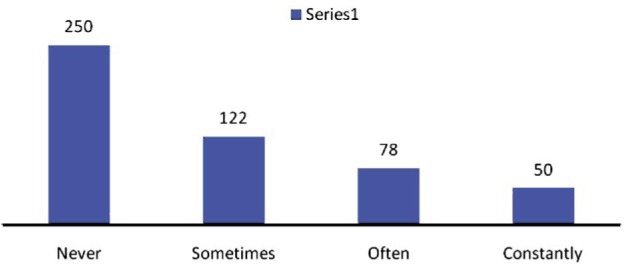
Out of a total of 500 patients, 129 (25.80%) complained of soreness in the eyes, 15 (3%) complained of grittiness, 100 (20.00%) complained of scratchiness in the eyes, 49 (9.80%) complained of dryness in the eyes, and 60 (12%) complained of burning in the eyes (Figure 3). In 122 (24.4%) patients, symptoms were present sometimes, while often in 78 (15.6%), and constantly (all the time) in 50 (10%) of the patients (Figure 4). Meibomian gland disease was present in 15.2% patients.
Figure 3. Number of patients complaining of various symptoms in study group.
Dry eye symptoms (n=500).
Figure 4. Frequency of symptoms among study subjects.
Symptom frequency (n=500).
Out of 500 patients, 40 (8%) gave history of alcohol consumption, out of which 25 (62.5%) complained that their eyes always felt dry and irritated through the day after drinking alcohol while 15 (37.5%) felt so sometimes. History of thyroid abnormality was found in 15 (3%) patients while 45 (9%) gave history of arthritis. The association between arthritis and Schirmer test result was statistically very significant (P<0.002) while that with thyroid abnormality was not significant (P=0.865). And 109 (21.8%) patients complained of dryness elsewhere in nose, mouth, throat, chest or vagina sometimes, 19 (3.8%) had these complaints often while 3 (0.6%) experienced dryness elsewhere constantly. The association between dryness elsewhere and Schirmer test result was statistically very significant (P<0.001). Thirty-two (6.4%) patients sometimes complained of eye irritation on waking up from sleep while 24 (4.8%) patients always complained of eye irritation on waking up.
A total of 12 (2.4%) patients were using soft contact lens, out of which 18.18% and 8.33% had positive Schirmer test with strip wetting of <10 mm and <5 mm, while out of 93 (18.6%), rigid gas permeable (RGP) lens users, 10.75% had <10mm and 7.53% had <5 mm wetting. The association between contact lens use and Schirmer test result was statistically very significant (P<0.002, Table 1).
Table 1. Relation between contact lens use, history of systemic drugs intake, and history of dry eye treatment with Schirmer test.
| Schirmer test results | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Total | P |
| Contact Lens | ||||||
| None | 344 (87.09) | 39 (9.87) | 11 (2.78) | 1 (0.25) | 395 | <0.002 |
| RGP | 75 (80.65) | 10 (10.75) | 7 (7.53) | 1 (1.08) | 93 | |
| Soft CL | 5 (41.67) | 6 (50.00) | 1 (8.33) | 0 | 12 | |
| Drugs | ||||||
| No drug | 330 (87.77) | 34 (9.04) | 11 (2.93) | 1 (0.27) | 376 | <0.0001 |
| Other drugs1 | 73 (78.49) | 15 (16.13) | 5 (5.38) | 0 | 93 | |
| Antihistamines (systemic/topical) | 19 (73.08) | 3 (11.54) | 3 (11.54) | 1 (3.85) | 26 | |
| Antihistamines+other drugs | 1 (25.00) | 3 (75.00) | 0 | 0 | 4 | |
| Eye-drops for dry eye | ||||||
| None | 378 (88.52) | 33 (7.73) | 14 (3.28) | 2 (0.47) | 427 | <0.0001 |
| Uncertain | 6 (100.00) | 0 | 0 | 0 | 6 | |
| Yes | 40 (59.70) | 22 (32.84) | 5 (7.46) | 0 | 67 |
1Diuretics, sleeping tablets, tranquillizers, oral contraceptives, medication for duodenal ulcer, antihypertensive, antidepressants.
n=500 (%)
Fifty-nine (11.8%) patients complained that their eyes were always unusually sensitive to cigarette smoke, smog, air conditioning, or central heating while 84 (16.8%) sometimes had these complaints. The association between these symptoms and Schirmer test result was statistically highly significant (P<0.001). Forty (8%) patients said that their eyes always became very red and irritated after swimming while 29 (5.8%) sometimes felt so. Other 29 (5.8%) swimmers were asymptomatic. The association between these symptoms and Schirmer test result was statistically highly significant (P<0.001).
