Abstract
Objectives
To evaluate latent heterogeneity in long-term trajectories of body weight in older adults.
Methods
We analyzed 14-year longitudinal data on 10,314 older adults from the Health and Retirement Study. Semiparametric mixture models identified latent subgroups of similar trajectories of body mass index (BMI).
Results
Five distinct trajectory subgroups emerged: normal starting-BMI with accelerated increase over time (trajectory #1), overweight and increasing (trajectory #2), borderline-obese and increasing (trajectory #3), obese and increasing (trajectory #4), and morbidly obese with decelerating gain (trajectory #5). Blacks and Hispanics had greater risk of membership in ascending high-BMI trajectory groups. Females had approximately half the risk of following overweight and obese increasing BMI trajectories compared with males.
Discussion
Distinct latent subgroups of BMI trajectories and significant racial/ethnic and gender trajectory heterogeneity exist in the older adult population. The propensity of men and minorities to experience high-risk BMI trajectories may exacerbate existing disparities in morbidity/ mortality in older age.
Keywords: BMI trajectories, latent subgroups, membership risk, racial/ethnic, gender, older adults
Introduction
Overweight and obesity are increasingly prevalent among middle-age and older adults (Flegal, Carroll, Ogden, & Curtin, 2010). Excess weight in older ages carries a higher risk for adverse physical and cognitive health outcomes (Field et al., 2001; Profenno, Porsteinsson, & Faraone, 2010) and poses an escalating economic burden for individuals and society (Finkelstein, Trogdon, Cohen, & Dietz, 2009).
Obesity tracks “from cradle to grave” (Eriksson, Forsen, Osmond, & Barker, 2003). Finding support in the life-course development of health paradigm (Ben-Shlomo & Kuh, 2002), extensive research has been focused on the relationship between obesity and aging; yet current knowledge on the progression of body weight starting in middle age is sparse. Cross-sectional and short-term longitudinal studies suggest that body weight increases up to the seventh decade of life, after which it levels off or declines (e.g., Kahng, Dunkle, & Jackson, 2004; McDowell, Fryar, Ogden, & Flegal, 2008; Newman et al., 2001; Seidell & Visscher, 2000). Such studies, however, cannot assess the underlying body weight growth curve (Rogosa, 1988), frequently do not account for cohort or mortality bias (Barone et al., 2006), and cannot distinguish between intrapersonal and interpersonal variations in body weight over time (Singer & Willett, 2003). Few studies have examined the basis for variation in the trajectory of body weight in older adults, with a majority anchored in an assumption of population homogeneity with respect to changes in body weight over time. Such studies focus on the population “average” pattern of change and explain the observed deviation of individuals from the “average” in terms of interindividual differences such as race, age, gender, or disease status (Botoseneanu & Liang, 2011; Kahng, Dunkle, & Jackson, 2004). However, significant heterogeneity and latent subgroups of body weight trajectories may exist within the larger population, reflecting qualitatively distinctive groups which vary in their patterns of body weight change. This alternative, person-centered approach, allows for multiple distinctive trajectories (i.e., statistically different intercept and slope) within a given population and defines the probability of individuals to belong to any such trajectory as a function of time-constant and/or time-varying individual characteristics (Nagin & Tremblay, 2005; Nagin, 2005).
The examination of heterogeneity in trajectories of body weight starting in middle age may be useful in several respects. First, each trajectory may hold distinct clinical significance (i.e., different etiology and/or associated disease risk) and may call for individualized targeted interventions. Second, it may assist in identifying socioeconomic, behavioral, or comorbidity factors associated with a higher propensity of experiencing “risky” weight trajectories. Third, the ability to recognize trajectories of body weight associated with a higher risk for or accelerated decline in physical, functional, or cognitive health may result in more efficient public health efforts aimed at reducing the long-term effects of obesity, by selectively focusing on those subgroups most likely to benefit from such interventions.
This study has two specific aims. First, we hypothesize that latent subgroups of trajectories of body mass index (BMI) exist within the middle-age and older adult population. Using group-based semiparametric mixture models (Jones & Nagin, 2007; Nagin, 2005), we aim to identify distinct subgroups of BMI trajectories over a 14-year period (1992-2006), in a nationally representative sample of Americans age 51 to 61 years old at the baseline, and to explore whether differences in these trajectories are mainly the result of variations in their intercept (i.e., differences in weight accumulation prior to the baseline middle age), slopes (i.e., differences in the rate-of-change during the examined study period), or both. As a second aim, we explore racial/ ethnic and gender differences in trajectory-group membership probabilities.
Method
Study population
We evaluated 14-year longitudinal data from the Health and Retirement Study (HRS) (http://hrsonline.isr.umich.edu/). To minimize the potential for bias due to cohort differences in BMI (Reynolds & Himes, 2007), respondents from a single cohort (1931-1941 birth years) were analyzed. Data were gathered every 2 years from 1992 to 2006, for up to eight repeated observations. Of the 13,565 individuals in the HRS cohort, we excluded 3,116 (22.9%) cohort-ineligible spouses (i.e., born before 1931 or after 1941) and 135 (0.9%) participants who did not respond to the health survey sections. The final analytic sample consisted of 10,314 individuals (6.4 mean number of interviews completed). Response rates ranged from 81.7% (1992) to 89.2% (1994); 55.7% of respondents completed all eight interviews. As of 2006, the cumulative mortality rate, validated through the National Death Index (NDI), was 18.7%. Proxy interviews were conducted when a respondent was unable to participate due to physical or cognitive limitations (range from 4.8% in 1992 to 9.0% in 2002).
Assessment of weight, height, and BMI
Self-reported weight was recorded at each wave; height was recorded at the first interview and verified in the second wave. BMI was calculated at each wave as follows: BMI = [Weight (lb.)/ Height (in.)2] × 703, using current weight and initial height. For validation purposes, interviewer-measured weight and height were available for the 2004 and 2006 waves.
