Abstract
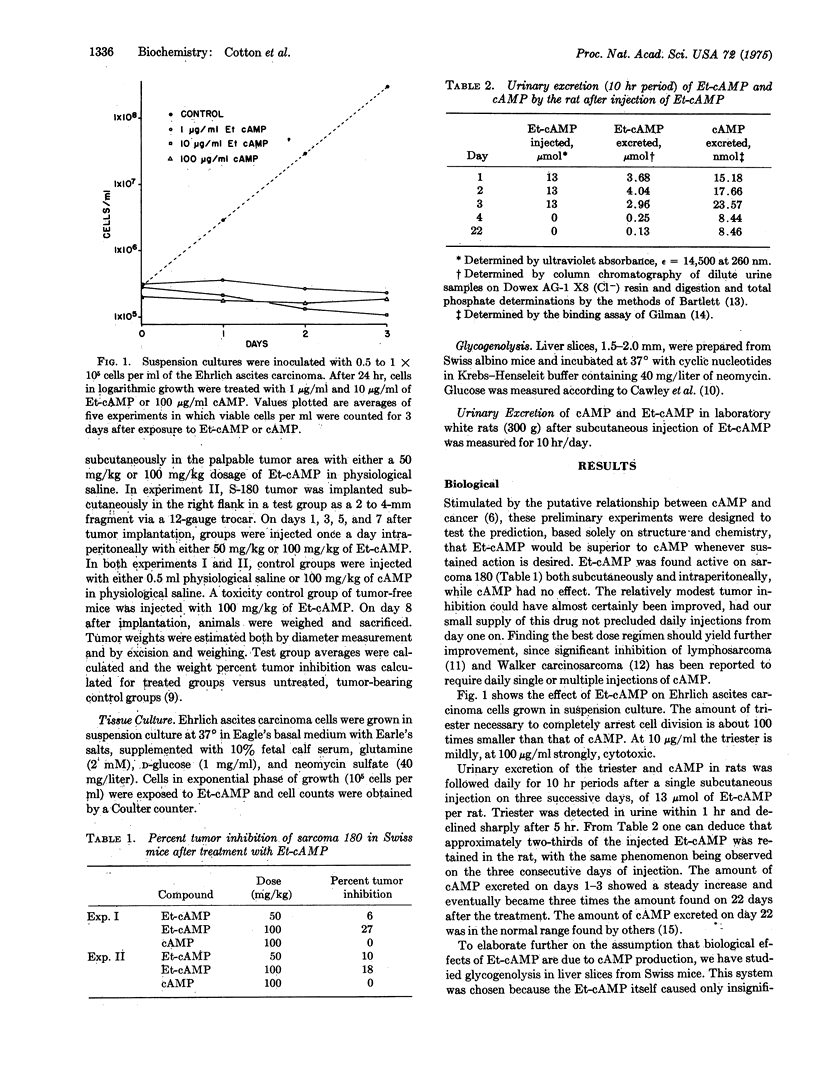
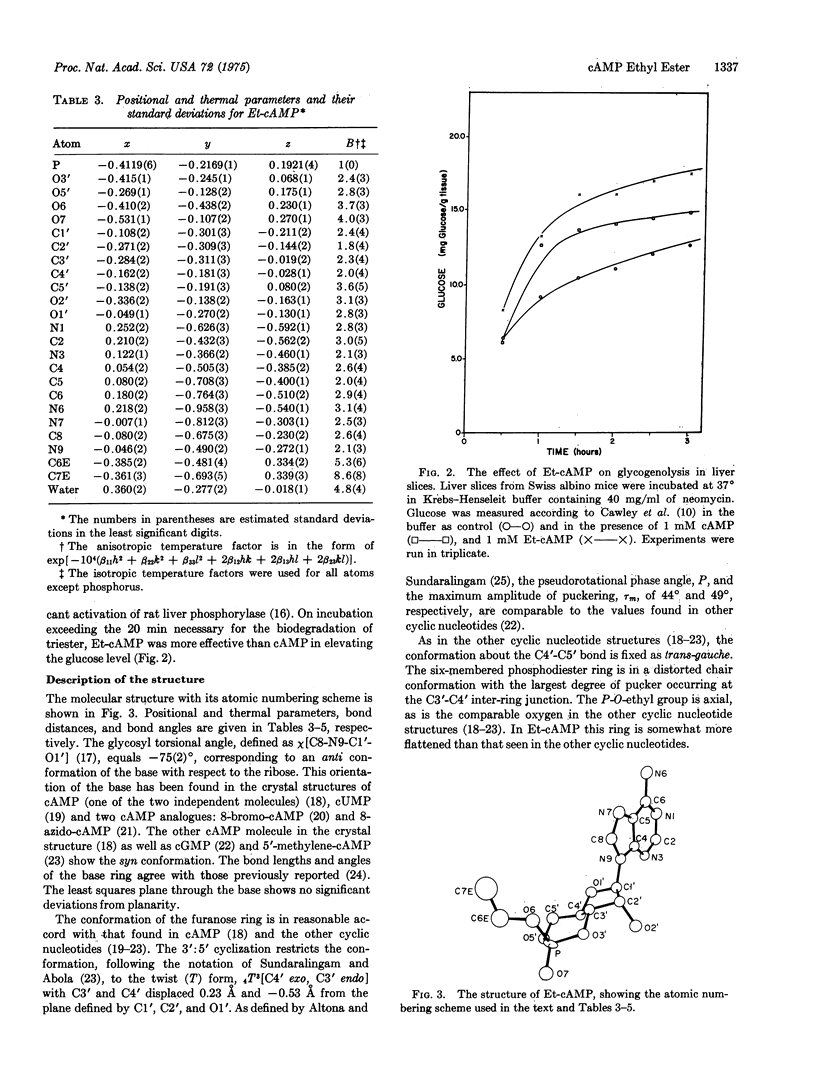
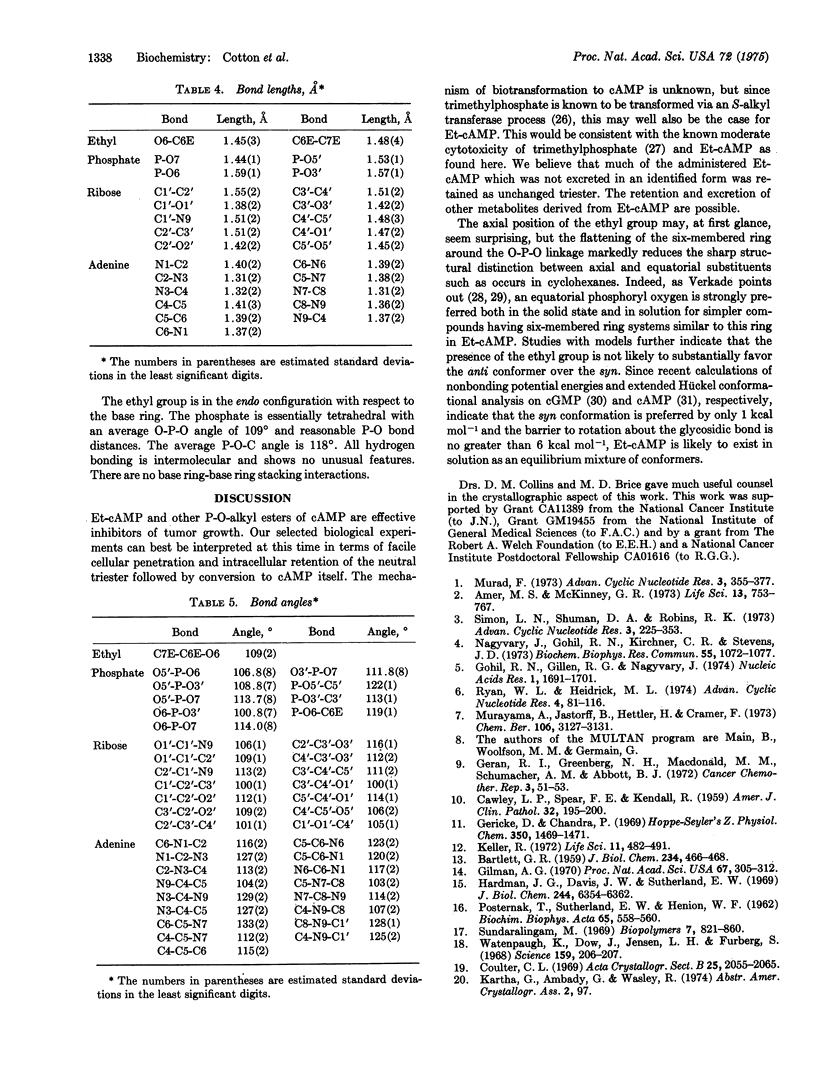
The P-O-ethyl ester of cAMP has been synthesized, its inhibition of solid and ascites tumors studied, and its pattern of urinary excretion followed. Et-cAMP is more effective than cAMP against solid sarcoma 180 in mice and against Ehrlich ascites carcinoma cells in tissue culture. The urinary excretion pattern of injected E-t-cAMP suggests that about two-thirds of the injected dose (13 mumol per animal) is retained in the rat rather than being promptly excreted. Liver slice studies of the effect on glycogenolysis suggest that the Et-cAMP is converted to cAMP intracellularly. The compound crystallizes in space group P21 with one molecule per asymmetric unit. The base ring has the anti conformation. The ethyl group is endo to the base ring and is axial in the flattened chair-conformer six-membered ring formed by the 3'-5' O-P-O cyclization. In most other respects the structure of the compound is closely similar to the known structures of other cyclic nucleotides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Amer M. S., McKinney G. R. Possibilities for drug development based on the cyclic AMP system. Life Sci. 1973 Oct 1;13(7):753–767. doi: 10.1016/0024-3205(73)90066-0. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Chwang A. K., Sundaralingam M. Molecular conformation of guanosine 3,'5-cyclic monophosphate. Nat New Biol. 1973 Aug 1;244(135):136–137. doi: 10.1038/newbio244136a0. [DOI] [PubMed] [Google Scholar]
- Coulter C. L. The crystal and molecular structure of the triethylammonium salt of cyclic uridine-3',5'-phosphate. Acta Crystallogr B. 1969 Oct 15;25(10):2055–2065. doi: 10.1107/s0567740869005140. [DOI] [PubMed] [Google Scholar]