Out of 26 patients on antihistamines, 7 (26.92%) had Schirmer test positive. Out of 93 patients on other drugs, 20 (21.5%) had Schirmer test positive. Out of 4 patients on both antihistamine and antihypertensive drug, 3 (75.00%) had Schirmer test positive. The association between systemic medication and Schirmer test was statistically highly significant (P<0.0001, Table 1).
Out of a total of 67 patients who were already using eye drops or any other treatment for dry eyes, 40 (59.7%) patients showed normal Schirmer test while 22 (32.84%) and 5 (7.46%) had positive Schirmer test with <10 mm and <5 mm wetting respectively which was statistically highly significant (P<0.0001, Table 1).
Thirty-five (7%) patients gave history of sleeping with eyes partly open sometimes, out of these 3 (8.57%) and 2 (5.71%) patients showed positive Schirmer test with <10 mm and <5 mm reading respectively. Fifteen (3%) patients always slept with their eyes partly open, out of these 7 (46.67%) and 2 (13.33%) had positive Schirmer test with <10 mm and <5 mm readings respectively. The association was statistically significant (P<0.002).
On comparing symptom scores with Rose Bengal staining, it can be seen that in Oxford grade 0 group, percentage of patients is decreasing as symptom score increase while in Oxford grade 1 and 2 groups, percentage of patients is increasing with increasing scores which indicates that there is a good correlation between patients symptoms and Rose Bengal staining and it was statistically highly significant (P<0.0001, Table 2).
Table 2. Relationship between McMonnies scores and Rose Bengal staining.
| Score group | Rose Bengal-Oxford grades |
Total | P | ||
| 0 | 1 | 2 | |||
| 1 (<10) Control group | 307 (92.47) | 23 (6.93) | 2 (0.60) | 332 | <0.0001 |
| 2 (10-20) Marginal dry eye | 71 (51.08) | 61 (43.88) | 7 (5.04) | 139 | |
| 3 (>20) Pathological dry eye | 4 (13.79) | 23 (79.31) | 2 (6.90) | 29 | |
n=500 (%)
On comparing McMonnies symptom scores with Lissamine green staining, it can be seen that in Oxford grade 0 group, percentage of patients is decreasing as symptom score increases while in Oxford grade 1 and 2 groups, percentage of patients is increasing with increasing scores and it was statistically highly significant (P<0.001) which indicates that there is a good correlation between patients symptoms and Lissamine green staining (Table 3).
Table 3. Relationship between McMonnies Scores and Lissamine green staining.
| Score Group | Lissamine green staining-Oxford grades |
Total | P | ||
| Grade 0 | Grade 1 | Grade 2 | |||
| 1 (<10) Control group | 308 (92.77) | 22 (6.63) | 2 (0.60) | 29 | <0.001 |
| 2 (10-20) Marginal dry eye | 74 (53.24) | 58 (41.73) | 7 (5.04) | 139 | |
| 3 (>20) Pathological dry eye | 5 (17.24) | 22 (75.86) | 2 (6.90) | 332 | |
n=500 (%)
Similarly on comparing McMonnies symptom scores with Schirmer test, it shows that with increase in symptom scores, severity of dry eye based on Schirmer test readings also increased and it was statistically highly significant (P<0.001) which shows symptom scores correlates well with Schirmer test results (Table 4).
Table 4. Relationship between McMonnies scores and Schirmer test.
| Score group | Schirmer test results1 |
Total | P | |||
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |||
| 1 (<10) Control group | 323 (97.29) | 4 (1.20) | 5 (1.51) | 0 (0.00) | 332 (100.00) | <0.001 |
| 2 (10-20) Marginal dry eye | 89 (64.03) | 38 (27.34) | 10 (7.19) | 2 (1.44) | 139 (100.00) | |
| 3 (>20) Pathological dry eye | 12 (41.38) | 13 (44.83) | 4 (13.79) | 0 (0.00) | 29 (100.00) | |
1Grade 0: >15 mm/5min; Grade 1: <10 mm/5min; Grade 2: <5 mm/5min; Grade 3: <2 mm/5min.
n=500 (%)
On comparing McMonnies symptom scores with TBUT, in control group (symptom score group 1 with <10 score), 9 (2.71%) patients showed TBUT positive; in score group 2 (marginal dry eye with score 10-20), 72 (51.80%) patients were TBUT positive; in score group 3 (pathological dry eye with score >20), 22 (75.86%) had positive break up time. It shows a decreasing number of patients in column 1 showing negative TBUT test results (i.e. >10s break-up time) with increasing McMonnies symptom scores while increasing percentages of patients in column 2 showing positive TBUT test results (<10s break-up time) with increasing symptom scores which indicates that symptom scores correlates well with TBUT test results and it was statistically highly significant (P<0.001, Table 5).