Statistical analysis
We conducted analyses in two stages. First, trajectories of BMI were determined by fitting semiparametric mixture models (SPMM) to the data using the Proc Traj procedure in SAS (Jones, Nagin, & Roeder, 2001). SPMMs identify distinct groups of individual trajectories within a population and, by use of posterior probabilities, assign individuals to the group to which they have the highest probability of belonging (probability of 0.9 or higher was considered excellent fit, a value of 0.7 or lower was considered poor fit; Nagin, 2005). Maximum likelihood estimation was used to estimate the group trajectory parameters. We assumed a censored normal distribution to account for potential floor and ceiling effects in BMI and evaluated the BMI growth curves plotted as a function of time (Alwin, Hofer, & McCammon, 2006), with control for interpersonal differences in age-at-baseline (Liang et al., 2008). Intercept-only, linear, quadratic, and cubic time functions for successive models with between 2 and 10 trajectories were tested. Time (t) was centered at its mean to minimize the possibility of multicollinearity when evaluating nonlinear time functions. The best-fitting model (i.e., the number of distinct trajectories) was specified, as recommended, using Bayesian Information Criterion (BIC) scores and examination of 95% CIs (Nagin, 2005).
In the second stage, potential predictors of trajectory membership were included in the models. The log odds for the effect of race/ethnicity and gender on the probability of membership in each trajectory relative to a designated comparison group were derived using specifications similar to multinomial logistic regression (Nagin, 2005). The Proc Traj procedure in SAS estimated the equation for stage 2 simultaneously with equation for stage 1 (Jones, Nagin, & Roeder, 2001; Jones & Nagin, 2007).
We chose not to weight the data because many of the variables used in the calculation of differential selection weights (e.g., race/ethnic group, gender, marital status) are explicitly included in the models, making unweighted ordinary least squares estimates less biased than weighted estimates (Winship & Radbill, 1994). Results from unweighted analyses are shown henceforth, except in Table 1, where in accordance with the consensus on the presentation of descriptive information we show weighted sample characteristics (Gelman, 2007).
Table 1.
Sample Baseline Characteristics and Attrition Status Indicatorsa (N = 10,314 Respondents).
| Covariates | Mean ± SD/% |
|---|---|
| Body Mass Index (BMI) | 26.98 ± 4.96 |
| Sociodemographic | |
| Age (1992) | 55.83 ± 3.17 |
| Female | 52.3% |
| Educationb | 12.34 ± 3.05 |
| Non-Hispanic Blackc | 10.3% |
| Hispanicc | 6.5% |
| Married | 0.77 ± 0.42 |
| Incomed | 2.57 ± 1.11 |
| Assetsd | 2.46 ± 1.21 |
| Health Status | |
| Self-rated health | 2.55 ± 1.18 |
| Index chronic diseases | 1.15 ± 1.11 |
| Nagi index | 1.44 ± 1.66 |
| CES-D score | 3.77 ± 1.57 |
| Loss to follow-up | |
| Mortalitye | 18.7% |
| Attritione | 7.1% |
| Proxy status | 0.05 ± 0.21 |
Note.
Weighted descriptives shown; respondent level 1992 weights from the HRS Cross Tracker 2006 File.
Education measured by “number of school-years completed.”
Racial/ethnic group “Whites” represents the default percentage up to 100%.
Income and Assets quartile categories shown; quartile range available upon request.
Mortality and attrition recorded between baseline 1992 and 2006.
The Proc Traj procedure allows for missing values on time-varying variables, but excludes from analyses all subjects with missing time-constant values. To minimize the loss of participants due to item-missing (Little & Rubin, 2002), 3 complete data sets were imputed using multiple imputation (MI) for multivariate normal data with 100 draws per missing item. The assumptions and procedure for MI have been described in detail elsewhere (Schafer & Olsen, 1998). Unit-missing data due to mortality and permanent attrition were not imputed and data were censored at the last wave prior to death or drop out. We ran Proc Traj analyses using each of the three imputed data sets. Parameter estimates and their standard errors were calculated by averaging across the three data sets and adjusting for their variance. For validation purposes, we ran the same analyses using the original nonimputed data and obtained similar results. Results of analyses using the three imputed datasets are shown below because they incorporate all the available information from all study participants.
Finally, due to the large HRS sample, a two-sided p-value of less than .01 was considered to indicate statistical significance.
Trajectory predictors and potential confounders
Race/ethnicity (mutually exclusive group designation—non-Hispanic White, non-Hispanic Black, and Hispanic) and gender (1 = female, 0 = male) were examined as predictors of trajectory membership.
Sociodemographic characteristics related to BMI and/or to the potential predictors were included as control variables: education (years of education completed; Ball & Crawford, 2005) and age (years; Clarke, O'Malley, Johnston, & Schulenberg, 2009) were measured at baseline in 1992 and included as time-constant covariates. Total household income (quartiles; Chang & Lauderdale, 2005), total household assets (quartiles; Fonda, Fultz, Jenkins, Wheeler, & Wray, 2004), and marital status (1 = married/living with a partner, 0 = single/divorced/widowed/ separated; Sobal, Rauschenbach, & Frongillo, 2003) were assessed at each wave and included as time-varying covariates (quartiles range for income and assets not shown; available upon request).
To account for potential “healthy survivor” bias in BMI estimation (Mehta & Chang, 2009) and for the changes in participants’ health status over the study period, time-varying measures of physical and mental health were included such as index of chronic diseases (count of seven chronic conditions— heart disease, stroke, high-blood pressure, diabetes, arthritis, chronic lung disease, and cancer; range = 0-7), self-rated health (single-item rating; range = 1 [excellent] −5 [poor]), Nagi index of functional limitations (count of six items representing reported difficulties with common activities; range = 0-6; Nagi, 1979), and depression CES-D score (previously validated short-form 9-item count from the Center for Epidemiological Studies Depression Scale; range = 0-9; Radloff, 1977).
All socioeconomic and health status time-varying covariates were represented by a lagged measure (i.e., observation from the previous wave) and a change term (i.e., difference between current and previous observation) to ensure clear time precedence between these and the dependent variable.
In older populations, mortality and attrition are sources of nonrandom missing data (Little & Rubin, 2002) and potentially bias the results toward healthier, longer-living subjects who may differ in their level and rate-of-change in body weight from those who die or drop out during the study. As such, mortality and attrition need to be addressed as confounders (Harel, Hofer, Hoffman, & Pedersen, 2007; Mroczek & Spiro, 2005). Subgroups of participants were identified based on patterns of missing data and group membership indicators were included in the models (for mortality: 1 = died, 0 = alive at end of study; for attrition: 1 = dropped out for reasons other than mortality and did not return, 0 = completed study) (Hedeker & Gibbons, 2006). Time-varying proxy status was represented by a lagged measure (1 = proxy respondent, 0 = self) and a change term (i.e., difference between current and previous wave).