- Epstein S. S., Bass W., Arnold E., Bishop Y. Mutagenicity of trimethylphosphate in mice. Science. 1970 May 1;168(3931):584–586. doi: 10.1126/science.168.3931.584. [DOI] [PubMed] [Google Scholar]
- Gericke D., Chandra P. Inhibition of tumor growth by nucleoside cyclic 3'-5'-monophosphates. Hoppe Seylers Z Physiol Chem. 1969 Nov;350(11):1469–1471. doi: 10.1515/bchm2.1969.350.2.1469. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil R. N., Gillen R. G., Nagyvary J. Synthesis and properties of some cyclic AMP alkyl phosphotriesters. Nucleic Acids Res. 1974 Dec;1(12):1691–1701. doi: 10.1093/nar/1.12.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbmann M. R., Gagné W. E., Williams S. N. Short-term toxicity studies of rats fed triethylphosphate in the diet. Toxicol Appl Pharmacol. 1968 May;12(3):360–371. doi: 10.1016/0041-008x(68)90145-2. [DOI] [PubMed] [Google Scholar]
- Hardman J. G., Davis J. W., Sutherland E. W. Effects of some hormonal and other factors on the excretion of guanosine 3',5'-monophosphate and adenosine 3',5'-monophosphate in rat urine. J Biol Chem. 1969 Dec 10;244(23):6354–6362. [PubMed] [Google Scholar]
- Lespinasse J. N., Vasilescu D. Conformational analysis of a cyclic nucleotide: 3'-5'-adenosine monophosphate. A study based on the extended Hückel theory. Biopolymers. 1974 Jan;13(1):63–75. doi: 10.1002/bip.1974.360130104. [DOI] [PubMed] [Google Scholar]
- Murad F. Clinical studies and applications of cyclic nucleotides. Adv Cyclic Nucleotide Res. 1973;3:355–383. [PubMed] [Google Scholar]
- Nagyvary J., Gohil R. N., Kirchner C. R., Stevens J. D. Studies on neutral esters of cyclic AMP. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1072–1077. doi: 10.1016/s0006-291x(73)80004-x. [DOI] [PubMed] [Google Scholar]