Table 5. Relationship between McMonnies scores and tear film break up time.
| Score group | Tear film breakup time test |
Total | P | ||
| Grade 0 | Grade 1 | Grade 2 | |||
| 1 (<10) Control group | 322 (96.99) | 9 (2.71) | 1 (0.30) | 332 (100.00) | <0.001 |
| 2 (10-20) Marginal dry eye | 67 (48.20) | 72 (51.80) | 0 (0.00) | 139 (100.00) | |
| 3 (>20) Pathological dry eye | 7 (24.14) | 22 (75.86) | 0 (0.00) | 29 (100.00) | |
Tear film break up time, Grade 0: >10s; Grade 1 <10s; Grade 2 <5s.
n=500 (%)
DISCUSSION
Dry eye disease is a major tear deficiency disorder that affects millions of people world-wide[4]. It is a distressing problem which is often overlooked and under-diagnosed. The recent International Dry Eye Workshop (DEWS) report defined dry eye as a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface[13]–[19].
A healthy tear film nourishes, lubricates and protects the ocular surface. Any dysfunction of the main or accessory lacrimal glands, the meibomian glands, eyelids, cornea, conjunctiva or the connecting neural reflex arcs (the components which together form the lacrimal functional unit) causes tear film instability, symptoms of grittiness and irritation, ocular surface inflammation and ultimately signs of ocular surface damage and visual impairment[2]. Whilst dry eye patients report similar symptoms of dryness, grittiness, irritation and burning, the causes can be diverse. Reduction in quality of life is inevitable when symptoms of dry eye occur. It can interfere with daily social and physical functioning, with a significant impact on work place productivity and is associated with a significant socio-economic burden[20],[21].
Tests of tear function including Schirmer test, TBUT, fluorescein staining, or Rose Bengal staining for determination of dry eye have found generally lower prevalence rates as compare to questionnaire based surveys of dry eye symptoms. This might include a history of contact lens wear, previous treatment for dry eye, frequency of symptoms, sensitivity to provocative stimuli, use of systemic medications, and co-morbidity. Borderline dry eye can become manifest in the presence of cigarette smoke, or in highly air-conditioned or centrally heated environments. Besides there is occupation based etiology (computer operators using computers for long hours), chlorine used to disinfect swimming pools is another known provocative stimulus, as is dehydration after alcohol consumption[13]. Left untreated, dry eye may result in decreased vision complicated by ulceration and scarring. We studied the prevalence of dry eye, effect of environmental and personal factors on dry eye symptoms and its correlation with clinical signs.
Literature review on previous epidemiological data reveals that prevalence of dry eye could be as high as 11%-30% of the general population and is found to be more common in Asians compared with other races[4],[22].
In our study prevalence of dry eye based on symptoms (often or all the time), Schirmer test, TBUT, Rose Bengal staining and Lissamine Green staining was 25.6%, 15.20 %, 20.80%, 23.60%, and 22.60% respectively. This indicates a good association between subjective symptoms and clinical tests. Lower prevalence by Schirmer test may be explained by the fact that many patients with symptoms of dry eye syndrome may have a normal aqueous tear production and normal Schirmer score. In such cases their symptoms may be caused by the common, but not always properly diagnosed, condition meibomian gland dysfunction. Another possibility is a poor mucin layer resulting in poor adherence of tears to the ocular surface. Yet another explanation for variable schirmer test results is the inter- and intra-patient variability, that is normal human tear volume may range from 7 µL to 30 µL, while tear turnover rates range from 0.5 µL/min to 2.2 µL/min[13],[23].
Another study reported that most of the patients presented with symptoms of foreign body sensation, discomfort, itching, tearing and photophobia. In our study, majority 25.8% complained of soreness, followed by scratchiness 20.0%, burning eyes 12% and dryness 9.8% in that order.