Results
Sample description
Baseline characteristics of the study population are shown below (additional time-varying sample statistics in Table 1A in Supplementary Appendix).
Trajectories of BMI over time
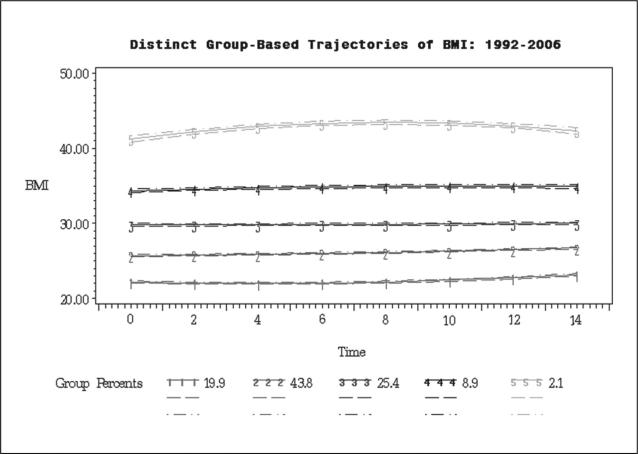
We estimated sequential SPMMs with between 2 and 10 trajectories of BMI. The cubic slope coefficients were not significant in all models. Hence, only quadratic time (t) functions were further analyzed (Table 2). For both the unconditional (M0) and covariate-adjusted models (M1–M3), the BIC revealed an improved model fit from 2 to 5 trajectories, with a plateau in fit improvement evident at specifications greater than 5 trajectories (not shown; available upon request). The narrow, nonoverlapping 95% CIs (Figure 1) provided further support. Thus, the analyses presented hereafter are based on the 5 trajectories model.
Table 2.
Estimates of Growth Curves Parameters for Distinct Trajectories of BMI: 1992-2006.
| Trajectory | M0 | M1 | M2 | M3 |
|---|---|---|---|---|
| Trajectory 1 | ||||
| Normal, increasing (accelerating) | ||||
| Intercept | 22.122*** | 22.015*** | 21.602*** | 21.505*** |
| Linear slope | 0.069*** | 0.064*** | 0.073*** | 0.073*** |
| Quadratic slope | 0.011*** | 0.011*** | 0.010*** | 0.012*** |
| Trajectory 2 | ||||
| Overweight, increasing (linear) | ||||
| Intercept | 26.067*** | 26.081*** | 26.208*** | 25.679*** |
| Linear slope | 0.070*** | 0.072*** | 0.073*** | 0.073*** |
| Quadratic slope | 0.003** | 0.002 | 0.002 | 0.001 |
| Trajectory 3 | ||||
| Borderline obese, increasing (linear) | ||||
| Intercept | 29.869*** | 29.974*** | 30.432*** | 29.484*** |
| Linear slope | 0.016* | 0.028*** | 0.039*** | 0.033*** |
| Quadratic slope | 0.001 | −0.001 | 0.000 | −0.001 |
| Trajectory 4 | ||||
| Obese, increasing (linear) | ||||
| Intercept | 34.905*** | 35.000*** | 36.208*** | 33.745*** |
| Linear slope | 0.042*** | 0.069*** | 0.039*** | 0.011 |
| Quadratic slope | −0.006* | −0.005* | −0.005* | −0.008*** |
| Trajectory 5 | ||||
| Morbidly obese, increasing (decelerating) | ||||
| Intercept | 43.441*** | 43.504** | 43.279*** | 42.239*** |
| Linear slope | 0.077*** | 0.073*** | 0.146*** | 0.097*** |
| Quadratic slope | −0.033*** | −0.030*** | −0.029*** | −0.033*** |
Note. M0 is the unconditional, time-only model; M1 adjusted for mortality, attrition, and proxy status; M2 adjusted for socioeconomic status measures; and M3 adjusted for health status indicators.
p < .05.
p < .01.
p < .001.
Figure 1.
BMI trajectories for five groups model with 95% CIs (1992-2006).
In the unconditional best-fitting model (M0, Table 2), the 5 distinct trajectories of BMI differ from each other primarily in terms of intercept: normal BMI (intercept: b = 22.12, p < .001; 19.9% of sample; trajectory #1), overweight (intercept: b = 26.07, p < .001; 43.8% of sample; trajectory #2), borderline-obese (intercept: b = 29.87, p < .001; 25.4% of sample; trajectory #3), obese (intercept: b = 34.91, p < .001; 8.9% of sample; trajectory #4), and morbidly obese (intercept: b = 43.44, p < .001; 2.1% of sample; trajectory #5). The results pertaining to intercept differences did not substantially change after sequential adjustment for potential confounders (M1, 2, 3). As for the rate-of-change, all 5 trajectories were characterized by small but significant increases in BMI over the period of observation (Table 2). After adjustment for mortality (M1), socioeconomic characteristics (M2) and health indicators (M3), the normal BMI trajectory displayed an accelerating pattern of increase (positive quadratic slope), while the obese and morbidly obese trajectories showed a diminishing rate of increase in BMI (negative quadratic slope). For the other two groups (overweight and borderline-obese), the best-fitting models showed a linear increase throughout the period of observation. For each trajectory, the predicted BMI values (±SD) at the end of the study period (calculated as a quadratic function of time (t), using the intercept, linear slope and quadratic slope corresponding to each trajectory in Table 2) were as follows: 22.71 (±0.17) for trajectory #1, 26.82 (±0.16) for trajectory #2, 30.71 (±0.20) for trajectory #3, 36.48 (±0.25) for trajectory #4, and 42.9 (±0.38) for trajectory #5.
Table 3 shows the characteristics of the five identified subgroups of participants, defined according to their propensity to follow one of the five distinct trajectories of BMI.
Table 3.