- POSTERNAK T., SUTHERLAND E. W., HENION W. F. Derivatives of cyclic 3',5'-adenosine monophosphate. Biochim Biophys Acta. 1962 Dec 17;65:558–560. doi: 10.1016/0006-3002(62)90475-4. [DOI] [PubMed] [Google Scholar]
- Rohrer D. C., Sundaralingam M. Stereochemistry of nucleic acids and their constituents. XII. The crystal and molecular structure of alpha-D-2'-deoxyadenosine monohydrate. J Am Chem Soc. 1970 Aug 12;92(16):4956–4962. doi: 10.1021/ja00719a032. [DOI] [PubMed] [Google Scholar]
- Ryan W. L., Heidrick M. L. Role of cyclic nucleotides in cancer. Adv Cyclic Nucleotide Res. 1974;4(0):81–116. [PubMed] [Google Scholar]
- Simon L. N., Shuman D. A., Robins R. K. The chemistry and biological properties of nucleotides related to nucleoside 3',5'-cyclic phosphates. Adv Cyclic Nucleotide Res. 1973;3:225–353. [PubMed] [Google Scholar]
- Sundaralingam M., Abola J. Stereochemistry of nucleic acids and their constituents. XXVII. The crystal structure of 5'-methyleneadenosine 3',5'-cyclic monophosphonate monohydrate, a biologically active analog of the secondary hormonal messenger cyclic adenosine 3',5-monophosphate. Conformational "rigidity" of the furanose ring in cyclic nucleotides. J Am Chem Soc. 1972 Jul 12;94(14):5070–5076. doi: 10.1021/ja00769a047. [DOI] [PubMed] [Google Scholar]
- Verkade J. G. Phosphate basicity and nucleophilicity loss upon constraint: the role of the alkoxy oxygens. Bioinorg Chem. 1974 Jan;3(2):165–182. doi: 10.1016/s0006-3061(00)80040-x. [DOI] [PubMed] [Google Scholar]
- Watenpaugh K., Dow J., Jensen L. H., Furberg S. Crystal and molecular structure of adenosine 3',5'-cyclic phosphate. Science. 1968 Jan 12;159(3811):206–207. doi: 10.1126/science.159.3811.206. [DOI] [PubMed] [Google Scholar]