In an epidemiological study of dry eye in elderly Chinese in Taiwan, Lin et al[24] found that of the 1361 participating in the study, 33.7% had symptoms of the condition (reporting one or more dry eye symptoms often or all time). Among those with symptoms, 78.9% had a low TBUT (<10s), 62.5% had a low Schirmer's I test (<5 mm) 61.7% had abnormal anatomic features of the meibomian glands, and 85.4% had symptoms and either a low Schirmer I score or abnormal meibomian gland appearance. In a Japanese population study of dry eye disease, a high proportion (33%) was found to have symptoms of the condition. In an Indonesian study of dry eye, Lee et al[25] found that 27.5% of the 1058 participants aged 21y and over reported one or more dry eye symptoms often or all the time. In our study symptoms of the condition were present often or all the time in 25.8% of the patients. MS suggests 35.6% patients were having moderate to severe dry eye. Higher prevalence could be because of inclusion of scores given to positive history of provocative stimuli and presence of intrinsic or extrinsic risk factors for development of dry eye. Among those with severe symptoms (MS>20), 75.86% had a low TBUT (<10s), 58.62% had a low Schirmer I test (<10 mm), 86.20% had Rose Bengal staining score of ≥1, 79.31% had lissamine green staining score of ≥1. Our findings corroborate with findings of Lin et al[24]. Our findings differ from the study conducted by Nichols et al[26] who found poor relation between dry eye tests and symptoms.
We found statistically significant associations between positive Schirmer test and arthritis (P<0.002), dryness elsewhere in nose, mouth, throat, chest or vagina (P<0.001), contact lens use (P<0.002), systemic medication (P<0.0001), history of sleeping with eyes partly open (P<0.002), and history of using eye drops or any other treatment for dry eyes (P<0.0001). These findings are in agreement with prevailing literature. Johnson[27] in his review article suggested an intuitive causal link between the signs of dry eye observed by clinicians and the severity of symptoms experienced by patients.
Recent studies have shown the importance of environmental factors on pathophysiology of dry eye[13]. These symptoms seem to worsen in dry climates, in windy conditions, with higher temperatures, with lower humidity. In a population based survey conducted on 1890 patients in India, Bhatnagar et al[28] and others found a significant association between dry eye disease and exposure to excessive wind, sunlight, high temperature and air pollution[29],[30]. In present study, 28.2% patients reported unusual sensitivity to these environmental factors with statistically significant association with Schirmer test (P<0.001).
Chlorine used to disinfect swimming pools is another known provocative stimulus, as is dehydration after alcohol consumption[13]. We found statistically significant association between history of swimming with Schirmer test (P<0.001) but not with alcohol consumption.
Twenty-one percent of patients complained of dry eye symptoms particularly burning and ocular fatigue after watching television, reading or working on computer for more than 3-4h. When we read, watch television, computer use or perform a task that requires close attention with our eyes, we may not blink as often. If blinking is decreased or if the eyelids cannot be closed, the eyes may dry out because of tear evaporation[13].
On extensive literature search, we could not find any study where association between each item on McMonnies questionnaire was studied against clinical test for dry eye. We found a good association between symptoms and clinical signs of dry eye including Schirmer test, TBUT, Rose Bengal staining and Lissamine green staining.
Symptom assessment plays a large role in the diagnosis of dry eye[31]. We suggest meticulous symptom assessment including each item on the questionnaire should be done for dry eye screening to avoid under diagnosing a case. If patient's symptoms are affecting his day to day working he should be treated for dry eye after ruling out other causative factors. Of course, results of clinical tests including TBUT test, Schirmer test or diagnostic staining methods or newer non-invasive tests should be considered for grading severity of dry eye and choosing the treatment option.
Acknowledgments
We are grateful to Dr. Jadhav SL, Professor, Community Medicine for helping us with the statistical calculations.
Foundation: Supported partly by Indian Council of Medical Research (ICMR)
Conflicts of Interest: Bhatnagar KR, None; Pote S, None; Pujari S, None; Deka D, None.