Subgroup Characteristics According to BMI Trajectory.a
| Trajectory 1 | Trajectory 2 | Trajectory 3 | Trajectory 4 | Trajectory 5 | |
|---|---|---|---|---|---|
| Body mass index (BMI) | 22.0 ± 2.25 | 25.7 ± 2.90 | 29.64 ± 3.23 | 34.27 ± 3.70 | 41.56 ± 5.51 |
| Demographics | |||||
| Female (%) | 64.4 | 47.4 | 49.1 | 58.5 | 74.4 |
| Black (%)b | 10.6 | 15.7 | 21.1 | 23.8 | 31.2 |
| Hispanic (%)b | 6.1 | 11.7 | 12.6 | 10.5 | 7.7 |
| Age-at-baseline (years) | 55.86 ± 3.12 | 55.92 ± 3.16 | 55.71 ± 3.14 | 55.44 ± 3.16 | 55.44 ± 3.21 |
| Education (years) | 12.47 ± 3.07 | 11.98 ± 3.36 | 11.73 ± 3.33 | 11.41 ± 3.43 | 11.33 ± 3.05 |
| Health Status | |||||
| Self-rated health (range 1-5) | 2.61 ± 1.18 | 2.58 ± 1.20 | 2.57 ± 1.20 | 2.60 ± 1.21 | 2.70 ± 1.17 |
| Nagi index (range 0-6) | 1.29 ± 1.62 | 1.42 ± 1.67 | 1.59 ± 1.69 | 1.96 ± 1.74 | 2.82 ± 1.69 |
| CES-D score (range 0-9) | 4.88 ± 1.74 | 4.93 ± 1.77 | 4.99 ± 1.81 | 5.25 ± 1.80 | 5.57 ± 1.81 |
| Disease index (range 0-7) | 1.12 ± 1.07 | 1.10 ± 1.12 | 1.08 ± 1.12 | 1.07 ± 1.10 | 1.15 ± 1.12 |
| Died (%) | 17.3 | 22.1 | 19.6 | 14.3 | 15.8 |
| Dropped Out (%) | 6.1 | 9.2 | 7.6 | 3.4 | 3.0 |
| Overall % samplec | 18.4 | 41.3 | 27.6 | 10.2 | 2.5 |
Note.
Plus-minus represents means ± SD.
Non-Hispanic White racial/ethnic group represents the default percentage up to 100%.
Based on fully adjusted model (i.e., M3 in Table 3; model adjusted for socioeconomic and health status covariates); the percentages differ slightly from percentages derived from the unconditional time-only model.
*p < .05.
**p < .01.
***p < .001.
Predictors of trajectory membership
Next, predictors of BMI trajectory groups were examined (trajectory #1 as reference) in successive minimally adjusted (i.e., adjustment for mortality, attrition and proxy status; M2 in Table 4) and fully adjusted (i.e., adjustment for time-varying socioeconomic and health status covariates, M2_1; and baseline BMI, M2_2 in Table 4) models. Coefficient estimates (shown in Table 2A in Supplementary Appendix) were used to derive the relative odds ratios for membership in each trajectory (OR = eb, where b is the logistic regression coefficient). Results (Table 4) indicated significant racial/ethnic and gender differences in the likelihood of trajectory membership.
Table 4.
Odds Ratios of Trajectory Membership (Before and After Adjustment for Socioeconomic and Health Status Covariates): Proc Traj Results.
| Modela | M2 | M2_1 | M2_2 |
|---|---|---|---|
| Trajectory #1 | |||
| Reference group | |||
| Trajectory #2 | |||
| Mortality | 1.185 | 1.162 | 2.479*** |
| Attrition | 1.579*** | 1.660*** | 2.416*** |
| Black | 1.863*** | 1.763*** | 1.342 |
| Hispanic | 2.188*** | 2.149*** | 3.438*** |
| Female | 0.427*** | 0.434*** | 0.836 |
| Education | 0.960*** | 0.958*** | 0.394 |
| Age_1992 | 1.002 | 1.005 | 0.955** |
| BMI_1992 | 2.654*** | ||
| Trajectory #3 | |||
| Mortality | 0.955 | 0.886 | 1.132 |
| Attrition | 1.179 | 1.269 | 2.250** |
| Black | 2.651*** | 2.502*** | 1.412 |
| Hispanic | 2.300*** | 2.305*** | 3.117*** |
| Female | 0.450*** | 0.436*** | 0.847 |
| Education | 1.049*** | 1.041*** | 1.020 |
| Age_1992 | 0.980 | 0.975* | 0.878*** |
| BMI_1992 | 9.786*** | ||
| Trajectory #4 | |||
| Mortality | 0.758* | 0.698** | 0.238*** |
| Attrition | 0.623* | 0.736 | 1.161 |
| Black | 3.037*** | 3.133*** | 1.300 |
| Hispanic | 1.520* | 1.697** | 1.921* |
| Female | 0.683*** | 0.554*** | 0.641** |
| Education | 0.919*** | 0.936*** | 1.043 |
| Age_1992 | 0.955*** | 0.957** | 0.856*** |
| BMI_1992 | 17.236*** | ||
| Trajectory #5 | |||
| Mortality | 0.450*** | 0.394*** | 0.037*** |
| Attrition | 0.392* | 0.542 | 0.750 |
| Black | 3.228*** | 3.007** | 0.701 |
| Hispanic | 0.933 | 0.925 | 0.872 |
| Female | 0.999 | 0.812 | 0.505** |
| Education | 0.910*** | 0.927** | 1.121** |
| Age_1992 | 0.933*** | 0.939** | 0.815*** |
| BMI_1992 | 25.103*** | ||
| Group membership | |||
| Trajectory #1 | 19.11% | 18.39% | 16.82% |
| Trajectory #2 | 42.82% | 41.32% | 35.43% |
| Trajectory #3 | 26.51% | 27.55% | 30.98% |
| Trajectory #4 | 9.28% | 10.22% | 13.53% |
| Trajectory #5 | 2.28% | 2.52% | 3.24% |
Note.
M2 is the model without adjustment for socioeconomic and health covariates (i.e., minimally adjusted model); M2_1 is the model adjusted for time-varying socioeconomic and health covariates; and M2_2 is the model adjusted also for baseline BMI (i.e., fully adjusted model).
bFor each model, group membership represents the sample percentage in each trajectory group.
p < .05.
p < .01.
p < .001
Racial/Ethnic Differences in Trajectory Membership
Compared with Whites, Blacks and Hispanics had a significantly greater probability of belonging to the higher BMI trajectories relative to the normal BMI reference trajectory (M2; Table 4). Specifically, Blacks had a twofold increase in the risk of following overweight trajectories (OR = 1.86 for trajectory #2 and OR = 2.65 for trajectory #3) and a three-fold increase in the risk of following the obese trajectories (OR = 3.04 for trajectory #4 and OR = 3.23 for trajectory #5). On the other hand, differences in risk between Hispanics and Whites were greater for overweight trajectories (OR = 2.19 for trajectory #2 and OR = 2.30 for trajectory #3) than for obese trajectories (OR = 1.52 for trajectory #4 and nonsignificant for trajectory #5). Further, differences between Whites and Blacks in the relative odds of trajectory membership disappeared after control for socioeconomic differences and baseline BMI (M2_1 and M2_2; Table 4), while those between Hispanics and Whites increased, mainly in the overweight and borderline-obese trajectories (M2_2; Table 4).
Gender Differences in Trajectory Membership
In all models, females showed smaller odds of following the higher BMI trajectories (Table 4) compared with males. Females had approximately half the risk of belonging to overweight and obese trajectories (OR = 0.43, OR = 0.45, OR = 0.68 respectively, p < .001, for trajectories #2, #3, and #4 in M2; OR = 0.64 for trajectory #4 and OR = 0.51 for trajectory #5 respectively, p < .01 in M2_2). Baseline BMI explained the difference in group membership risk between females and males in overweight trajectories (trajectories #2, #3), but not in obese trajectory groups (trajectories #4, #5).
Effects of Other Covariates
Older respondents had a slightly lower risk of membership in the higher BMI trajectory groups as compared with the reference normal-weight trajectory (M2_2, Table 4). In SES and health-controlled models (M2, M2_1, Table 4), higher education was associated with a slightly lower risk of experiencing the higher BMI trajectories; this inverse association was fully explained by baseline BMI (M2_2, Table 4), with the exception of the risk of following the morbidly obese trajectory (Trajectory #5) for which the risk increased after control for baseline BMI.
Mortality, attrition, and proxy status were included as confounders to account for possible selection bias. Respondents who died during the study period had substantially lower probabilities of experiencing the obese or morbidly obese trajectories (trajectories #4 and #5, M2, M2_1 and M2_2, Table 4). After full adjustment for socioeconomic, health, and baseline BMI differences (M2_2, Table 4), attrition was significantly associated with a higher likelihood of membership in the overweight and borderline-obese trajectory groups (trajectory #2 and 3). The significant associations between mortality/ attrition and the probability of group membership indicate that the coefficients for trajectories estimates would have been incorrect if these sources of selection bias were not explicitly addressed in the models.
Discussion
A major finding of our work is the underlying heterogeneity in trajectories of BMI in middle-aged and older adults. In this cohort, we identified five clusters of BMI growth trajectories and considerable racial/ethnic and gender differences in the propensity to experience each trajectory. In contrast to the conventional approach focusing on the population “average” trajectory of BMI, the group-based approach used here assumes and identifies a number of distinct trajectories, each with a distinct intercept, rate-of-change and estimated population prevalence (Jones & Nagin, 2007). We offer three key observations: (a) as indicated in Table 4, only approximately 20% of the study cohort observes a “normal” BMI trajectory (i.e., normal BMI intercept and weight gain within the normal BMI range), with the rest divided between overweight (43%) and obese or morbidly obese (37%); (b) the main observed differences between the identified trajectories are in their intercepts, suggesting that the accumulation of body weight prior to entering middle age is a critical determinant of the later BMI trajectory; and (c) all trajectory groups gain weight over the 14 years of observation, with the morbidly obese subgroup experiencing a slight deceleration in weight gain over time.
These are troubling findings for two reasons. First, the 20% prevalence of normal BMI trajectory is even lower than recent cross-sectional estimates, which put the prevalence of normal BMI among middle age and older adults at 22% for men and 32% for women (Flegal, Carroll, Ogden, & Curtin, 2010). Second, for two of the groups, the rates of increase justify an upward change in categorization over the study period (from borderline-obese to overtly obese category 1 for trajectory #3 and from obese category 1 to obese category 2 for trajectory #4) and signify a substantial increase in obesity-associated disease risks (National Heart Lung and Blood Institute, National Institutes of Health, 2010). While we expected a more pronounced dissimilarity between the trajectory subgroups (e.g., raising vs. falling vs. stable BMI), the results are in line with observations from population-average longitudinal studies (Barone et al., 2006; Dugravot et al., 2010; Jacobsen et al., 2001), which suggested that BMI increases into older age beyond the point previously believed to represent the age of peak body weight (i.e., around age 65).
Racial/ethnic differences in weight status and weight change have been previously documented primarily in young adults (Clarke et al., 2009; Mujahid, Diez Roux, Borrell, & Nieto, 2005; Sanchez-Vaznaugh, Kawachi, Subramanian, Sanchez, & Acevedo-Garcia, 2009). We found that the course of BMI also differed considerably between the racial/ethnic groups of older adults considered in our study. Notably, the relative distribution of trajectory membership risk by race/ethnicity was sensitive to variations in baseline BMI, but not to variations in socioeconomic or health status indicators. Regardless of SES and health status, Blacks were more likely (compared with Whites) to follow each of the higher BMI trajectories, yet these differences were entirely explained by the BMI with which they enter middle age. Conversely, trajectory risk differences between Hispanics and Whites increased substantially after accounting for baseline BMI. These findings suggest that differences in trajectory risk between Blacks and Whites are established before middle age, while those between Hispanics and Whites persist and even increase from middle to old age. It is quite possible that factors other than the conventional measures of SES (income, assets or education), such as early-life behavioral and cultural factors (Abraido-Lanza, Chao, & Florez, 2005; Akresh, 2007) and birthplace or immigration status differences (Sanchez-Vaznaugh et al., 2009) between the groups, partially explain the timing of weight disadvantage initiation. This is a consequential finding, suggesting that the critical periods for interventions to reduce racial/ ethnic inequalities in weight status are prior to entering middle age among Blacks, and continue into middle and older age among Hispanics.