REFERENCES
- 1.Levin LA. Adler's Physiology of the eye. 11th. Amsterdam: Elsevier; 2011. [Google Scholar]
- 2.American Academy of Ophthalmology . Fundamental and principle of Ophthalmology: section two, Basic and Clinical Science Course. San Francisco: American Academy of Ophthalmology; 2011–2012. [Google Scholar]
- 3.Horwath-Winter J, Gerghold A, Schmut O. Evaluation of the clinical course of dry eye syndrome. Pak J Ophthalmol. 2004;20:43–45. doi: 10.1001/archopht.121.10.1364. [DOI] [PubMed] [Google Scholar]
- 4.Asbell PA, lemp MA. Dry Eye Disease: The Clinician's Guide to Diagnosis and Treatment. New York: Thieme Medical publishers; 2007. [Google Scholar]
- 5.Pult H, Purslow C, Murphy PJ. The relationship between clinical signs and dry eye symptoms. Eye (Lond) 2011;25(4):502–510. doi: 10.1038/eye.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finis D, Pischel N, König C, Hayajneh J, Borrelli M, Schrader S, Geerling G. Comparison of the OSDI and SPEED questionnaires for the evaluation of dry eye disease in clinical routine. Ophthalmologe. 2014;111(11):1050–1056. doi: 10.1007/s00347-014-3042-z. [DOI] [PubMed] [Google Scholar]
- 7.Gothwal VK, Pesudovs K, Wright TA, MacMonnies CW. McMonnies questionnaire: enhancing screening for dry eye syndromes with Rasch analysis. Invest Ophthalmol Vis Sci. 2010;51(3):1401–1407. doi: 10.1167/iovs.09-4180. [DOI] [PubMed] [Google Scholar]
- 8.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Nichols KK, Smith JA. Association of clinical diagnostic tests and dry eye surveys: the NEI-VFQ-25 and the OSDI. Adv Exp Med Biol. 2002;506(Pt B):1177–1181. doi: 10.1007/978-1-4615-0717-8_166. [DOI] [PubMed] [Google Scholar]
- 10.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 11.Vitale S, Goodman LA, Reed GF, Smith JA. Comparison of the NEI-VFQ and OSDI questionnaires in patients with Sjogren's syndrome-related dry eye. Health Qual Life Outcomes. 2004;2:44. doi: 10.1186/1477-7525-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 13.The definition and classification of dry eye disease: report of the definition and classification subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 14.Gayton JL. Etiology, prevalence and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, Lemp MA, Sullivan DA. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, Foulks GN. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–1737. doi: 10.1167/iovs.10-6997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asbell PA, Stapleton FJ, Wickström K, Akpek EK, Aragona P, Dana R, Lemp MA, Nichols KK. The international workshop on meibomian gland dysfunction: report of the clinical trials subcommittee. Invest Ophthalmol Vis Sci. 2011;4(52):2065–2085. doi: 10.1167/iovs.10-6997h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O'Brien T, Rolando M, Tsubota K, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;4(52):2050–2064. doi: 10.1167/iovs.10-6997g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, Yee R, Yokoi N, Arita R, Dogru M. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;4(52):2006–2049. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14(3 Suppl):S102–106. [PubMed] [Google Scholar]
- 21.Waduthantri S, Yong SS, Tan CH, Shen L, Lee MX, Nagarajan S, Hla MH, Tong L. Cost of dry eye treatment in an Asian clinic setting. PLos One. 2012;7(6):e37711. doi: 10.1371/journal.pone.0037711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waduthantri S. Environmental stress and dry eye. Medical Journal of Dr. D. Y. Patil University. 2014;7(1):18–19. [Google Scholar]
- 23.Uchino M, Dogru M, Yagi Y, Goto E, Tomita M, Kon T, Saiki M, Matsumoto Y, Uchino Y, Yokoi N, Kinoshita S, Tsubota K. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006;83(11):797–802. doi: 10.1097/01.opx.0000232814.39651.fa. [DOI] [PubMed] [Google Scholar]
- 24.Lin PY, Cheng CY, Hsu WM, Tsai SY, Lin MW, Liu JH, Chou P. Association between symptoms and signs of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2005;46(5):1593–598. doi: 10.1167/iovs.04-0864. [DOI] [PubMed] [Google Scholar]
- 25.Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, Widjaja D, Tan DT. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86(12):1347–1351. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols JJ, Ziegler C, Mitchell GL, Nichols KK. Self-reported dry eye disease across refractive modalities. Invest Ophthalmol Vis Sci. 2005;46(6):1911–1914. doi: 10.1167/iovs.04-1294. [DOI] [PubMed] [Google Scholar]
- 27.Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surf. 2009;7(4):199–211. doi: 10.1016/s1542-0124(12)70187-8. [DOI] [PubMed] [Google Scholar]
- 28.Bhatnagar KR, Sapovadkar A, Gupta D, Jasani H. Dry eye: a rising occupational hazard. Medical Journal of Dr. D. Y. Patil University. 2014;7(1):13–18. [Google Scholar]
- 29.Rozanova E, Heilig P, Godnić-Cvar J. The eye-a neglected organ in environment and occupational medicine: an overview of known environment and occupational non-traumatic effects on the eyes. Arh Hig Rada Toksikol. 2009;60(2):205–215. doi: 10.2478/10004-1254-60-2009-1869. [DOI] [PubMed] [Google Scholar]
- 30.Savini G, Prabhawasat P, Kojima T, Grueterich M, Espana E, Goto E. The challenge of dry eye diagnosis. Clin Ophthalmol. 2008;2(1):31–55. doi: 10.2147/opth.s1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahai A, Malik P. Dry eye: prevalence and attributable risk factors in a hospital-based population. Indian J Ophthalmol. 2005;53(2):87–91. doi: 10.4103/0301-4738.16170. [DOI] [PubMed] [Google Scholar]