The analysis also revealed considerable gender differences in trajectory risk— women showing a substantially lower propensity to follow the high-BMI trajectories. The initial association between gender and trajectory risk was partially explained by the baseline BMI (for the overweight and borderline-obese trajectories only), but not by educational or health status differences. It is somewhat difficult to place our results in the context of existing literature, mainly because studies on gender differences in trajectories of body weight are scarce and the results from other studies (cross-sectional or 2-point transitions) are mixed. While some investigations point to a higher prevalence of overweight in men (He & Meng, 2008; Wang & Beydoun, 2007) and a higher prevalence of obesity and higher variability in body weight in women (Jenkins, Fultz, Fonda, & Wray, 2003), others find no such differences (Ogden et al., 2006; Sobal & Rauschenbach, 2003). A recent examination of the average long-term trajectory of BMI in a sample of middle age and older adults found no significant gender differences in either BMI intercept or rate-of-change (Botoseneanu & Liang, 2011). This, coupled with the present finding of differences favorable to women in the obese and morbidly obese BMI trajectories, but not in the overweight trajectories, suggests that significant effects may be overlooked under the classic average-trajectory approach. When such models are employed and distinct trajectories are collapsed into an “average” trajectory, substantial differences on particular trajectories not observed on others may be lost. In contrast, group-based modeling allows not only for discrete trajectories, but also for discrete predictors of each trajectory. Future studies linking gender heterogeneity in body weight evolution to specific health outcomes are needed and may partially explain the observed gender-related disparities in morbidity and mortality in old age (Oksuzyan et al., 2008).
One of the main goals of identifying heterogeneous trajectories of BMI within a given population is to assess whether such trajectories carry differential morbidity and mortality potential. While this kind of analysis is outside the scope of our present study, we can use the results to draw some inferences and suggestions for future research. For example, the female advantage in mortality in older age is well documented. Overweight and obesity have been associated with an increased mortality risk in the same age group (Berrington de Gonzalez et al., 2010; Villareal, Apovian, Kushner, & Klein, 2005). In this context, our results showing that women have a substantially lower risk of following the obese BMI trajectories compared with men imply that the gender gap in mortality may potentially increase in the future. This may also hold true for other health outcomes. Over time and barring effective population-level weight-control interventions, detrimental health consequences associated with obesity will disproportionately accrue in those groups (i.e., racial minorities and men) more likely to follow the high-BMI trajectories and will result in an increase in obesity-related racial and gender health disparities. Additional research linking relevant health outcomes to various trajectories of body weight is warranted; we suggest that priority be given to those conditions which carry a heavy morbidity burden for individuals and society, and for which racial/ethnic or gender disparities have been well documented, such as diabetes (Duru et al., 2009; Saydah, Cowie, Eberhardt, De Rekeneire, & Narayan, 2007), stroke (Glymour, Avendaño, Haas, & Berkman, 2008; Kleindorfer, 2009), dementia (Glymour & Manly, 2008; Husaini et al., 2003), or functional decline and disability (Alley & Chang, 2007; Chen & Guo, 2008; Dunlop, Song, Manheim, Daviglus, & Chang, 2007; Fuller-Thomson, Nuru-Jeter, Minkler, & Guralnik, 2009).
It is paramount to acknowledge that the results derived from group-based modeling are approximations of population differences in BMI trajectories, which are based on subgroup means over a specific period of time (Nagin, 2005). In other words, the results should not be construed to mean that individuals actually “belong” to a trajectory group, but rather that individual trajectories can be clustered into a finite number of subgroups, which is not immutable, but arrived at through assessments of model-fitting indices, to approximate a continuous distribution within a population (Nagin & Tremblay, 2005). Further, while this technique allows for the identification of population risk factors, one should refrain from predicting any particular individual's trajectory membership group based on ex ante individual characteristics. This reflects the fact that even if a set of characteristics increase the probability of individuals following a particular trajectory, not all individuals with those characteristics will follow that trajectory (Nagin & Tremblay, 2005).
Despite the considerable advantages this study offers, some limitations should be acknowledged. First, self-reported BMI was used for trajectory calculation. Individuals are known to overreport height and underreport weight (Nawaz, Chan, Abdulrahman, Larson, & Katz, 2001). As such, self-reported BMI tends to reflect a conservative estimate of the “true” BMI. We performed a comparison of self-reported and interviewer-measured height and weight (available only for the 2004 and 2006 waves) and, consistent with others studies (Fillenbaum et al., 2010; Weir, 2008) found only small differences. Second, BMI may not be the optimal body weight indicator in older age (Seidell & Visscher, 2000). Other anthropometric and body-composition measures (e.g., waist circumference or intra-visceral fat mass) offer more accurate assessments of disease risk (Janssen, Katzmarzyk, & Ross, 2004) and are not affected by the potential age-related loss of height. This limitation is partially mitigated by the observation that the age range of a majority of our respondents was below the age of accelerated height loss (Dey, Rothenberg, Sundh, Bosaeus, & Steen, 1999; Sorkin, Muller, & Andres, 1999). However, some respondents were followed up to age 75, so we cannot entirely rule out the possibility of an upward bias in BMI in later waves. We were unable to perform similar analyses of other body-composition indicators or to assess the potential for artifactual changes in BMI due to loss of height because HRS does not collect such data. Similar studies on heterogeneity in trajectories of other body-composition indicators are needed, as they may prove more accurate predictors of associated disease risk in old age. Lastly, because data were not suitable for age-based analyses (Alwin, Hofer, & McCammon, 2006), the BMI trajectories were estimated as a function of time with adjustment for age-at-baseline, to minimize the potential for age-cohort confounding. Consequently, the results should not be interpreted to represent the effect of age on BMI, but rather the evolution of BMI over time in this specific age group.
The present study identified five distinct trajectories of BMI in a middle age and older population and substantial racial/ethnic and gender differences in the propensity of following each trajectory. Yet this is only an initial step toward a better understanding of the risk factors associated with variability in body weight course starting in middle age. Awareness of discrete BMI trajectories may allow clinicians and policy professionals to tailor programs to specific groups who are at risk for poor aging outcomes due to obesity and to intervene at an early stage to alter the path of risky trajectories.
Supplementary Material
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants T32-AG027708 (to A.B.) and R01-AG154124 and R01-AG028116 (to J.L) from the National Institute on Aging at the National Institutes of Health. The Japanese Ministry of Health, Labor and welfare Longevity Foundation, the Tokyo Metropolitan Institute of Gerontology, and the Michigan Claude D. Pepper Older Americans Independence Center (P60-AG08808) provided additional support (to J.L.).
Footnotes
Authors’ Contribution
All authors meet the criteria for authorship stated in the “Uniform Requirements for Manuscripts Submitted to Biomedical Journals.” A. B. planned the study, performed data management and statistical analysis, interpreted the results, and drafted and revised the manuscript. Jersey Liang helped in planning the study, supervised the data analysis and interpretation of results, and contributed to writing and revising the manuscript. Both authors gave their final approval for the version submitted for publication.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abraido-Lanza AF, Chao MT, Florez KR. Do healthy behaviors decline with greater acculturation? Implications for the Latino mortality paradox. Social Science & Medicine. 2005;61:1243–1255. doi: 10.1016/j.socscimed.2005.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akresh IR. Dietary assimilation and health among Hispanic immigrants to the United States. Journal of Health and Social Behavior. 2007;48:404–417. doi: 10.1177/002214650704800405. [DOI] [PubMed] [Google Scholar]
- Alley DE, Chang VW. The changing relationship of obesity and disability, 1988-2004. Journal of American Medical Association. 2007;298:2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- Alwin DF, Hofer SM, McCammon RJ. Modeling the effects of time: Integrating demographic and developmental perspectives. In: Binstock RH, George LK, editors. Handbook of aging and the social sciences. 6th ed. Elsevier; San Diego, CA: 2006. pp. 20–38. [Google Scholar]
- Ball K, Crawford D. Socioeconomic status and weight change in adults: A review. Social Science & Medicine. 2005;60:1987–2010. doi: 10.1016/j.socscimed.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Barone BB, Clark JM, Wang NY, Meoni LA, Klag MJ, Brancati FL. Lifetime weight patterns in male physicians: The effects of cohort and selective survival. Obesity. 2006;14:902–908. doi: 10.1038/oby.2006.104. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31:285–293. [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Freeman LB. Body-mass index and mortality among 1.46 million White adults. New England Journal of Medicine. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botoseneanu A, Liang J. Social stratification of body weight trajectory in middle-age and older Americans: Results from a 14-year longitudinal study. Journal of Aging and Health. 2011;23:454–480. doi: 10.1177/0898264310385930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang VW, Lauderdale DS. Income disparities in body mass index and obesity in the United States, 1971-2002. Archives of Internal Medicine. 2005;165:2122–2128. doi: 10.1001/archinte.165.18.2122. [DOI] [PubMed] [Google Scholar]
- Chen H, Guo X. Obesity and functional disability in elderly Americans. Journal of American Geriatrics Society. 2008;56:689–694. doi: 10.1111/j.1532-5415.2007.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, O'Malley PM, Johnston LD, Schulenberg JE. Social disparities in BMI trajectories across adulthood by gender, race/ethnicity and lifetime socioeconomic position: 1986-2004. International Journal of Epidemiology. 2009;38:499–509. doi: 10.1093/ije/dyn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D, Rothenberg E, Sundh V, Bosaeus I, Steen B. Height and body weight in the elderly. A 25-year longitudinal study of a population aged 70 to 95 years. European Journal of Clinical Nutrition. 1999;53:905–914. doi: 10.1038/sj.ejcn.1600852. [DOI] [PubMed] [Google Scholar]
- Dugravot A, Sabia S, Stringhini S, Kivimaki M, Westerlund H, Vahtera J, Nabi H. Do socioeconomic factors shape weight and obesity trajectories over the transition from midlife to old age? Results from the French GAZEL cohort study. American Journal of Clinical Nutrition. 2010;92(1):16–23. doi: 10.3945/ajcn.2010.29223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop DD, Song J, Manheim LM, Daviglus ML, Chang RW. Racial/ethnic differences in the development of disability among older adults. American Journal of Public Health. 2007;97:2209–2215. doi: 10.2105/AJPH.2006.106047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru OK, Gerzoff RB, Selby JV, Brown AF, Ackermann RT, Karter AJ, Waitzfelder B. Identifying risk factors for racial disparities in diabetes outcomes: the Translating Research Into Action for Diabetes (TRIAD) Study. Medical Care. 2009;47:700–706. doi: 10.1097/mlr.0b013e318192609d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J, Forsen T, Osmond C, Barker D. Obesity from cradle to grave. International Journal of Obesity. 2003;27:722–727. doi: 10.1038/sj.ijo.0802278. [DOI] [PubMed] [Google Scholar]
- Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Archives of Internal Medicine. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- Fillenbaum GG, Kuchibhatla MN, Whitson HE, Batch BC, Svetkey LP, Pieper CF, Blazer DG. Accuracy of self-reported height and weight in a community-based sample of older African Americans and Whites. Journal of Gerontology: Medical Sciences. 2010;65:1123–1129. doi: 10.1093/gerona/glq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Affairs. 2009;28:822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Journal of American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Fonda SJ, Fultz NH, Jenkins KR, Wheeler LM, Wray LA. Relationship of body mass and net worth for retirement-aged men and women. Research on Aging. 2004;26:153–176. [Google Scholar]
- Fuller-Thomson E, Nuru-Jeter A, Minkler M, Guralnik JM. Black— White disparities in disability among older Americans. Journal of Aging and Health. 2009;21:677–698. doi: 10.1177/0898264309338296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A. Struggles with survey weighting and regression modeling. Statistical Science. 2007;22:153–164. [Google Scholar]
- Glymour MM, Avendaño M, Haas S, Berkman LF. Lifecourse social conditions and racial disparities in incidence of first stroke. Annals of Epidemiology. 2008;18:904–912. doi: 10.1016/j.annepidem.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review. 2008;18:223–254. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- Harel O, Hofer SM, Hoffman L, Pedersen NL. Population inference with mortality and attrition in longitudinal studies on aging: A two-stage multiple imputation method. Experimental Aging Research. 2007;33:187–203. doi: 10.1080/03610730701239004. [DOI] [PubMed] [Google Scholar]
- He XZ, Meng H. Changes in weight among U.S. adults aged 70 and over, 1993 to 2002. Preventive Medicine. 2008;47:489–493. doi: 10.1016/j.ypmed.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Hedeker DR, Gibbons RD. Longitudinal data analysis. John Wiley & Sons; Hoboken, NJ: 2006. [Google Scholar]
- Husaini BA, Sherkat DE, Moonis M, Levine R, Holzer C, Cain VA. Racial differences in the diagnosis of dementia and in its effects on the use and costs of health care services. Psychiatric Services. 2003;54:92–96. doi: 10.1176/appi.ps.54.1.92. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Njolstad I, Thune I, Wilsgaard T, Lochen ML, Schirmer H. Increase in weight in all birth cohorts in a general population: The Tromso Study, 1974-1994. Archives of Internal Medicine. 2001;161:466–472. doi: 10.1001/archinte.161.3.466. [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. American Journal of Clinical Nutrition. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- Jenkins KR, Fultz NH, Fonda SJ, Wray LA. Patterns of body weight in middle-aged and older Americans, by gender and race, 1993-2000. Social and Preventive Medicine. 2003;48:257–268. doi: 10.1007/s00038-003-2053-3. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods and Research. 2007;35:542–571. [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Kahng SK, Dunkle RE, Jackson JS. The relationship between the trajectory of body mass index and health trajectory among older adults: Multilevel modeling analyses. Research on Aging. 2004;26(1):31–61. [Google Scholar]
- Kleindorfer D. Sociodemographic groups at risk: Race/Ethnicity. Stroke. 2009;40(Supplement 1):S75–S78. doi: 10.1161/STROKEAHA.108.534909. [DOI] [PubMed] [Google Scholar]
- Liang J, Bennett JM, Shaw BA, Quinones AR, Ye W, Xu X, Ofstedal MB. Gender differences in functional status in middle and older age: Are there any age variations? Journal of Gerontology: Social Sciences. 2008;63:S282–292. doi: 10.1093/geronb/63.5.s282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. Wiley-Interscience; Hoboken, NJ: 2002. [Google Scholar]
- McDowell MA, Fryar CD, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2003-2006 (National Health Statistics Reports (NHSR, No.10) National Center for Health Statistics; Hyattsville, MD: 2008. [PubMed] [Google Scholar]
- Mehta NK, Chang VW. Mortality attributable to obesity among middle-aged adults in the United States. Demography. 2009;46:851–872. doi: 10.1353/dem.0.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A. Change in life satisfaction during adulthood: Findings from the Veterans Affairs Normative Aging Study. Journal of Personality and Social Psychology. 2005;88(1):189–202. doi: 10.1037/0022-3514.88.1.189. [DOI] [PubMed] [Google Scholar]
- Mujahid MS, Diez Roux AV, Borrell LN, Nieto FJ. Cross-sectional and longitudinal associations of BMI with socioeconomic characteristics. Obesity Research. 2005;13:1412–1421. doi: 10.1038/oby.2005.171. [DOI] [PubMed] [Google Scholar]
- Nagi SZ. The concept and measurement of disability. Praeger; New York, NY: 1979. [Google Scholar]
- Nagin DS. Group-based modeling of development over the life course. Harvard University Press; Cambridge, MA: 2005. [Google Scholar]
- Nagin DS, Tremblay RE. Developmental trajectory groups: Fact or a useful statistical fiction? Criminology. 2005;43:873–904. [Google Scholar]
- National Heart Lung and Blood Institute, & National Institutes of Health Classification of overweight and obesity by BMI, waist circumference, and associated disease risks. Retrieved from http://www.nhlbi.nih.gov/health/public/heart/obesity/lose_wt/bmi_dis.htm.
- Nawaz H, Chan W, Abdulrahman M, Larson D, Katz DL. Self-reported weight and height implications for obesity research. American Journal of Preventive Medicine. 2001;20:294–298. doi: 10.1016/s0749-3797(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP, Cardiovascular Study Research Group Weight change in old age and its association with mortality. Journal of American Geriatrics Society. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. Journal of American Medical Association. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Oksuzyan A, Juel K, Vaupel JW, Christensen K, Denmark S, Copenhagen D. Men: Good health and high mortality. Sex differences in health and aging. Aging Clinical and Experimental Research. 2008;20(2):91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biological Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reynolds SL, Himes CL. Cohort differences in adult obesity in the United States: 1982-2002. Journal of Aging and Health. 2007;19:831–850. doi: 10.1177/0898264307305182. [DOI] [PubMed] [Google Scholar]
- Rogosa DR. Myths about longitudinal research. In: Schaie KW, Campbell RT, Meredith WM, Rawlings SC, editors. Methodological issues in aging research. Springer; New York, NY: 1988. pp. 171–209. [Google Scholar]
- Sanchez-Vaznaugh EV, Kawachi I, Subramanian SV, Sanchez BN, Acevedo-Garcia D. Do socioeconomic gradients in body mass index vary by race/ethnicity, gender, and birthplace? American Journal of Epidemiology. 2009;169:1102–1112. doi: 10.1093/aje/kwp027. [DOI] [PubMed] [Google Scholar]
- Saydah S, Cowie C, Eberhardt MS, De Rekeneire N, Narayan KMV. Race and ethnic differences in glycemic control among adults with diagnosed diabetes in the United States. Ethnicity & Disease. 2007;17:529–535. [PubMed] [Google Scholar]
- Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A data analyst's perspective. Multivariate Behavioral Research. 1998;33:545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- Seidell JC, Visscher TL. Body weight and weight change and their health implications for the elderly. European Journal of Clinical Nutrition. 2000;54(3):33–39. doi: 10.1038/sj.ejcn.1601023. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press, USA; New York, NY: 2003. [Google Scholar]
- Sobal J, Rauschenbach BS. Gender, marital status, and body weight in older US adults. Gender Issues. 2003;21(3):75–94. [Google Scholar]
- Sobal J, Rauschenbach B, Frongillo EA. Marital status changes and body weight changes: A US longitudinal analysis. Social Science & Medicine. 2003;56:1543–1555. doi: 10.1016/s0277-9536(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Sorkin JD, Muller DC, Andres R. Longitudinal change in height of men and women: Implications for interpretation of the body mass index: The Baltimore Longitudinal Study of Aging. American Journal of Epidemiology. 1999;150:969–977. doi: 10.1093/oxfordjournals.aje.a010106. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, the Obesity Society. American Journal of Clinical Nutrition. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA. The obesity epidemic in the United States— gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiologic Reviews. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- Weir D. Elastic powers: The integration of biomarkers into the Health and Retirement Study. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial surveys. Committee on advances in collecting and utilizing biological indicators and genetic information in social science surveys. National Academies Press; Washington, DC: 2008. pp. 78–95. [Google Scholar]
- Winship C, Radbill L. Sampling weights and regression analysis. Sociological Methods & Research. 1994;23:230–257. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.